The MNK1/2-eIF4E Axis as a Potential Therapeutic Target in Melanoma
Abstract
1. Classification of Melanoma Molecular Subtypes
2. Frequently Occurring Mutations in Melanoma and Molecular Significance
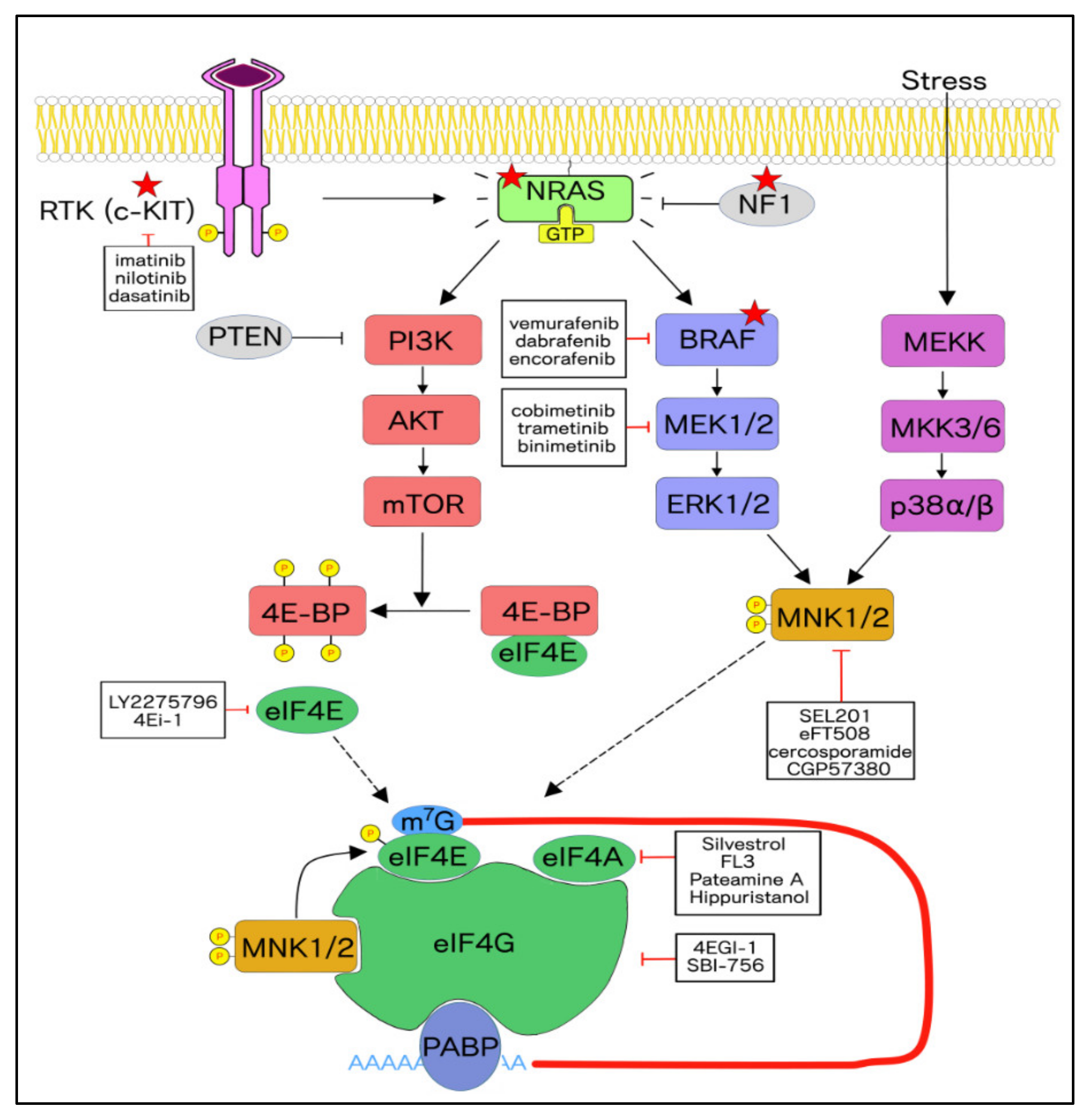
3. Targeted Therapies for Specific Molecular Subtypes in Melanoma
3.1. Rationale for Targeting the MNK1/2-eIF4E Axis in Cancer
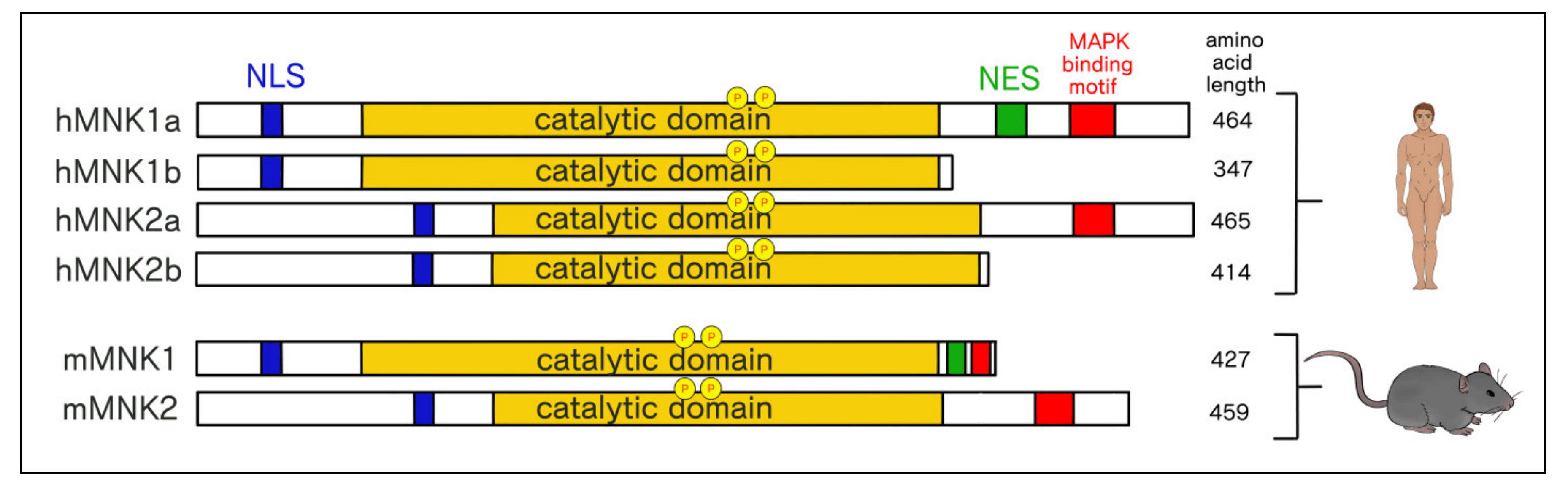
3.2. Structural and Functional Differences Between MNK1/2 Isoforms
3.3. Differential Cellular Localization of MNK1a/b and MNK2a/b
3.4. Identified Substrates of MNK1/2
4. Translational Targets of the MNK1/2-eIF4E Axis
5. Therapeutic Targeting of the Cap-Dependent Translational Machinery
5.1. Directly Targeting the eIF4F Complex
5.2. Putting a Cap on eIF4E
5.3. Disrupting the eIF4E:eIF4G Interaction
5.4. Inhibitors of eIF4A
5.5. Toggling the Regulation of eIF4E by Targeting Upstream Kinases
6. Immunotherapy and Melanoma
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Curtin, J.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Carvajal, R.D. GNAQ and GNA11 mutations in uveal melanoma. Melanoma Res. 2014, 24, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Carr, M.J.; Khushalani, N.I. Principles of Targeted Therapy for Melanoma. Surg. Clin. North Am. 2019, 100, 175–188. [Google Scholar] [CrossRef]
- Pratilas, C.A.; Taylor, B.S.; Ye, Q.; Viale, A.; Sander, C.; Solit, D.B.; Rosen, N. (V600E) BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc. Natl. Acad. Sci. USA 2009, 106, 4519–4524. [Google Scholar] [CrossRef] [PubMed]
- Nassar, K.W.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Boil. 2020, 61, 139–148. [Google Scholar] [CrossRef]
- Devitt, B.; Liu, W.; Salemi, R.; Wolfe, R.; Kelly, J.; Tzen, C.-Y.; Dobrovic, A.; McArthur, G. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment. Cell Melanoma Res. 2011, 24, 666–672. [Google Scholar] [CrossRef]
- Jakob, J.A.; Bassett, R.L.; Ng, C.S.; Curry, J.L.; Joseph, R.W.; Alvarado, G.C.; Rohlfs, M.L.; Richard, J.; Gershenwald, J.E.; Kim, K.B.; et al. NRAS mutation status is an independent prognostic factor in metastatic melanoma. Cancer 2011, 118, 4014–4023. [Google Scholar] [CrossRef]
- Babaei, M.A.; Goh, Y.-M.; Saleem, M.; Huri, H.Z.; Ahmadipour, F. Receptor tyrosine kinase (c-Kit) inhibitors: A potential therapeutic target in cancer cells. Drug Des. Dev. Ther. 2016, 10, 2443–2459. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; Dummer, R.; Garbe, C.; Testori, A.; Maio, M.; et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Yang, A.S.; Chapman, P.B. The History and Future of Chemotherapy for Melanoma. Hematol. Clin. North Am. 2009, 23, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Sosman, J.A.; Kim, K.B.; Schuchter, L.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.; Hutson, T.E.; Moschos, S.J.; Flaherty, K.T.; et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. New Engl. J. Med. 2012, 366, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Hugo, W.; Kong, X.; Hong, A.; Koya, R.C.; Moriceau, G.; Chodon, T.; Guo, R.; Johnson, D.B.; Dahlman, K.B.; et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2013, 4, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Heidorn, S.J.; Milagre, C.; Whittaker, S.R.; Nourry, A.; Niculescu-Duvas, I.; Dhomen, N.; Hussain, J.; Reis-Filho, J.S.; Springer, C.J.; Pritchard, C.; et al. Kinase-Dead BRAF and Oncogenic RAS Cooperate to Drive Tumor Progression through CRAF. Cell 2010, 140, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Das Thakur, M.; Salangsang, F.; Landman, A.S.; Sellers, W.R.; Pryer, N.K.; Levesque, M.P.; Dummer, R.; McMahon, M.; Stuart, D.D. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013, 494, 251–255. [Google Scholar] [CrossRef]
- Broman, K.K.; Dossett, L.; Sun, J.; Eroglu, Z.; Zager, J.S. Update on BRAF and MEK inhibition for treatment of melanoma in metastatic, unresectable, and adjuvant settings. Expert Opin. Drug Saf. 2019, 18, 381–392. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. New Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Long, G.V.; Hauschild, A.; Santinami, M.; Atkinson, V.; Mandalà, M.; Sileni, V.C.; Larkin, J.; Nyakas, M.; Dutriaux, C.; Haydon, A.; et al. Adjuvant Dabrafenib plus Trametinib in Stage IIIBRAF-Mutated Melanoma. New Engl. J. Med. 2017, 377, 1813–1823. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.; Arance, A.; Mandalà, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsová, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF -mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Kakadia, S.; Yarlagadda, N.; Awad, R.; Kundranda, M.; Niu, J.; Naraev, B.; Mina, L.; Dragovich, T.; Gimbel, M.; Mahmoud, F. Mechanisms of resistance to BRAF and MEK inhibitors and clinical update of US Food and Drug Administration-approved targeted therapy in advanced melanoma. Oncol. Targets Ther. 2018, 11, 7095–7107. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Brunton, H.; Rowling, E.J.; Ferguson, J.; Arozarena, I.; Miskolczi, Z.; Lee, J.L.; Girotti, M.R.; Marais, R.; Levesque, M.P.; et al. Inhibiting Drivers of Non-mutational Drug Tolerance Is a Salvage Strategy for Targeted Melanoma Therapy. Cancer Cell 2016, 29, 270–284. [Google Scholar] [CrossRef]
- Kiuru, M.; Busam, K.J. The NF1 gene in tumor syndromes and melanoma. Lab. Investig. 2017, 97, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Dankort, D.; Curley, D.P.; Cartlidge, R.A.; Nelson, B.; Karnezis, A.N.; Damsky, W.E.; You, M.J.; Depinho, R.A.; McMahon, M.; Bosenberg, M.W. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat. Genet. 2009, 41, 544–552. [Google Scholar] [CrossRef]
- Singh, H.; Longo, D.L.; Chabner, B.A. Improving Prospects for Targeting RAS. J. Clin. Oncol. 2015, 33, 3650–3659. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Salama, A.K.; Niedzwiecki, D.; Johnson, J.; Linette, G.; Bucher, C.; Blaskovich, M.A.; Sebti, S.M.; Haluska, F. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma (CALGB 500104). J. Transl. Med. 2012, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Dummer, R.; Schadendorf, D.; Ascierto, P.A.; Arance, A.; Dutriaux, C.; di Giacomo, A.M.; Rutkowski, P.; del Vecchio, M.; Gutzmer, R.; Mandalà, M.; et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 435–445. [Google Scholar] [CrossRef]
- Najem, A.; Krayem, M.; Salès, F.; Hussein, N.; Badran, B.; Robert, C.; Awada, A.; Journe, F.; Ghanem, G. P53 and MITF/Bcl-2 identified as key pathways in the acquired resistance of NRAS-mutant melanoma to MEK inhibition. Eur. J. Cancer 2017, 83, 154–165. [Google Scholar] [CrossRef]
- Nakamura, A.; Arita, T.; Tsuchiya, S.; Donelan, J.; Chouitar, J.; Carideo, E.; Galvin, K.; Okaniwa, M.; Ishikawa, T.; Yoshida, S. Antitumor Activity of the Selective Pan-RAF Inhibitor TAK-632 in BRAF Inhibitor-Resistant Melanoma. Cancer Res. 2013, 73, 7043–7055. [Google Scholar] [CrossRef]
- Wong, D.J.; Robert, L.; Atefi, M.; Lassen, A.; Avarappatt, G.; Cerniglia, M.; Avramis, E.; Tsoi, J.; Foulad, D.; Graeber, T.; et al. Antitumor activity of the ERK inhibitor SCH772984 [corrected] against BRAF mutant, NRAS mutant and wild-type melanoma. Mol. Cancer 2014, 13, 194. [Google Scholar] [CrossRef]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef]
- Guo, J.; Si, L.; Kong, Y.; Flaherty, K.T.; Xu, X.; Zhu, Y.; Corless, C.L.; Li, L.; Li, H.; Sheng, X.; et al. Phase II, Open-Label, Single-Arm Trial of Imatinib Mesylate in Patients With Metastatic Melanoma Harboring c-Kit Mutation or Amplification. J. Clin. Oncol. 2011, 29, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Lee, S.; Rubin, K.M.; Lawrence, D.P.; Iafrarte, A.J.; Borger, D.R.; Margolin, K.; Leitao, M.M.; Tarhini, A.A.; Koon, H.B.; et al. A phase 2 trial of dasatinib in patients with locally advanced or stage IV mucosal, acral, or vulvovaginal melanoma: A trial of the ECOG-ACRIN Cancer Research Group (E2607). Cancer 2017, 123, 2688–2697. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.-A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a Therapeutic Target in Metastatic Melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gu, Z.; Wu, J.; Huang, X.; Zhou, R.; Shi, C.; Tao, W.; Wang, L.; Wang, Y.; Zhou, G.; et al. Repurposing Ponatinib as a Potent Agent against KIT Mutant Melanomas. Theranostics 2019, 9, 1952–1964. [Google Scholar] [CrossRef]
- Casali, P.; le Cesne, A.; Velasco, A.P.; Kotasek, D.; Rutkowski, P.; Hohenberger, P.; Fumagalli, E.; Judson, I.; Italiano, A.; Gelderblom, H.; et al. Time to Definitive Failure to the First Tyrosine Kinase Inhibitor in Localized GI Stromal Tumors Treated With Imatinib As an Adjuvant: A European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Intergroup Randomized Trial in Collaboration With the Australasian Gastro-Intestinal Trials Group, UNICANCER, French Sarcoma Group, Italian Sarcoma Group, and Spanish Group for Research on Sarcomas. J. Clin. Oncol. 2015, 33, 4276–4283. [Google Scholar] [CrossRef] [PubMed]
- Minor, D.R.; Kashani-Sabet, M.; Garrido, M.; O’Day, S.J.; Hamid, O.; Bastian, B.C. Sunitinib Therapy for Melanoma Patients withKITMutations. Clin. Cancer Res. 2012, 18, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.R.; Becker, T.M.; Kefford, R.; Rizos, H. Secondary c-Kit mutations confer acquired resistance to RTK inhibitors in c-Kit mutant melanoma cells. Pigment. Cell Melanoma Res. 2013, 26, 518–526. [Google Scholar] [CrossRef]
- Conca, E.; Negri, T.; Gronchi, A.; Fumagalli, E.; Tamborini, E.; Pavan, G.M.; Fermeglia, M.; Pierotti, M.; Pricl, S.; Pilotti, S. Activate and resist: L576P-KIT in GIST. Mol. Cancer Ther. 2009, 8, 2491–2495. [Google Scholar] [CrossRef][Green Version]
- Woodman, S.E.; Trent, J.C.; Stemke-Hale, K.; Lazar, A.; Pricl, S.; Pavan, G.M.; Fermeglia, M.; Gopal, Y.V.; Yang, D.; Podoloff, N.A.; et al. Activity of dasatinib against L576P KIT mutant melanoma: Molecular, cellular, and clinical correlates. Mol. Cancer Ther. 2009, 8, 2079–2085. [Google Scholar] [CrossRef]
- Sabnis, A.J.; Bivona, T.G. Principles of Resistance to Targeted Cancer Therapy: Lessons from Basic and Translational Cancer Biology. Trends Mol. Med. 2019, 25, 185–197. [Google Scholar] [CrossRef]
- Marcotrigiano, J.; Gingras, A.-C.; Sonenberg, N.; Burley, S.K. Cap-Dependent Translation Initiation in Eukaryotes Is Regulated by a Molecular Mimic of eIF4G. Mol. Cell 1999, 3, 707–716. [Google Scholar] [CrossRef]
- Ueda, T.; Watanabe-Fukunaga, R.; Fukuyama, H.; Nagata, S.; Fukunaga, R. Mnk2 and Mnk1 Are Essential for Constitutive and Inducible Phosphorylation of Eukaryotic Initiation Factor 4E but Not for Cell Growth or Development. Mol. Cell. Boil. 2004, 24, 6539–6549. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.; Proud, C.G. Serine 209, Not Serine 53, Is the Major Site of Phosphorylation in Initiation Factor eIF-4E in Serum-treated Chinese Hamster Ovary Cells. J. Boil. Chem. 1995, 270, 21684–21688. [Google Scholar] [CrossRef] [PubMed]
- Waskiewicz, A.J.; Johnson, J.C.; Penn, B.; Mahalingam, M.; Kimball, S.R.; Cooper, J. Phosphorylation of the Cap-Binding Protein Eukaryotic Translation Initiation Factor 4E by Protein Kinase Mnk1 In Vivo. Mol. Cell. Boil. 1999, 19, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Holm, N.; Byrnes, K.; Johnson, L.; Abreo, F.; Sehon, K.; Alley, J.; Meschonat, C.; Chu, Q.; Li, B.D.L. A Prospective Trial on Initiation Factor 4E (eIF4E) Overexpression and Cancer Recurrence in Node-Negative Breast Cancer. Ann. Surg. Oncol. 2008, 15, 3207–3215. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, S.; Tam, K.J.; Ardekani, G.S.; Martinka, M.; McElwee, K.J.; Ong, C.J. EIF4E Is an Adverse Prognostic Marker of Melanoma Patient Survival by Increasing Melanoma Cell Invasion. J. Investig. Dermatol. 2015, 135, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.R.; Konicek, B.W.; Lynch, R.L.; Dumstorf, C.A.; Dowless, M.S.; McNulty, A.M.; Parsons, S.H.; Brail, L.H.; Colligan, B.M.; Koop, J.W.; et al. eIF4E Activation Is Commonly Elevated in Advanced Human Prostate Cancers and Significantly Related to Reduced Patient Survival. Cancer Res. 2009, 69, 3866–3873. [Google Scholar] [CrossRef]
- Fang, D.; Peng, J.; Wang, G.; Zhou, D.; Geng, X. Upregulation of eukaryotic translation initiation factor 4E associates with a poor prognosis in gallbladder cancer and promotes cell proliferation in vitro and in vivo. Int. J. Mol. Med. 2019, 44, 1325–1332. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Tsai, H.-P.; Wu, C.-C.; Wang, J.-Y.; Chai, C.-Y. Eukaryotic translation initiation factor 4E (eIF-4E) expressions are associated with poor prognosis in colorectal adenocarcinoma. Pathol. Res. Pr. 2017, 213, 490–495. [Google Scholar] [CrossRef]
- Jiang, X.-M.; Yu, X.-N.; Huang, R.-Z.; Zhu, H.-R.; Chen, X.-P.; Xiong, J.; Chen, Z.-Y.; Huang, X.-X.; Shen, X.; Zhu, J.-M. Prognostic significance of eukaryotic initiation factor 4E in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 2016, 142, 2309–2317. [Google Scholar] [CrossRef]
- Salehi, Z.; Mashayekhi, F.; Shahosseini, F. Significance of eIF4E expression in skin squamous cell carcinoma. Cell Boil. Int. 2007, 31, 1400–1404. [Google Scholar] [CrossRef] [PubMed]
- Salehi, Z.; Mashayekhi, F. Expression of the eukaryotic translation initiation factor 4E (eIF4E) and 4E-BP1 in esophageal cancer. Clin. Biochem. 2006, 39, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Wheater, M.J.; Johnson, P.W.M.; Blaydes, J.P. The role of MNK proteins and eIF4E phosphorylation in breast cancer cell proliferation and survival. Cancer Boil. Ther. 2010, 10, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Konicek, B.W.; Stephens, J.R.; McNulty, A.M.; Robichaud, N.; Peery, R.B.; Dumstorf, C.A.; Dowless, M.S.; Iversen, P.W.; Parsons, S.; Ellis, K.E.; et al. Therapeutic Inhibition of MAP Kinase Interacting Kinase Blocks Eukaryotic Initiation Factor 4E Phosphorylation and Suppresses Outgrowth of Experimental Lung Metastases. Cancer Res. 2011, 71, 1849–1857. [Google Scholar] [CrossRef]
- Fan, W.; Wang, W.; Mao, X.; Chu, S.; Feng, J.; Xiao, D.; Zhou, J.; Fan, S. Elevated levels of p-Mnk1, p-eIF4E and p-p70S6K proteins are associated with tumor recurrence and poor prognosis in astrocytomas. J. Neuro-Oncology 2016, 131, 485–493. [Google Scholar] [CrossRef]
- Lu, J.; Zang, H.; Zheng, H.; Zhan, Y.; Yang, Y.; Zhang, Y.; Liu, S.; Feng, J.; Wen, Q.; Long, M.; et al. Overexpression of p-Akt, p-mTOR and p-eIF4E proteins associates with metastasis and unfavorable prognosis in non-small cell lung cancer. PLoS ONE 2020, 15, e0227768. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, J.; Xu, L.; Xie, G.; Wen, Q.; Luo, J.; Li, D.; Huang, N.; Fan, S. Phosphorylated Mnk1 and eIF4E Are Associated with Lymph Node Metastasis and Poor Prognosis of Nasopharyngeal Carcinoma. PLoS ONE 2014, 9, e89220. [Google Scholar] [CrossRef]
- Carter, J.H.; Deddens, J.; Iv, N.R.S.; Lucas, D.; Colligan, B.M.; Lewis, T.G.; Hawkins, E.; Jones, J.; O Pemberton, J.; E Douglass, L.; et al. Phosphorylation of eIF4E serine 209 is associated with tumour progression and reduced survival in malignant melanoma. Br. J. Cancer 2016, 114, 444–453. [Google Scholar] [CrossRef]
- Furic, L.; Rong, L.; Larsson, O.; Koumakpayi, I.H.; Yoshida, K.; Brueschke, A.; Petroulakis, E.; Robichaud, N.; Pollak, M.; Gaboury, L.A.; et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc. Natl. Acad. Sci. USA 2010, 107, 14134–14139. [Google Scholar] [CrossRef]
- Fan, S.; Ramalingam, S.S.; Kauh, J.; Xu, Z.; Khuri, F.R.; Sun, S.-Y. Phosphorylated eukaryotic translation initiation factor 4 (eIF4E) is elevated in human cancer tissues. Cancer Boil. Ther. 2009, 8, 1463–1469. [Google Scholar] [CrossRef]
- Hou, S.; Du, P.; Wang, P.; Liu, P. Significance of MNK1 in prognostic prediction and chemotherapy development of epithelial ovarian cancer. Clin. Transl. Oncol. 2017, 61, 69–1116. [Google Scholar] [CrossRef]
- Zhan, Y.; Guo, J.; Yang, W.; Goncalves, C.; Rzymski, T.; Dreas, A.; Żyłkiewicz, E.; Mikulski, M.; Brzózka, K.; Golas, A.; et al. MNK1/2 inhibition limits oncogenicity and metastasis of KIT-mutant melanoma. J. Clin. Investig. 2017, 127, 4179–4192. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q. MNK1/NODAL signaling promotes invasive progression of breast ductal carcinoma in situ. Cancer Res. 2019, 79, 1646–1657. [Google Scholar] [CrossRef]
- Agüeras, S.R.Y.C.; de Mattos-Arruda, L.; Sonenberg, N.; Cortes, J.; Peg, V. The intra-tumor heterogeneity of cell signaling factors in breast cancer: p4E-BP1 and peIF4E are diffusely expressed and are real potential targets. Clin. Transl. Oncol. 2014, 16, 937–941. [Google Scholar] [CrossRef]
- Truitt, M.L.; Conn, C.S.; Shi, Z.; Pang, X.; Tokuyasu, T.; Coady, A.M.; Seo, Y.; Barna, M.; Ruggero, D. Differential Requirements for eIF4E Dose in Normal Development and Cancer. Cell 2015, 162, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.J.; Corrie, P.G. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther. Adv. Med Oncol. 2015, 7, 122–136. [Google Scholar] [CrossRef] [PubMed]
- O’Loghlen, A.; González, V.M.; Piñeiro, D.; Pérez-Morgado, M.; Salinas, M.; Martín, M. Identification and molecular characterization of Mnk1b, a splice variant of human MAP kinase-interacting kinase Mnk1. Exp. Cell Res. 2004, 299, 343–355. [Google Scholar] [CrossRef]
- Slentz-Kesler, K.; Moore, J.T.; Lombard, M.; Zhang, J.; Hollingsworth, R.; Weiner, M.P. Identification of the Human Mnk2 Gene (MKNK2) through Protein Interaction with Estrogen Receptor β. Genomics 2000, 69, 63–71. [Google Scholar] [CrossRef]
- Fukunaga, R.; Hunter, T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 1997, 16, 1921–1933. [Google Scholar] [CrossRef]
- Scheper, G.C.; Parra, J.L.; Wilson, M.; van Kollenburg, B.; Vertegaal, A.C.O.; Han, Z.; Proud, C.G. The N and C Termini of the Splice Variants of the Human Mitogen-Activated Protein Kinase-Interacting Kinase Mnk2 Determine Activity and Localization. Mol. Cell. Boil. 2003, 23, 5692–5705. [Google Scholar] [CrossRef]
- Parra-Palau, J.-L.; Scheper, G.C.; Wilson, M.L.; Proud, C.G. Features in the N and C Termini of the MAPK-interacting Kinase Mnk1 Mediate Its Nucleocytoplasmic Shuttling. J. Boil. Chem. 2003, 278, 44197–44204. [Google Scholar] [CrossRef] [PubMed]
- Pyronnet, S.; Imataka, H.; Gingras, A.-C.; Fukunaga, R.; Hunter, T.; Sonenberg, N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999, 18, 270–279. [Google Scholar] [CrossRef]
- McKendrick, L.; Thompson, E.; Ferreira, J.; Morley, S.J.; Lewis, J.D. Interaction of Eukaryotic Translation Initiation Factor 4G with the Nuclear Cap-Binding Complex Provides a Link between Nuclear and Cytoplasmic Functions of the m7Guanosine Cap. Mol. Cell. Boil. 2001, 21, 3632–3641. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.L.; Buxade, M.; Proud, C.G. Features of the Catalytic Domains and C Termini of the MAPK Signal-integrating Kinases Mnk1 and Mnk2 Determine Their Differing Activities and Regulatory Properties. J. Boil. Chem. 2005, 280, 37623–37633. [Google Scholar] [CrossRef] [PubMed]
- Waskiewicz, A.J.; Flynn, A.; Proud, C.G.; Cooper, J.A. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997, 16, 1909–1920. [Google Scholar] [CrossRef]
- Jauch, R.; Cho, M.-K.; Jäkel, S.; Netter, C.; Schreiter, K.; Aicher, B.; Zweckstetter, M.; Jäckle, H.; Wahl, M.C. Mitogen-activated protein kinases interacting kinases are autoinhibited by a reprogrammed activation segment. EMBO J. 2006, 25, 4020–4032. [Google Scholar] [CrossRef]
- Kannan, S. Small molecules targeting the inactive form of the Mnk1/2 kinases. ACS Omega 2017, 2, 7881–7891. [Google Scholar] [CrossRef]
- Karni, R.; de Stanchina, E.; Lowe, S.W.; Sinha, R.; Mu, D.; Krainer, A.R. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat. Struct. Mol. Boil. 2007, 14, 185–193. [Google Scholar] [CrossRef]
- Adesso, L. Gemcitabine triggers a pro-survival response in pancreatic cancer cells through activation of the MNK2/eIF4E pathway. Oncogene 2013, 32, 2848–2857. [Google Scholar] [CrossRef]
- Maimon, A.; Mogilevsky, M.; Shilo, A.; Golan-Gerstl, R.; Obiedat, A.; Ben-Hur, V.; Lebenthal-Loinger, I.; Stein, I.; Reich, R.; Beenstock, J.; et al. Mnk2 Alternative Splicing Modulates the p38-MAPK Pathway and Impacts Ras-Induced Transformation. Cell Rep. 2014, 7, 501–513. [Google Scholar] [CrossRef]
- Pinto, C.; García-Recio, E.M.; Pérez-Morgado, M.I.; García-Hernández, M.; Sanz-Criado, L.; Sacristán, S.; Toledo-Lobo, M.V.; Pérez-Mies, B.; Esteban-Rodriguez, I.; Pascual, A.; et al. Increased expression of MNK1b, the spliced isoform of MNK1, predicts poor prognosis and is associated with triple-negative breast cancer. Oncotarget 2018, 9, 13501–13516. [Google Scholar] [CrossRef] [PubMed]
- García-Recio, E.M.; Pinto, C.; Pérez-Morgado, M.I.; García-Hernández, M.; Fernández, G.; Martín, M.; González, V.M. Characterization of MNK1b DNA Aptamers That Inhibit Proliferation in MDA-MB231 Breast Cancer Cells. Mol. Ther. Nucleic Acids 2016, 5, e275. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Yao, Z.; Proud, C.G. The C-terminal domain of Mnk1a plays a dual role in tightly regulating its activity. Biochem. J. 2009, 423, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Sharma, M.; Kentsis, A.; Perez, J.M.; Strudwick, S.; Borden, K.L. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J. 2001, 20, 4547–4559. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, D.; Kaspar, R.; Rosenwald, I.; Gehrke, L.; Sonenberg, N. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc. Natl. Acad. Sci. USA 1996, 93, 1065–1070. [Google Scholar] [CrossRef]
- Guo, Z.; Peng, G.; Li, E.; Xi, S.; Zhang, Y.; Li, Y.; Lin, X.; Li, G.; Wu, Q.; He, J. MAP kinase-interacting serine/threonine kinase 2 promotes proliferation, metastasis, and predicts poor prognosis in non-small cell lung cancer. Sci. Rep. 2017, 7, 10612. [Google Scholar] [CrossRef]
- Linkous, A.; Yazlovitskaya, E.M. Cytosolic phospholipase A2 as a mediator of disease pathogenesis. Cell. Microbiol. 2010, 12, 1369–1377. [Google Scholar] [CrossRef]
- Hefner, Y.; Borsch-Haubold, A.G.; Murakami, M.; Wilde, J.I.; Pasquet, S.; Schieltz, D.; Ghomashchi, F.; Yates, I.J.R.; Armstrong, C.G.; Paterson, A.; et al. Serine 727 Phosphorylation and Activation of Cytosolic Phospholipase A2by MNK1-related Protein Kinases. J. Boil. Chem. 2000, 275, 37542–37551. [Google Scholar] [CrossRef]
- Buxade, M.; Morrice, N.; Krebs, D.L.; Proud, C.G. The PSF {middle dot} p54nrb Complex Is a Novel Mnk Substrate That Binds the mRNA for Tumor Necrosis Factor. J. Boil. Chem. 2007, 283, 57–65. [Google Scholar] [CrossRef]
- Mitobe, Y.; Iino, K.; Takayama, K.-I.; Ikeda, K.; Suzuki, T.; Aogi, K.; Kawabata, H.; Suzuki, Y.; Horie-Inoue, K.; Inoue, S. PSF promotes ER-positive breast cancer progression via posttranscriptional regulation of ESR1 and SCFD2. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Tsukahara, T.; Haniu, H.; Matsuda, Y. PTB-associated splicing factor (PSF) is a PPARgamma-binding protein and growth regulator of colon cancer cells. PLoS One 2013, 8, e58749. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara, T.; Matsuda, Y.; Haniu, H. PSF Knockdown Enhances Apoptosis via Downregulation of LC3B in Human Colon Cancer Cells. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Buxadé, M.; Parra, J.L.; Rousseau, S.; Shpiro, N.; Marquez, R.; Morrice, N.; Bain, J.; Espel, E.; Proud, C.G. The Mnks Are Novel Components in the Control of TNFα Biosynthesis and Phosphorylate and Regulate hnRNP A1. Immunity 2005, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Huang, Y.; Seckl, M.J.; Pardo, O.E. Emerging roles of hnRNPA1 in modulating malignant transformation. Wiley Interdiscip. Rev. RNA 2017, 8, e1431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, J.; Wang, W.; Xiang, L.; Wang, J.; Liu, S.; Zhou, H.; Guo, Z. High expression of hnRNPA1 promotes cell invasion by inducing EMT in gastric cancer. Oncol. Rep. 2018, 39, 1693–1701. [Google Scholar] [CrossRef] [PubMed]
- Edwin, F.; Anderson, K.; Patel, T.B. HECT Domain-containing E3 Ubiquitin Ligase Nedd4 Interacts with and Ubiquitinates Sprouty2. J. Boil. Chem. 2009, 285, 255–264. [Google Scholar] [CrossRef]
- Da Silva, J.; Xu, L.; Kim, H.J.; Miller, W.T.; Bar-Sagi, D. Regulation of Sprouty Stability by Mnk1-Dependent Phosphorylation. Mol. Cell. Boil. 2006, 26, 1898–1907. [Google Scholar] [CrossRef]
- Park, J.-W.; Wollmann, G.; Urbiola, C.; Fogli, B.; Florio, T.; Geley, S.; Klimaschewski, L. Sprouty2 enhances the tumorigenic potential of glioblastoma cells. Neuro-Oncology 2018, 20, 1044–1054. [Google Scholar] [CrossRef]
- Saini, M.; Verma, A.; Mathew, S. SPRY2 is a novel MET interactor that regulates metastatic potential and differentiation in rhabdomyosarcoma. Cell Death Dis. 2018, 9, 237. [Google Scholar] [CrossRef]
- Lama, D.; Verma, C.S. Deciphering the mechanistic effects of eIF4E phosphorylation on mRNA-cap recognition. Protein Sci. 2019, 29, 1373–1386. [Google Scholar] [CrossRef]
- Marcotrigiano, J.; Gingras, A.-C.; Sonenberg, N.; Burley, S.K. Cocrystal Structure of the Messenger RNA 5′ Cap-Binding Protein (eIF4E) Bound to 7-methyl-GDP. Cell 1997, 89, 951–961. [Google Scholar] [CrossRef]
- Scheper, G.C.; van Kollenburg, B.; Hu, J.; Luo, Y.; Goss, D.J.; Proud, C.G. Phosphorylation of Eukaryotic Initiation Factor 4E Markedly Reduces Its Affinity for Capped mRNA. J. Boil. Chem. 2001, 277, 3303–3309. [Google Scholar] [CrossRef] [PubMed]
- Slepenkov, S.V.; Darzynkiewicz, E.; Rhoads, R.E. Stopped-flow Kinetic Analysis of eIF4E and Phosphorylated eIF4E Binding to Cap Analogs and Capped Oligoribonucleotides. J. Boil. Chem. 2006, 281, 14927–14938. [Google Scholar] [CrossRef] [PubMed]
- Topisirovic, I.; Ruiz-Gutierrez, M.; Borden, K.L.B. Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res. 2004, 64, 8639–8642. [Google Scholar] [CrossRef]
- Robichaud, N.; del Rincon, S.V.; Huor, B.; Alain, T.; A Petruccelli, L.; Hearnden, J.; Goncalves, C.; Grotegut, S.; Spruck, C.H.; Furic, L.; et al. Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3. Oncogene 2014, 34, 2032–2042. [Google Scholar] [CrossRef]
- Lim, S.; Saw, T.Y.; Zhang, M.; Janes, M.R.; Nacro, K.; Hill, J.; Lim, A.Q.; Chang, C.-T.; Fruman, D.A.; Rizzieri, D.A.; et al. Targeting of the MNK–eIF4E axis in blast crisis chronic myeloid leukemia inhibits leukemia stem cell function. Proc. Natl. Acad. Sci. USA 2013, 110, 2298–2307. [Google Scholar] [CrossRef]
- Geter, P.A.; Ernlund, A.W.; Bakogianni, S.; Alard, A.; Arju, R.; Giashuddin, S.; Gadi, A.; Bromberg, J.; Schneider, R.J. Hyperactive mTOR and MNK1 phosphorylation of eIF4E confer tamoxifen resistance and estrogen independence through selective mRNA translation reprogramming. Genome Res. 2017, 31, 2235–2249. [Google Scholar] [CrossRef]
- Robichaud, N.; Hsu, B.E.; Istomine, R.; Alvarez, F.; Blagih, J.; Ma, E.H.; Morales, S.V.; Dai, D.L.; Li, G.; Souleimanova, M.; et al. Translational control in the tumor microenvironment promotes lung metastasis: Phosphorylation of eIF4E in neutrophils. Proc. Natl. Acad. Sci. USA 2018, 115, 2202–2209. [Google Scholar] [CrossRef]
- Eberle, J.; Krasagakis, K.; Orfanos, C.E. Translation initiation factor eIF-4A1 mRNA is consistently overexpressed in human melanoma cells in vitro. Int. J. Cancer 1997, 71, 396–401. [Google Scholar] [CrossRef]
- Yang, S.X.; Hewitt, S.M.; Steinberg, S.M.; Liewehr, D.J.; Swain, S. Expression levels of eIF4E, VEGF, and cyclin D1, and correlation of eIF4E with VEGF and cyclin D1 in multi-tumor tissue microarray. Oncol. Rep. 2007, 17, 281–287. [Google Scholar] [CrossRef]
- De Benedetti, A.; Joshi-Barve, S.; Rinker-Schaeffer, C.; Rhoads, R.E. Expression of antisense RNA against initiation factor eIF-4E mRNA in HeLa cells results in lengthened cell division times, diminished translation rates, and reduced levels of both eIF-4E and the p220 component of eIF-4F. Mol. Cell. Boil. 1991, 11, 5435–5445. [Google Scholar] [CrossRef] [PubMed]
- De Fatta, R.J.; Nathan, C.-A.O.; De Benedetti, A. Antisense RNA to eIF4E Suppresses Oncogenic Properties of a Head and Neck Squamous Cell Carcinoma Cell Line. Laryngoscope 2000, 110, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Graff, J.R.; Konicek, B.W.; Vincent, T.M.; Lynch, R.L.; Monteith, D.; Weir, S.N.; Schwier, P.; Capen, A.; Goode, R.L.; Dowless, M.S.; et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J. Clin. Investig. 2007, 117, 2638–2648. [Google Scholar] [CrossRef] [PubMed]
- Rinker-Schaeffer, C.W.; Graff, J.R.; De Benedetti, A.; Zimmer, S.G.; Rhoads, R.E. Decreasing the level of translation initiation factor 4E with antisense rna causes reversal ofras-mediated transformation and tumorigenesis of cloned rat embryo fibroblasts. Int. J. Cancer 1993, 55, 841–847. [Google Scholar] [CrossRef]
- Hong, D.S.; Kurzrock, R.; Oh, Y.; Wheler, J.; Naing, A.; Brail, L.; Callies, S.; André, V.; Kadam, S.K.; Nasir, A.; et al. A phase 1 dose escalation, pharmacokinetic, and pharmacodynamic evaluation of eIF-4E antisense oligonucleotide LY2275796 in patients with advanced cancer. Clin. Cancer Res. 2011, 17, 6582–6591. [Google Scholar] [CrossRef]
- Jiang, C.C.; Croft, A.; Tseng, H.-Y.; Guo, S.T.; Jin, L.; Hersey, P.; Zhang, X.D. Repression of microRNA-768-3p by MEK/ERK signalling contributes to enhanced mRNA translation in human melanoma. Oncogene 2013, 33, 2577–2588. [Google Scholar] [CrossRef]
- Wendel, H.-G. Dissecting eIF4E action in tumorigenesis. Genes Dev. 2007, 21, 3232–3237. [Google Scholar] [CrossRef]
- Wagner, C.R.; Iyer, V.; Mcintee, E.J. Pronucleotides: Toward thein vivo delivery of antiviral and anticancer nucleotides. Med. Res. Rev. 2000, 20, 417–451. [Google Scholar] [CrossRef]
- Ghosh, B.; Benyumov, A.O.; Ghosh, P.; Jia, Y.; Avdulov, S.; Dahlberg, P.S.; Peterson, M.; Smith, K.; Polunovsky, V.A.; Bitterman, P.; et al. Nontoxic Chemical Interdiction of the Epithelial-to-Mesenchymal Transition by Targeting Cap-Dependent Translation. ACS Chem. Boil. 2009, 4, 367–377. [Google Scholar] [CrossRef]
- Braziunas, J. In B110. Genomics, Metabolonics, and Epigenetics in Lung Disease: Late Breaking Abstracts. Direct Sci 2012, 117, 6820. [Google Scholar]
- Li, S.; Jia, Y.; Jacobson, B.; McCauley, J.; Kratzke, R.; Bitterman, P.; Wagner, C.R. Treatment of Breast and Lung Cancer Cells with a N-7 Benzyl Guanosine Monophosphate Tryptamine Phosphoramidate Pronucleotide (4Ei-1) Results in Chemosensitization to Gemcitabine and Induced eIF4E Proteasomal Degradation. Mol. Pharm. 2013, 10, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Z.; Jacobson, B.A.; Patel, M.; Okon, A.M.; Li, S.; Xiong, K.; Vaidya, A.J.; Bitterman, P.; Wagner, C.R.; Kratzke, R.A. Small-molecule inhibition of oncogenic eukaryotic protein translation in mesothelioma cells. Investig. New Drugs 2014, 32, 598–603. [Google Scholar] [CrossRef] [PubMed]
- McCauley, J. In D74. Molecular Targets and Model Therapies for Lung Cancer A6291–A6291. Chin. J. Cancer. 2012. [Google Scholar] [CrossRef]
- Mader, S.; Lee, H.; Pause, A.; Sonenberg, N. The translation initiation factor eIF-4E binds to a common motif shared by the translation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol. Cell. Boil. 1995, 15, 4990–4997. [Google Scholar] [CrossRef]
- Yanagiya, A.; Svitkin, Y.V.; Shibata, S.; Mikami, S.; Imataka, H.; Sonenberg, N. Requirement of RNA Binding of Mammalian Eukaryotic Translation Initiation Factor 4GI (eIF4GI) for Efficient Interaction of eIF4E with the mRNA Cap. Mol. Cell. Boil. 2008, 29, 1661–1669. [Google Scholar] [CrossRef]
- Cencic, R.; Desforges, M.; Hall, D.R.; Kozakov, D.; Du, Y.; Min, J.; Dingledine, R.; Fu, H.; Vajda, S.; Talbot, P.J.; et al. Blocking eIF4E-eIF4G Interaction as a Strategy To Impair Coronavirus Replication. J. Virol. 2011, 85, 6381–6389. [Google Scholar] [CrossRef]
- Moerke, N.J.; Aktas, H.; Chen, H.; Cantel, S.; Reibarkh, M.; Fahmy, A.; Gross, J.D.; Degterev, A.; Yuan, J.; Chorev, M.; et al. Small-Molecule Inhibition of the Interaction between the Translation Initiation Factors eIF4E and eIF4G. Cell 2007, 128, 257–267. [Google Scholar] [CrossRef]
- Chen, L.; Aktas, B.H.; Wang, Y.; He, X.; Sahoo, R.; Zhang, Y.N.; Denoyelle, S.; Kabha, E.; Yang, H.; Freedman, R.Y.; et al. Tumor suppression by small molecule inhibitors of translation initiation. Oncotarget 2012, 3, 869–881. [Google Scholar] [CrossRef]
- Croft, A.; Tay, K.H.; Boyd, S.C.; Guo, S.T.; Jiang, C.C.; Lai, F.; Tseng, H.-Y.; Jin, L.; Rizos, H.; Hersey, P.; et al. Oncogenic Activation of MEK/ERK Primes Melanoma Cells for Adaptation to Endoplasmic Reticulum Stress. J. Investig. Dermatol. 2014, 134, 488–497. [Google Scholar] [CrossRef]
- Feng, Y.; Pinkerton, A.B.; Hulea, L.; Zhang, T.; Davies, M.A.; Grotegut, S.; Cheli, Y.; Yin, H.; Lau, E.L.; Kim, H.; et al. SBI-0640756 Attenuates the Growth of Clinically Unresponsive Melanomas by Disrupting the eIF4F Translation Initiation Complex. Cancer Res. 2015, 75, 5211–5218. [Google Scholar] [CrossRef]
- Cai, W.; Ye, Q.; She, Q.-B. Loss of 4E-BP1 function induces EMT and promotes cancer cell migration and invasion via cap-dependent translational activation of snail. Oncotarget 2014, 5, 6015–6027. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Kondoh, N.; Tanaka, K.; Ryo, A.; Wakatsuki, T.; Hada, A.; Goseki, N.; Igari, T.; Hatsuse, K.; Aihara, T.; et al. Enhanced expression of translation factor mRNAs in hepatocellular carcinoma. Anticancer. Res. 2000, 20, 2489–2494. [Google Scholar] [PubMed]
- Matsuhashi, S.; Manirujjaman, M.; Hamajima, H.; Ozaki, I. Control Mechanisms of the Tumor Suppressor PDCD4: Expression and Functions. Int. J. Mol. Sci. 2019, 20, 2304. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.; Garces, R.G.; Edmonds, K.A.; Hiller, S.; Hyberts, S.G.; Marintchev, A.; Wagner, G. PDCD4 inhibits translation initiation by binding to eIF4A using both its MA3 domains. Proc. Nat. Acad. Sci. USA 2008, 105, 3274–3279. [Google Scholar] [CrossRef] [PubMed]
- Sadlish, H.; Vazquez, G.G.; Paris, C.G.; Aust, T.; Bhullar, B.; Chang, L.; Helliwell, S.B.; Hoepfner, D.; Knapp, B.; Riedl, R.; et al. Evidence for a Functionally Relevant Rocaglamide Binding Site on the eIF4A–RNA Complex. ACS Chem. Boil. 2013, 8, 1519–1527. [Google Scholar] [CrossRef]
- Iwasaki, S.; Iwasaki, W.; Takahashi, M.; Sakamoto, A.; Watanabe, C.; Shichino, Y.; Floor, S.; Fujiwara, K.; Mito, M.; Dodo, K.; et al. The Translation Inhibitor Rocaglamide Targets a Bimolecular Cavity between eIF4A and Polypurine RNA. Mol. Cell 2019, 73, 738–748. [Google Scholar] [CrossRef]
- Polier, G.; Neumann, J.; Thuaud, F.; Ribeiro, N.; Gelhaus, C.; Schmidt, H.; Giaisi, M.; Köhler, R.; Müller, W.W.; Proksch, P.; et al. The Natural Anticancer Compounds Rocaglamides Inhibit the Raf-MEK-ERK Pathway by Targeting Prohibitin 1 and 2. Chem. Boil. 2012, 19, 1093–1104. [Google Scholar] [CrossRef]
- Hawkins, B.C.; Lindqvist, L.M.; Nhu, D.; Sharp, P.P.; Segal, D.; Powell, A.; Campbell, M.; Ryan, E.; Chambers, J.M.; White, J.M.; et al. Simplified Silvestrol Analogues with Potent Cytotoxic Activity. ChemMedChem 2014, 9, 1556–1566. [Google Scholar] [CrossRef]
- Hwang, B.Y.; Su, B.-N.; Chai, H.; Mi, Q.; Kardono, L.B.S.; Afriastini, J.J.; Riswan, S.; Santarsiero, B.D.; Mesecar, A.D.; Wild, R.; et al. Silvestrol and Episilvestrol, Potential Anticancer Rocaglate Derivatives fromAglaia silvestris. J. Org. Chem. 2004, 69, 3350–3358. [Google Scholar] [CrossRef]
- Chen, W.-L.; Pan, L.; Kinghorn, A.D.; Swanson, S.M.; Burdette, J.E. Silvestrol induces early autophagy and apoptosis in human melanoma cells. BMC Cancer 2016, 16, 17. [Google Scholar] [CrossRef]
- Shen, S.; Faouzi, S.; Bastide, A.; Martineau, S.; Malka-Mahieu, H.; Fu, Y.; Sun, X.; Mateus, C.; Routier, E.; Roy, S.; et al. An epitranscriptomic mechanism underlies selective mRNA translation remodelling in melanoma persister cells. Nat. Commun. 2019, 10, 5713–5714. [Google Scholar] [CrossRef] [PubMed]
- Boussemart, L.; Malka-Mahieu, H.; Girault, I.; Allard, D.; Hemmingsson, O.; Tomasic, G.; Thomas, M.; Basmadjian, C.; Ribeiro, N.; Thuaud, F.; et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 2014, 513, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Malka-Mahieu, H.; Girault, I.; Rubington, M.; Leriche, M.; Welsch, C.; Kamsu-Kom, N.; Zhao, Q.; Desaubry, L.; Vagner, S.; Robert, C. Synergistic effects of eIF4A and MEK inhibitors on proliferation of NRAS-mutant melanoma cell lines. Cell Cycle 2016, 15, 2405–2409. [Google Scholar] [CrossRef] [PubMed]
- Cerezo, M.; Guemiri, R.; Druillennec, S.; Girault, I.; Malka-Mahieu, H.; Shen, S.; Allard, D.; Martineau, S.; Welsch, C.; Agoussi, S.; et al. Translational control of tumor immune escape via the eIF4F–STAT1–PD-L1 axis in melanoma. Nat. Med. 2018, 24, 1877–1886. [Google Scholar] [CrossRef]
- Gupta, S.V. Resistance to the translation initiation inhibitor silvestrol is mediated by ABCB1/P-glycoprotein overexpression in acute lymphoblastic leukemia cells. AAPS J. 2011, 13, 357. [Google Scholar] [CrossRef]
- Thompson, P.A.; Young, N.P.; Strumpf, C.R.; Eam, B.; Goel, V.K.; Chen, J.; Fish, S.; Parker, G.S.; Gerson-Gurwitz, A.; Barrera, M.; et al. Abstract B133: eFT226, a first in class inhibitor of eIF4A1, targets FGFR1/2 and HER2 driven cancers. AACR 2019, 18, 12. [Google Scholar]
- Young, N.P.; Craig, R.S.; Chen, J.; Chiang, G.G.; Thompson, P.A.; Webster, K.R. A focused CRISPR screen to identify synthetic lethal interactions with the novel eIF4A inhibitor eFT226 in KRAS driven NSCLC. AACR 2019, 79, 13. [Google Scholar]
- Bordeleau, M.-E.; Matthews, J.; Wojnar, J.M.; Lindqvist, L.; Novac, O.; Jankowsky, E.; Sonenberg, N.; Northcote, P.; Teesdale-Spittle, P.; Pelletier, J. Stimulation of mammalian translation initiation factor eIF4A activity by a small molecule inhibitor of eukaryotic translation. Proc. Nat. Acad. Sci. USA 2005, 102, 10460–10465. [Google Scholar] [CrossRef]
- Low, W.-K.; Dang, Y.; Schneider-Poetsch, T.; Shi, Z.; Choi, N.S.; Merrick, W.C.; Romo, D.; Liu, J.O. Inhibition of Eukaryotic Translation Initiation by the Marine Natural Product Pateamine A. Mol. Cell 2005, 20, 709–722. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Xu, Q.; Rudolph-Owen, L.; TenDyke, K.; Liu, J.; Towle, M.; Zhao, N.; Marsh, J.; Agoulnik, S.; Twine, N.; et al. Potent in vitro and in vivo anticancer activities of des-methyl, des-amino pateamine A, a synthetic analogue of marine natural product pateamine A. Mol. Cancer Ther. 2009, 8, 1250–1260. [Google Scholar] [CrossRef]
- Bordeleau, M.-E.; Mori, A.; Oberer, M.; Lindqvist, L.; Chard, L.; Higa, T.; Belsham, G.J.; Wagner, G.; Tanaka, J.; Pelletier, J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat. Methods 2006, 2, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Liwak, U.; Thakor, N.; Jordan, L.E.; Roy, R.; Lewis, S.M.; Pardo, O.E.; Seckl, M.; Holcik, M. Tumor Suppressor PDCD4 Represses Internal Ribosome Entry Site-Mediated Translation of Antiapoptotic Proteins and Is Regulated by S6 Kinase 2. Mol. Cell. Boil. 2012, 32, 1818–1829. [Google Scholar] [CrossRef] [PubMed]
- Carayol, N.; Katsoulidis, E.; Sassano, A.; Altman, J.K.; Druker, B.J.; Platanias, L.C. Suppression of programmed cell death 4 (PDCD4) protein expression by BCR-ABL-regulated engagement of the mTOR/p70 S6 kinase pathway. J. Boil. Chem. 2008, 283, 8601–8610. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Pfeffer, S.R.; Handorf, C.R.; Pfeffer, L. MicroRNA miR-21 Regulates the Metastatic Behavior of B16 Melanoma Cells. J. Boil. Chem. 2011, 286, 39172–39178. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Pedone, C.; Sahebkar, A. Curcumin and treatment of melanoma: The potential role of microRNAs. Biomed. Pharmacother. 2017, 88, 832–834. [Google Scholar] [CrossRef]
- Yang, C.H.; Yue, J.; Sims, M.; Pfeffer, L. The Curcumin Analog EF24 Targets NF-κB and miRNA-21, and Has Potent Anticancer Activity In Vitro and In Vivo. PLoS ONE 2013, 8, e71130. [Google Scholar] [CrossRef]
- Alain, T.; Morita, M.; Fonseca, B.D.; Yanagiya, A.; Siddiqui, N.; Bhat, M.; Zammit, D.; Marcus, V.; Metrakos, P.; Voyer, L.-A.; et al. eIF4E/4E-BP Ratio Predicts the Efficacy of mTOR Targeted Therapies. Cancer Res. 2012, 72, 6468–6476. [Google Scholar] [CrossRef]
- Hsu, P.P.; Kang, S.A.; Rameseder, J.; Zhang, Y.; Ottina, K.A.; Lim, D.; Peterson, T.R.; Choi, Y.; Gray, N.S.; Yaffe, M.B.; et al. The mTOR-Regulated Phosphoproteome Reveals a Mechanism of mTORC1-Mediated Inhibition of Growth Factor Signaling. Science 2011, 332, 1317–1322. [Google Scholar] [CrossRef]
- Yu, Y.; Yoon, S.-O.; Poulogiannis, G.; Yang, Q.; Ma, X.M.; Villen, J.; Kubica, N.; Hoffman, G.R.; Cantley, L.C.; Gygi, S.P.; et al. Phosphoproteomic Analysis Identifies Grb10 as an mTORC1 Substrate That Negatively Regulates Insulin Signaling. Science 2011, 332, 1322–1326. [Google Scholar] [CrossRef]
- Choo, A.Y.; Blenis, J. Not all substrates are treated equally: Implications for mTOR, rapamycin-resistance, and cancer therapy. Cell Cycle 2009, 8, 567–572. [Google Scholar] [CrossRef]
- Harrington, L.S.; Findlay, G.M.; Lamb, R.F. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem. Sci. 2005, 30, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Fukuoka, J.; Shimizu, S.; Shilo, K.; Franks, T.J.; Hewitt, S.M.; Fujii, T.; Cordon-Cardo, C.; Jen, J.; Travis, W.D. Overexpression of Phospho-eIF4E Is Associated with Survival through AKT Pathway in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2009, 16, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, E.; Peg, V.; Ortega-Aznar, A.; Martinez-Ricarte, F.; Camacho, J.; Hernández-Losa, J.; Piñas, J.C.F.; Agüeras, S.R.Y.C. peIF4E as an independent prognostic factor and a potential therapeutic target in diffuse infiltrating astrocytomas. Cancer Med. 2016, 5, 2501–2512. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S. The Novel Mnk1/2 Degrader and Apoptosis Inducer VNLG-152 Potently Inhibits TNBC Tumor Growth and Metastasis. Cancers 2019, 11, 299. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; Mclauchlan, H.; Klevernic, I.; Arthur, J.S.C.; Alessi, D.R.; Cohen, P. The selectivity of protein kinase inhibitors: A further update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Khoury, E.; Guo, Q.; Prabhu, S.A.; Emond, A.; Huang, F.; Gonçalves, C.; Zhan, Y.; Plourde, D.; Nichol, J.N.; et al. MNK1 signaling induces an ANGPTL4-mediated gene signature to drive melanoma progression. Oncogene 2020, 39, 3650–3665. [Google Scholar] [CrossRef] [PubMed]
- Kosciuczuk, E.M.; Kar, A.K.; Blyth, G.T.; Fischietti, M.; Abedin, S.; Mina, A.A.; Siliezar, R.; Rzymski, T.; Brzozka, K.; Eklund, E.A.; et al. Inhibitory effects of SEL201 in acute myeloid leukemia. Oncotarget 2019, 10, 7112–7121. [Google Scholar] [CrossRef][Green Version]
- Lock, R.; Ingraham, R.; Maertens, O.; Miller, A.L.; Weledji, N.; Legius, E.; Konicek, B.M.; Yan, S.-C.B.; Graff, J.R.; Cichowski, K. Cotargeting MNK and MEK kinases induces the regression of NF1-mutant cancers. J. Clin. Investig. 2016, 126, 2181–2190. [Google Scholar] [CrossRef]
- Cherian, J.; Nacro, K.; Poh, Z.Y.; Guo, S.; Jeyaraj, D.A.; Wong, Y.X.; Ho, M.; Yang, H.Y.; Joy, J.K.; Kwek, Z.P.; et al. Structure–Activity Relationship Studies of Mitogen Activated Protein Kinase Interacting Kinase (MNK) 1 and 2 and BCR-ABL1 Inhibitors Targeting Chronic Myeloid Leukemic Cells. J. Med. Chem. 2016, 59, 3063–3078. [Google Scholar] [CrossRef]
- Diab, S.A.H.; Abdelaziz, A.M.; Li, P.; Teo, T.; Basnet, S.K.; Noll, B.; Rahaman, M.H.; Lu, J.; Hou, J.; Yu, M.; et al. Dual Inhibition of Mnk2 and FLT3 for potential treatment of acute myeloid leukaemia. Eur. J. Med. Chem. 2017, 139, 762–772. [Google Scholar] [CrossRef]
- Li, P.; Diab, S.A.H.; Yu, M.; Adams, J.; Islam, S.; Basnet, S.K.C.; Albrecht, H.; Milne, R.; Wang, S. Inhibition of Mnk enhances apoptotic activity of cytarabine in acute myeloid leukemia cells. Oncotarget 2016, 7, 56811–56825. [Google Scholar] [CrossRef] [PubMed]
- Lineham, E.; Tizzard, G.J.; Coles, S.J.; Spencer, J.; Morley, S.J. Synergistic effects of inhibiting the MNK-eIF4E and PI3K/AKT/ mTOR pathways on cell migration in MDA-MB-231 cells. Oncotarget 2018, 9, 14148–14159. [Google Scholar] [CrossRef] [PubMed]
- Eckerdt, F.; Beauchamp, E.; Bell, J.; Iqbal, A.; Su, B.; Fukunaga, R.; Lulla, R.R.; Goldman, S.; Platanias, L.C. Regulatory effects of a Mnk2-eIF4E feedback loop during mTORC1 targeting of human medulloblastoma cells. Oncotarget 2014, 5, 8442–8451. [Google Scholar] [CrossRef] [PubMed]
- Grzmil, M.; Huber, R.M.; Hess, D.; Frank, S.; Hynx, D.; Moncayo, G.; Klein, M.; Merlo, A.; A Hemmings, B. MNK1 pathway activity maintains protein synthesis in rapalog-treated gliomas. J. Clin. Investig. 2014, 124, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.T.; Liu, X.; Wysocka, M.; Rook, A.H.; Odum, N.; A Wasik, M. Simultaneous Inhibition of mTOR-Containing Complex 1 (mTORC1) and MNK Induces Apoptosis of Cutaneous T-Cell Lymphoma (CTCL) Cells. PLoS ONE 2011, 6, e24849. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-B.; Yang, C.-M.; Han, Q.-M.; Ye, X.; Lei, W.; Qian, W.-B. MNK1 inhibitor CGP57380 overcomes mTOR inhibitor-induced activation of eIF4E: The mechanism of synergic killing of human T-ALL cells. Acta Pharmacol. Sin. 2018, 39, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Santag, S.; Siegel, F.; Wengner, A.M.; Lange, C.; Bömer, U.; Eis, K.; Pühler, F.; Lienau, P.; Bergemann, L.; Michels, M.; et al. BAY 1143269, a novel MNK1 inhibitor, targets oncogenic protein expression and shows potent anti-tumor activity. Cancer Lett. 2017, 390, 21–29. [Google Scholar] [CrossRef]
- Santag, S.; Siegel, F.; Wegner, A.M.; Schneider, C.; Boemer, U.; Eis, K.; Puehler, F.; Michels, M.; von Nussbaum, F.; Ziegelbauer, K.; et al. Preclinical anti-tumor efficacy and mode of action of a novel, orally bioavailable, selective MKNK1 inhibitor [BAY 1143269]. AACR 2015, 75, 15. [Google Scholar]
- Hubbard, J.M.; Patel, M.R.; Bekaii-Saab, T.; Falchook, G.S.; Freilich, B.L.; Dasari, A.; Knisely, B.T.; Anderson, M.; Chiang, G.G.; Webster, K.R.; et al. A phase II, open label, randomized, noncomparative study of eFT508 (tomivosertib) alone or in combination with avelumab in subjects with relapsed/refractory microsatellite stable colorectal cancer (MSS CRC). Am. Soc. Clin. Oncol. 2019, 15. [Google Scholar] [CrossRef]
- Joshi, S.; Platanias, L.C. Mnk kinases in cytokine signaling and regulation of cytokine responses. Biomol. Concepts 2012, 3, 127–139. [Google Scholar] [CrossRef]
- Rowlett, R.M.; Chrestensen, C.A.; Nyce, M.; Harp, M.G.; Pelo, J.W.; Cominelli, F.; Ernst, P.; Pizarro, T.T.; Sturgill, T.W.; Worthington, M.T. MNK kinases regulate multiple TLR pathways and innate proinflammatory cytokines in macrophages. Am. J. Physiol. Liver Physiol. 2008, 294, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Fortin, C.F.; Mayer, T.Z.; Cloutier, A.; McDonald, P.P. Translational control of human neutrophil responses by MNK1. J. Leukoc. Boil. 2013, 94, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Noubade, R.; Krementsov, D.; del Rio, R.; Thornton, T.; Nagaleekar, V.K.; Saligrama, N.; Spitzack, A.; Spach, K.; Sabio, G.; Davis, R.J.; et al. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood 2011, 118, 3290–3300. [Google Scholar] [CrossRef] [PubMed]
- Muranski, P.; Boni, A.; Antony, P.A.; Cassard, L.; Irvine, K.R.; Kaiser, A.; Paulos, C.M.; Palmer, D.C.; Touloukian, C.E.; Ptak, K.; et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008, 112, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, N.; Muranski, P.; Chung, Y.; Yang, X.O.; Yamazaki, T.; Lu, S.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T Helper 17 Cells Promote Cytotoxic T Cell Activation in Tumor Immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef]
- Wang, L.; Yi, T.; Kortylewski, M.; Pardoll, D.M.; Zeng, D.; Yu, H. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 2009, 206, 1457–1464. [Google Scholar] [CrossRef]
- Nikolcheva, T. A translational rheostat for RFLAT-1 regulates RANTES expression in T lymphocytes. J. Clin. Investig. 2002, 110, 119–126. [Google Scholar] [CrossRef]
- Gorentla, B.K.; Krishna, S.; Shin, J.; Inoue, M.; Shinohara, M.L.; Grayson, J.M.; Fukunaga, R.; Zhong, X.-P. Mnk1 and 2 are dispensable for T cell development and activation but important for the pathogenesis of experimental autoimmune encephalomyelitis. J. Immunol. 2012, 190, 1026–1037. [Google Scholar] [CrossRef]
- Ueda, T.; Sasaki, M.; Elia, A.J.; Chio, I.I.C.; Hamada, K.; Fukunaga, R.; Mak, T.W. Combined deficiency for MAP kinase-interacting kinase 1 and 2 (Mnk1 and Mnk2) delays tumor development. Proc. Nat. Acad. Sci. USA 2010, 107, 13984–13990. [Google Scholar] [CrossRef]
- Atkins, M.B.; Tarhini, A.; Rael, M.; Gupte-Singh, K.; O’Brien, E.; Ritchings, C.; Rao, S.; McDermott, D.F. Comparative efficacy of combination immunotherapy and targeted therapy in the treatment of BRAF-mutant advanced melanoma: A matching-adjusted indirect comparison. Immunotherapy 2019, 11, 617–629. [Google Scholar] [CrossRef]
- Hodi, F.S.; Sileni, V.C.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S.; Mok, S.; Moreno, B.H.; Tsoi, J.; Robert, L.; Goedert, L.; Pinheiro, E.M.; Koya, R.C.; Graeber, T.; Comin-Anduix, B.; et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors inBRAFV600Emelanoma. Sci. Transl. Med. 2015, 7, 279ra41. [Google Scholar] [CrossRef] [PubMed]
- Cooper, Z.A.; Juneja, V.R.; Sage, P.T.; Frederick, D.T.; Piris, A.; Mitra, D.; Lo, J.A.; Hodi, F.S.; Freeman, G.J.; Bosenberg, M.W.; et al. Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol. Res. 2014, 2, 643–654. [Google Scholar] [CrossRef]
- McArthur, G.S.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. CT012 - Evaluation of atezolizumab (A), cobimetinib (C), and vemurafenib (V) in previously untreated patients with BRAFV600 mutation-positive advanced melanoma: Primary results from the phase 3 IMspire150 trial. In Proceedings of the AACR Virtual Annual Meeting, 22–24 June 2020. online.
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genome Res. 2018, 32, 1267–1284. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Xu, Y.; Poggio, M.; Jin, H.Y.; Shi, Z.; Forester, C.M.; Wang, Y.; Stumpf, C.R.; Xue, L.; Devericks, E.; So, L.; et al. Translation control of the immune checkpoint in cancer and its therapeutic targeting. Nat. Med. 2019, 25, 301–311. [Google Scholar] [CrossRef]

| Immunotherapy Target | Immunotherapy | Targeted Inhibitors | Clinical Trial Identifier | Clinical Phase | Clinical Trial Status |
|---|---|---|---|---|---|
| CTLA4 | Ipilimumab | Vemurafenib | NCT01400451 | Phase I | Terminated |
| Ipilimumab | Dabrafenib | NCT02200562 | Phase I | Terminated | |
| Ipilimumab | Dabrafenib; Dabrafenib + trametinib | NCT01767454 | Phase I | Completed | |
| Ipilimumab | BMS-908662 | NCT01245556 | Phase I | Completed | |
| Ipilimumab | Vemurafenib | NCT01673854 | Phase II | Completed | |
| CTLA4 + PD1 | Ipilimumab; Nivolumab; Ipilimumab + nivolumab | Dabrafenib; Trametinib; Dabrafenib + trametinib | NCT01940809 | Phase I | Active, not recruiting |
| Ipilimumab + nivolumab | Encorafenib + binimetinib | NCT03235245 | Phase II | Recruiting | |
| Ipilimumab + nivolumab | Vemurafenib + cobimetinib | NCT02968303 | Phase II | Active, not recruiting | |
| Ipilimumab + nivolumab | Encorafenib + binimetinib | NCT02631447 | Phase II | Active, not recruiting | |
| Ipilimumab + nivolumab | Dabrafenib + trametinib | NCT02224781 | Phase III | Recruiting | |
| PD-1 | Nivolumab | Dabrafenib; Trametinib; Dabrafenib + trametinib | NCT02357732 | Phase I | Withdrawn |
| Nivolumab | Dabrafenib + trametinib | NCT02910700 | Phase II | Recruiting | |
| Pembrolizumab | Vemurafenib + cobimetinib | NCT02818023 | Phase I | Active, not recruiting | |
| Pembrolizumab | Trametinib + dabrafenib | NCT02130466 | Phase I/II | Active, not recruiting | |
| Pembrolizumab | Encorafenib + binimetinib | NCT02902042 | Phase I/II | Recruiting | |
| Pembrolizumab | Dabrafenib + trametinib | NCT02858921 | Phase II | Recruiting | |
| Pembrolizumab | Dabrafenib + trametinib | NCT02625337 | Phase II | Unknown/Completed | |
| Spartalizumab | Dabrafenib + Trametinib | NCT02967692 | Phase III | Active, not recruiting | |
| PD-L1 | Atezolizumab | Vemurafenib; Vemurafenib + cobimetinib | NCT01656642 | Phase I | Active, not recruiting |
| Atezolizumab | Cobimetinib | NCT03178851 | Phase I | Active, not recruiting | |
| Durvalumab (MEDI4736) | Dabrafenib; trametinib; Dabrafenib + trametinib | NCT02027961 | Phase I/II | Completed | |
| Atezolizumab | Vemurafenib + cobimetinib; Cobimetinib | NCT03554083 | Phase II | Recruiting | |
| Atezolizumab | Vemurafenib + cobimetinib | NCT02902029 | Phase II | Active, not recruiting | |
| Atezolizumab | Cobimetinib | NCT01988896 | Phase I | Completed | |
| Atezolizumab | Vemurafenib + cobimetinib | NCT02908672 | Phase III | Active, not recruiting |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabhu, S.A.; Moussa, O.; Miller, W.H., Jr.; del Rincón, S.V. The MNK1/2-eIF4E Axis as a Potential Therapeutic Target in Melanoma. Int. J. Mol. Sci. 2020, 21, 4055. https://doi.org/10.3390/ijms21114055
Prabhu SA, Moussa O, Miller WH Jr., del Rincón SV. The MNK1/2-eIF4E Axis as a Potential Therapeutic Target in Melanoma. International Journal of Molecular Sciences. 2020; 21(11):4055. https://doi.org/10.3390/ijms21114055
Chicago/Turabian StylePrabhu, Sathyen A., Omar Moussa, Wilson H. Miller, Jr., and Sonia V. del Rincón. 2020. "The MNK1/2-eIF4E Axis as a Potential Therapeutic Target in Melanoma" International Journal of Molecular Sciences 21, no. 11: 4055. https://doi.org/10.3390/ijms21114055
APA StylePrabhu, S. A., Moussa, O., Miller, W. H., Jr., & del Rincón, S. V. (2020). The MNK1/2-eIF4E Axis as a Potential Therapeutic Target in Melanoma. International Journal of Molecular Sciences, 21(11), 4055. https://doi.org/10.3390/ijms21114055




