Production of Active Recombinant Hyaluronidase Inclusion Bodies from Apis mellifera in E. coli Bl21(DE3) and characterization by FT-IR Spectroscopy
Abstract
1. Introduction
2. Results
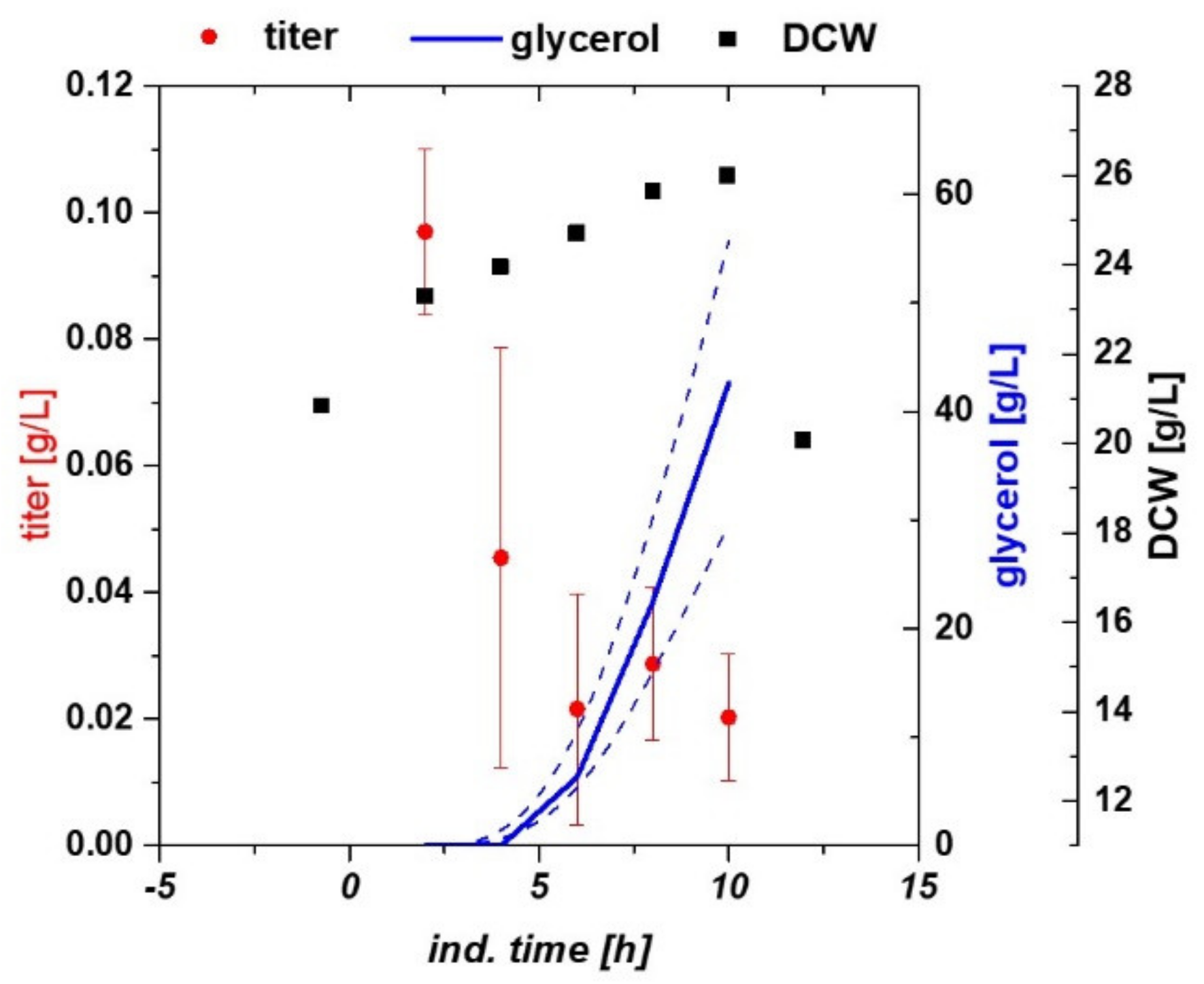
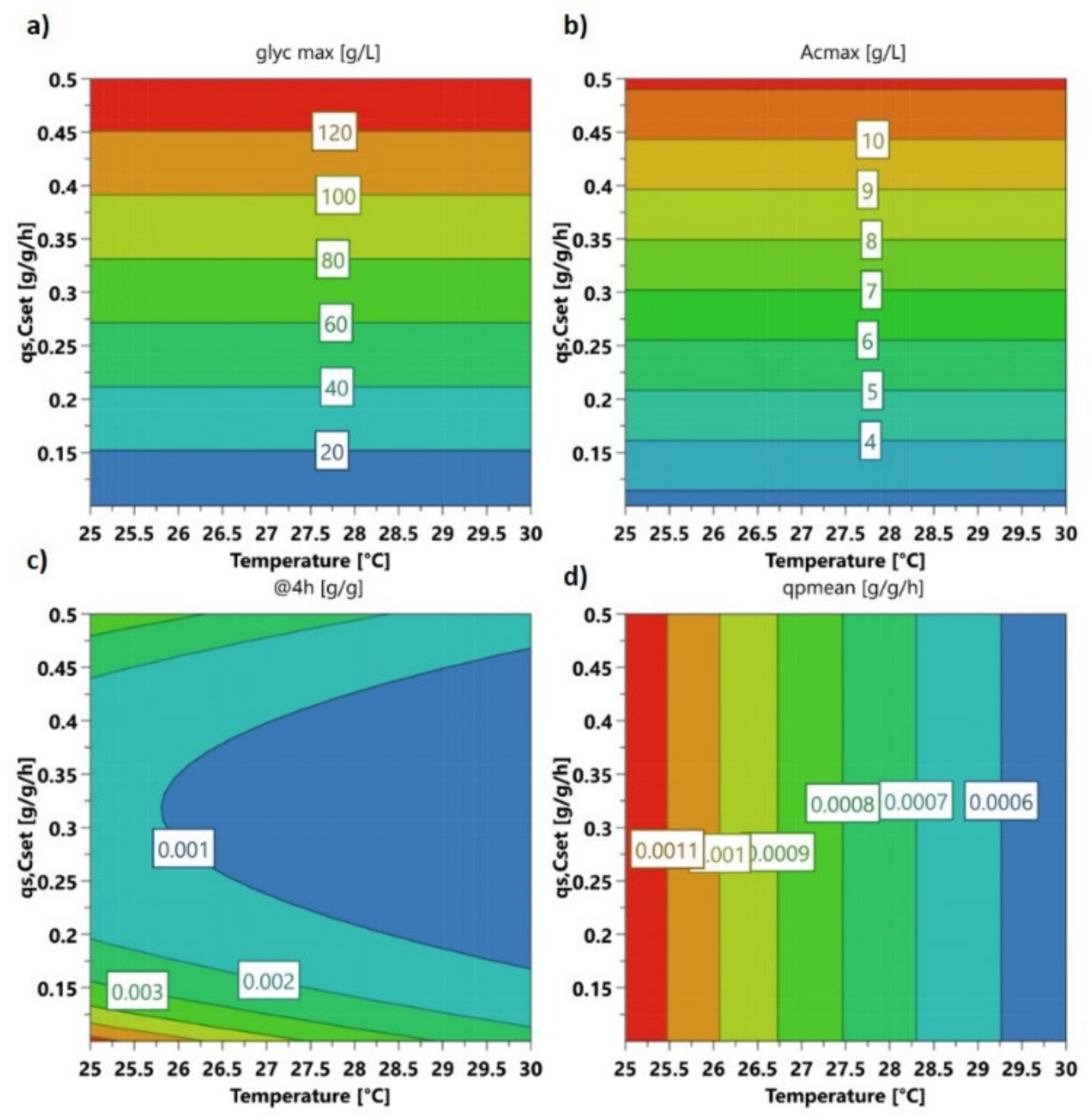
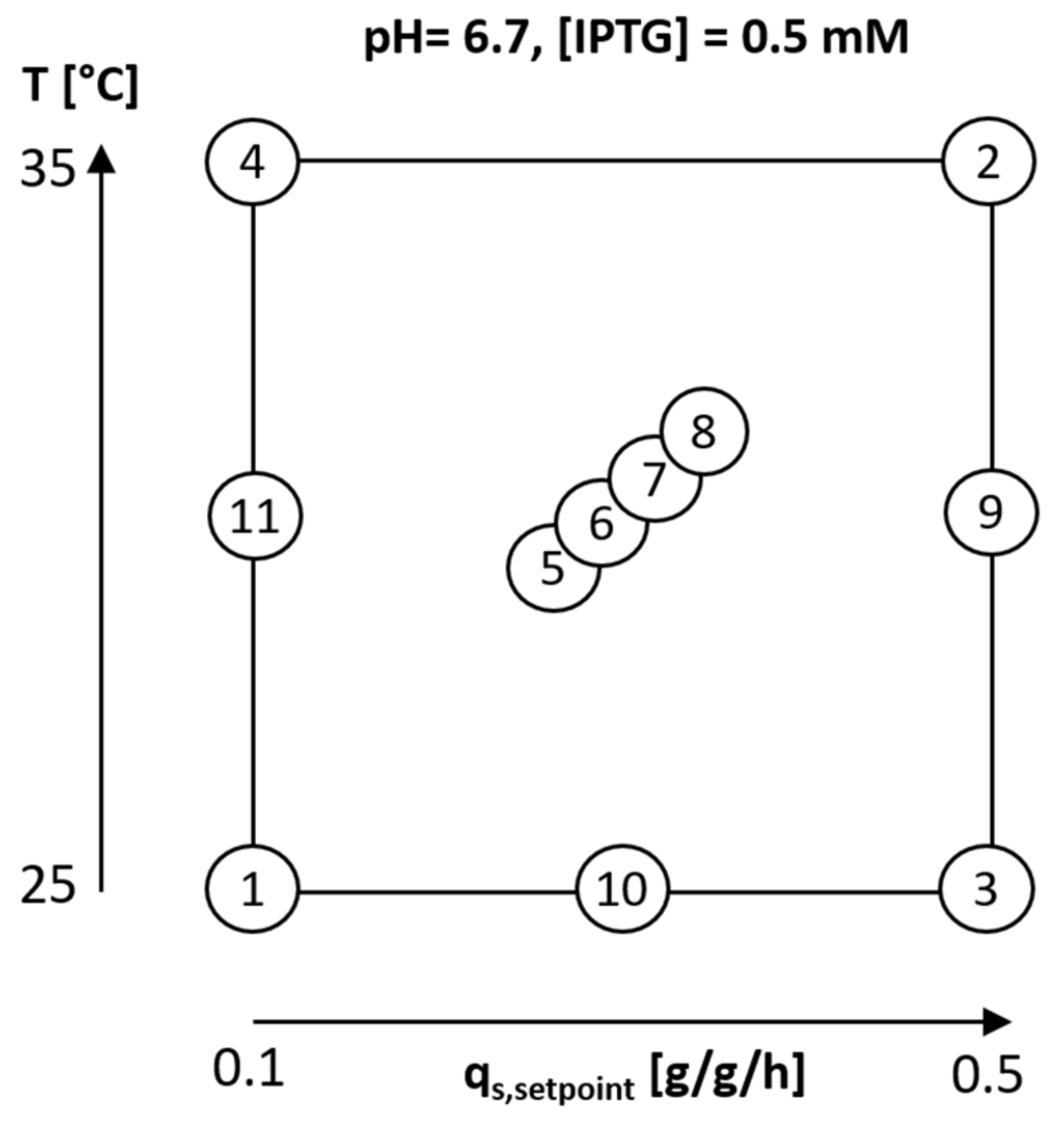
2.1. Variation of Specific Substrate Uptake Rate and Temperature Using a DoE Approach
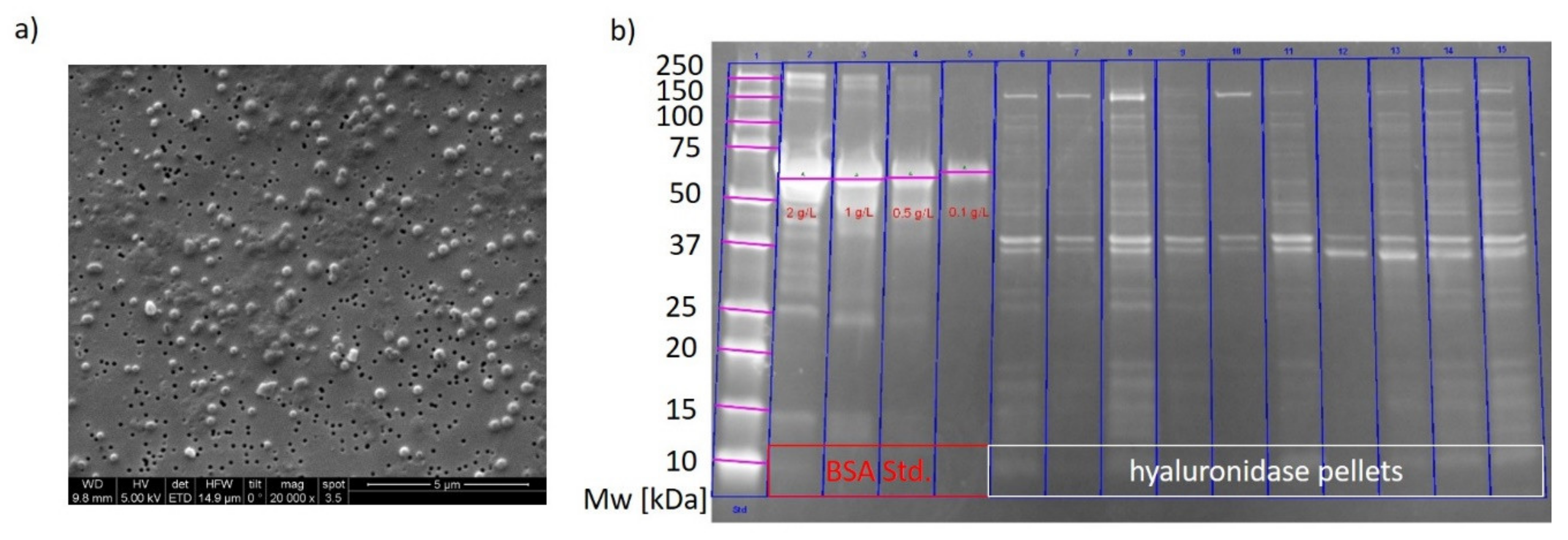
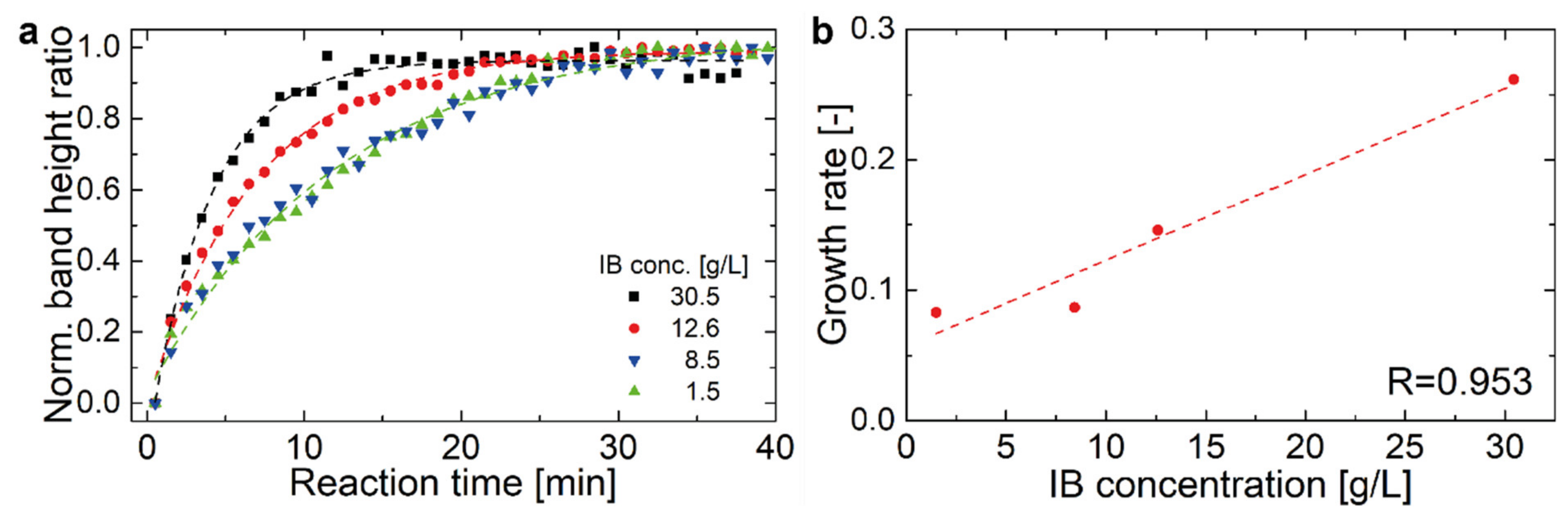
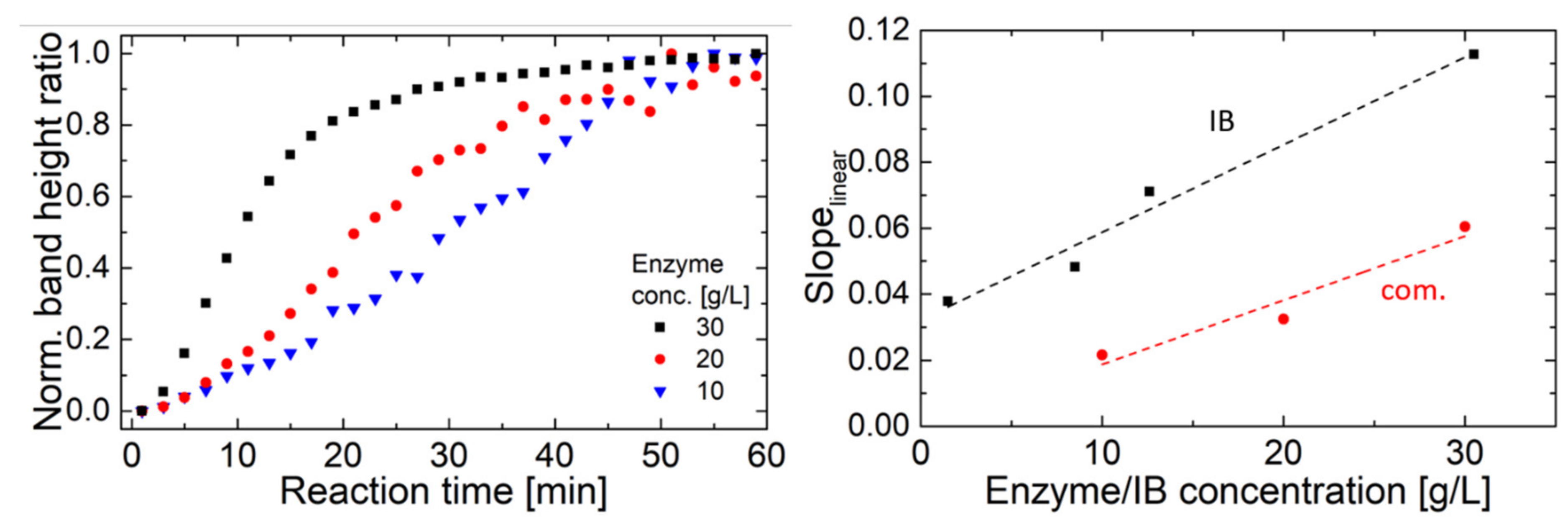
2.2. Hyaluronidase IB Activity Measured by FT-IR Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Bioreactor Cultivations
4.3. Cultivation Analytics
4.3.1. Biomass
4.3.2. Sugar Analytics
4.4. Product Analytics
4.4.1. IB Preparation
4.4.2. IB Size
4.4.3. IB Titer
4.4.4. SDS-PAGE
4.4.5. FT-IR Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| DCW | Dry cell weight |
| dO2 | Dissolved oxygen |
| DoE | Design of experiments |
| CCF | Face centered composite design |
| IB | Inclusion Body |
| IPTG | isopropyl β-D-1 thiogalactopyranoside |
| FT-IR | Fourier Transformed – Infrared (Spectroscopy) |
| MQ | Ultrapure water |
| qs,C [g/g/h] | specific substrate uptake rate |
| qp [g/g/h] | Specific production rate |
| SEM | Scanning electron microscopy |
References
- Baneyx, F. Recombinant protein expression in Escherichia coli. Curr. Opin. Biotechnol. 1999, 10, 411–421. [Google Scholar] [CrossRef]
- Huang, C.-J.; Lin, H.; Yang, X. Industrial production of recombinant therapeutics in Escherichia coli and its recent advancements. J. Ind. Microbiol. Biotechnol. 2012, 39, 383–399. [Google Scholar] [CrossRef] [PubMed]
- Sahdev, S.; Khattar, S.K.; Saini, K.S. Production of active eukaryotic proteins through bacterial expression systems: A review of the existing biotechnology strategies. Mol. Cell. Biochem. 2007, 307, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Walsh, G. Biopharmaceutical benchmarks 2010. Nat. Biotechnol. 2010, 28, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Baeshen, M.N.; Al-Hejin, A.M.; Bora, R.S.; Ahmed, M.M.; Ramadan, H.A.; Saini, K.S.; Baeshen, N.A.; Redwan, E.M. Production of Biopharmaceuticals in E. coli: Current Scenario and Future Perspectives. J. Microbiol. Biotechnol. 2015, 25, 953–962. [Google Scholar] [CrossRef]
- Spadiut, O.; Capone, S.; Krainer, F.; Glieder, A.; Herwig, C. Microbials for the production of monoclonal antibodies and antibody fragments. Trends Biotechnol. 2014, 32, 54–60. [Google Scholar] [CrossRef]
- Studier, F.W.; Moffatt, B.A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 1986, 189, 113–130. [Google Scholar] [CrossRef]
- Kopp, J.; Slouka, C.; Ulonska, S.; Kager, J.; Fricke, J.; Spadiut, O.; Herwig, C. Impact of Glycerol as Carbon Source onto Specific Sugar and Inducer Uptake Rates and Inclusion Body Productivity in E. coli BL21(DE3). Bioengineering 2018, 5, 1. [Google Scholar] [CrossRef]
- Dubendorff, J.W.; Studier, F.W. Controlling basal expression in an inducible T7 expression system by blocking the target T7 promoter with lac repressor. J. Mol. Biol. 1991, 219, 45–59. [Google Scholar] [CrossRef]
- García-Fruitós, E. Inclusion bodies: A new concept. Microb. Cell Fact. 2010, 9, 80. [Google Scholar] [CrossRef]
- García-Fruitós, E.; Arís, A.; Villaverde, A. Localization of functional polypeptides in bacterial inclusion bodies. Appl. Environ. Microbiol. 2007, 73, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Baneyx, F.; Mujacic, M. Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 2004, 22, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.; Villaverde, A. Protein quality in bacterial inclusion bodies. Trends Biotechnol. 2006, 24. [Google Scholar] [CrossRef] [PubMed]
- Peternel, Š.; Grdadolnik, J.; Gaberc-Porekar, V.; Komel, R. Engineering inclusion bodies for non denaturing extraction of functional proteins. Microb. Cell Fact. 2008, 7, 34. [Google Scholar] [CrossRef]
- Rinas, U.; Garcia-Fruitós, E.; Corchero, J.L.; Vázquez, E.; Seras-Franzoso, J.; Villaverde, A. Bacterial inclusion bodies: Discovering their better half. Trends Biochem. Sci. 2017, 42, 726–737. [Google Scholar] [CrossRef]
- Peternel, S.; Komel, R. Active protein aggregates produced in Escherichia coli. Int. J. Mol. Sci. 2011, 12, 8275–8287. [Google Scholar] [CrossRef]
- De Marco, A.; Ferrer-Miralles, N.; Garcia-Fruitós, E.; Mitraki, A.; Peternel, S.; Rinas, U.; Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Vázquez, E.; Villaverde, A. Bacterial inclusion bodies are industrially exploitable amyloids. FEMS Microbiol. Rev. 2019, 43, 53–72. [Google Scholar] [CrossRef]
- Krauss, U.; Jager, V.D.; Diener, M.; Pohl, M.; Jaeger, K.E. Catalytically-active inclusion bodies-Carrier-free protein immobilizates for application in biotechnology and biomedicine. J. Biotechnol. 2017, 258, 136–147. [Google Scholar] [CrossRef]
- Hrabárová, E.; Achbergerová, L.; Nahálka, J. Insoluble protein applications: The use of bacterial inclusion bodies as biocatalysts. In Insoluble Proteins; Springer: New York, NY, USA, 2015; pp. 411–422. [Google Scholar]
- Carrio, M.; Gonzalez-Montalban, N.; Vera, A.; Villaverde, A.; Ventura, S. Amyloid-like properties of bacterial inclusion bodies. J. Mol. Biol. 2005, 347, 1025–1037. [Google Scholar] [CrossRef]
- Morell, M.; Bravo, R.; Espargaro, A.; Sisquella, X.; Aviles, F.X.; Fernandez-Busquets, X.; Ventura, S. Inclusion bodies: Specificity in their aggregation process and amyloid-like structure. Biochim. Biophys. Acta 2008, 1783, 1815–1825. [Google Scholar] [CrossRef]
- Carrio, M.M.; Villaverde, A. Protein aggregation as bacterial inclusion bodies is reversible. FEBS Lett. 2001, 489, 29–33. [Google Scholar] [CrossRef]
- Groot, N.S.d.; Sabate, R.; Ventura, S. Amyloids in bacterial inclusion bodies. Trends Biochem. Sci. 2009, 34, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Xing, L.; Zhou, B.; Lin, Z. Active protein aggregates induced by terminally attached self-assembling peptide ELK16 in Escherichia coli. Microb. Cell Fact. 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xing, L.; Wu, W.; Zhang, X.-E.; Lin, Z. Small surfactant-like peptides can drive soluble proteins into active aggregates. Microb. Cell Fact. 2012, 11, 1–8. [Google Scholar] [CrossRef]
- Talafová, K.; Hrabárová, E.; Chorvát, D.; Nahálka, J. Bacterial inclusion bodies as potential synthetic devices for pathogen recognition and a therapeutic substance release. Microb. Cell Fact. 2013, 12, 16. [Google Scholar] [CrossRef]
- Vazquez, E.; Corchero, J.L.; Burgueno, J.F.; Seras-Franzoso, J.; Kosoy, A.; Bosser, R.; Mendoza, R.; Martinez-Lainez, J.M.; Rinas, U.; Fernandez, E.; et al. Functional inclusion bodies produced in bacteria as naturally occurring nanopills for advanced cell therapies. Adv. Mater. 2012, 24, 1742–1747. [Google Scholar] [CrossRef]
- Liovic, M.; Ozir, M.; Zavec, A.B.; Peternel, S.; Komel, R.; Zupancic, T. Inclusion bodies as potential vehicles for recombinant protein delivery into epithelial cells. Microb. Cell Fact. 2012, 11, 67. [Google Scholar] [CrossRef]
- Rueda, F.; Cano-Garrido, O.; Mamat, U.; Wilke, K.; Seras-Franzoso, J.; García-Fruitós, E.; Villaverde, A. Production of functional inclusion bodies in endotoxin-free Escherichia coli. Appl. Microbiol. Biotechnol. 2014, 98, 9229–9238. [Google Scholar] [CrossRef]
- Vázquez, E.; Villaverde, A. Microbial biofabrication for nanomedicine: Biomaterials, nanoparticles and beyond. Nanomedicine (Lond.) 2013, 8, 1895–1898. [Google Scholar] [CrossRef]
- Seras-Franzoso, J.; Steurer, C.; Roldan, M.; Vendrell, M.; Vidaurre-Agut, C.; Tarruella, A.; Saldana, L.; Vilaboa, N.; Parera, M.; Elizondo, E.; et al. Functionalization of 3D scaffolds with protein-releasing biomaterials for intracellular delivery. J. Control. Release 2013, 171, 63–72. [Google Scholar] [CrossRef]
- Céspedes, M.V.; Fernández, Y.; Unzueta, U.; Mendoza, R.; Seras-Franzoso, J.; Sánchez-Chardi, A.; Álamo, P.; Toledo-Rubio, V.; Ferrer-Miralles, N.; Vázquez, E.; et al. Bacterial mimetics of endocrine secretory granules as immobilized in vivo depots for functional protein drugs. Sci. Rep. 2016, 6, 35765. [Google Scholar] [CrossRef] [PubMed]
- Unzueta, U.; Seras-Franzoso, J.; Cespedes, M.V.; Saccardo, P.; Cortes, F.; Rueda, F.; Garcia-Fruitos, E.; Ferrer-Miralles, N.; Mangues, R.; Vazquez, E.; et al. Engineering tumor cell targeting in nanoscale amyloidal materials. Nanotechnology 2017, 28, 015102. [Google Scholar] [CrossRef]
- Sánchez, J.M.; López-Laguna, H.; Álamo, P.; Serna, N.; Sánchez-Chardi, A.; Nolan, V.; Cano-Garrido, O.; Casanova, I.; Unzueta, U.; Vazquez, E.; et al. Artificial Inclusion Bodies for Clinical Development. Adv. Sci. 2020, 7, 1902420. [Google Scholar] [CrossRef] [PubMed]
- Slouka, C.; Kopp, J.; Spadiut, O.; Herwig, C. Perspectives of inclusion bodies for bio-based products: Curse or blessing? Appl. Microbiol. Biotechnol. 2019, 103, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Steele, D. Infrared Spectroscopy: Theory. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; pp. 44–70. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Et Biophys. Actabioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Wiercigroch, E.; Szafraniec, E.; Czamara, K.; Pacia, M.Z.; Majzner, K.; Kochan, K.; Kaczor, A.; Baranska, M.; Malek, K. Raman and infrared spectroscopy of carbohydrates: A review. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 317–335. [Google Scholar] [CrossRef] [PubMed]
- Schindler, R.; Lendl, B.; Kellner, R. Determination of amyloglucosidase activity using flow injection analysis with Fourier transform infrared spectrometric detection. Analyst 1997, 122, 531–534. [Google Scholar] [CrossRef]
- Schindler, R.; Lendl, B.; Kellner, R. Simultaneous determination of alpha-amylase and amyloglucosidase activities using flow injection analysis with Fourier transform infrared spectroscopic detection and partial least-squares data treatment. Anal. Chim. Acta 1998, 366, 35–43. [Google Scholar] [CrossRef]
- Schindler, R.; Lendl, B. Simultaneous determination of enzyme activities by FTIR-spectroscopy in an one-step assay. Anal. Chim. Acta 1999, 391, 19–28. [Google Scholar] [CrossRef]
- Wurm, D.J.; Quehenberger, J.; Mildner, J.; Eggenreich, B.; Slouka, C.; Schwaighofer, A.; Wieland, K.; Lendl, B.; Rajamanickam, V.; Herwig, C.; et al. Teaching an old pET new tricks: Tuning of inclusion body formation and properties by a mixed feed system in E. coli. Appl. Microbiol. Biotechnol. 2018, 102, 667–676. [Google Scholar] [CrossRef]
- Doglia, S.M.; Ami, D.; Natalello, A.; Gatti-Lafranconi, P.; Lotti, M. Fourier transform infrared spectroscopy analysis of the conformational quality of recombinant proteins within inclusion bodies. Biotechnol. J. 2008, 3, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Slouka, C.; Kopp, J.; Hutwimmer, S.; Strahammer, M.; Strohmer, D.; Eitenberger, E.; Schwaighofer, A.; Herwig, C. Custom Made Inclusion Bodies: Impact of classical process parameters and physiological parameters on Inclusion Body quality attributes. Microb. Cell Fact. 2018, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- El-Safory, N.S.; Fazary, A.E.; Lee, C.-K.J.C.P. Hyaluronidases, a group of glycosidases: Current and future perspectives. Carbohydr. Polym. 2010, 81, 165–181. [Google Scholar] [CrossRef]
- Menzel, E.J.; Farr, C. Hyaluronidase and its substrate hyaluronan: Biochemistry, biological activities and therapeutic uses. Cancer Lett. 1998, 131, 3–11. [Google Scholar] [CrossRef]
- Guo, X.; Liu, F.; Zhu, X.; Su, Y.; Ling, P. Expression of a novel hyaluronidase from Streptococcus zooepidemicus in Escherichia coli and its application for the preparation of HA oligosaccharides. Carbohydr. Polym. 2009, 77, 254–260. [Google Scholar] [CrossRef]
- Rahmanian, M.; Pertoft, H.; Kanda, S.; Christofferson, R.; Claesson-Welsh, L.; Heldin, P. Hyaluronan Oligosaccharides Induce Tube Formation of a Brain Endothelial Cell Linein Vitro. Exp. Cell Res. 1997, 237, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Reichelt, W.N.; Brillmann, M.; Thurrold, P.; Keil, P.; Fricke, J.; Herwig, C. Physiological capacities decline during induced bioprocesses leading to substrate accumulation. Biotechnol. J. 2017, 12, 1600547. [Google Scholar] [CrossRef] [PubMed]
- Kopp, J.; Slouka, C.; Strohmer, D.; Kager, J.; Spadiut, O.; Herwig, C. Inclusion Body Bead Size in E. coli Controlled by Physiological Feeding. Microorganisms 2018, 6, 116. [Google Scholar]
- Soldatova, L.N.; Crameri, R.; Gmachl, M.; Kemeny, D.M.; Schmidt, M.; Weber, M.; Mueller, U.R. Superior biologic activity of the recombinant bee venom allergen hyaluronidase expressed in baculovirus-infected insect cells as compared with Escherichia coli. J. Allergy Clin. Immunol. 1998, 101, 691–698. [Google Scholar] [CrossRef]
- Astériou, T.; Vincent, J.-C.; Tranchepain, F.; Deschrevel, B. Inhibition of hyaluronan hydrolysis catalysed by hyaluronidase at high substrate concentration and low ionic strength. Matrix Biol. 2006, 25, 166–174. [Google Scholar] [CrossRef]
- Haxaire, K.; Maréchal, Y.; Milas, M.; Rinaudo, M. Hydration of hyaluronan polysaccharide observed by IR spectrometry. II. Definition and quantitative analysis of elementary hydration spectra and water uptake. Biopolymers 2003, 72, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Gilli, R.; Kacurakova, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. Ftir Studies of Sodium Hyaluronate and Its Oligomers in the Amorphous Solid-Phase and in Aqueous-Solution. Carbohyd. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef]
- Nahálka, J.; Vikartovská, A.; Hrabárová, E. A crosslinked inclusion body process for sialic acid synthesis. J. Biotechnol. 2008, 134, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2005; pp. 571–607. [Google Scholar]
- Ceroni, A.; Passerini, A.; Vullo, A.; Frasconi, P. DISULFIND: A disulfide bonding state and cysteine connectivity prediction server. Nucleic Acids Res. 2006, 34, W177–W181. [Google Scholar] [CrossRef] [PubMed]
- Dudler, T.; Chen, W.-Q.; Wang, S.; Schneider, T.; Annand, R.R.; Dempcy, R.O.; Crameri, R.; Gmachl, M.; Suter, M.; Gelb, M.H. High-level expression in Escherichia coli and rapid purification of enzymatically active honey bee venom phospholipase A2. Biochim. Biophys. Acta 1992, 1165, 201–210. [Google Scholar] [CrossRef]
- Ceroni, F.; Algar, R.; Stan, G.B.; Ellis, T. Quantifying cellular capacity identifies gene expression designs with reduced burden. Nat. Methods 2015, 12, 415–418. [Google Scholar] [CrossRef]
- Dvorak, P.; Chrast, L.; Nikel, P.I.; Fedr, R.; Soucek, K.; Sedlackova, M.; Chaloupkova, R.; de Lorenzo, V.; Prokop, Z.; Damborsky, J. Exacerbation of substrate toxicity by IPTG in Escherichia coli BL21(DE3) carrying a synthetic metabolic pathway. Microb. Cell Fact. 2015, 14, 201. [Google Scholar] [CrossRef]
- Silva, F.; Queiroz, J.A.; Domingues, F.C. Evaluating metabolic stress and plasmid stability in plasmid DNA production by Escherichia coli. Biotechnol. Adv. 2012, 30, 691–708. [Google Scholar] [CrossRef]
- Heyland, J.; Blank, L.M.; Schmid, A. Quantification of metabolic limitations during recombinant protein production in Escherichia coli. J. Biotechnol. 2011, 155, 178–184. [Google Scholar] [CrossRef]
- Malakar, P.; Venkatesh, K.V. Effect of substrate and IPTG concentrations on the burden to growth of Escherichia coli on glycerol due to the expression of Lac proteins. Appl. Microbiol. Biotechnol. 2012, 93, 2543–2549. [Google Scholar] [CrossRef]
- Haddadin, F.T.; Harcum, S.W. Transcriptome profiles for high-cell-density recombinant and wild-type Escherichia coli. Biotechnol. Bioeng. 2005, 90, 127–153. [Google Scholar] [CrossRef] [PubMed]
- Shiloach, J.; Fass, R.J.B.a. Growing E. coli to high cell density—a historical perspective on method development. Biotechnol. Adv. 2005, 23, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, M.; Shiloach, J. Proposed mechanism of acetate accumulation in two recombinant Escherichia coli strains during high density fermentation. Biotechnol. Bioeng. 1998, 57, 71–78. [Google Scholar] [CrossRef]
- Peternel, Š.; Jevševar, S.; Bele, M.; Gaberc-Porekar, V.; Menart, V. New properties of inclusion bodies with implications for biotechnology. Biotechnol. Appl. Biochem. 2008, 49, 239–246. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme assays. Perspect. Sci. 2014, 1, 41–55. [Google Scholar] [CrossRef]
- Lenormand, H.; Amar-Bacoup, F.; Vincent, J.-C. Reaction–complexation coupling between an enzyme and its polyelectrolytic substrate: Determination of the dissociation constant of the hyaluronidase–hyaluronan complex from the hyaluronidase substrate-dependence. Biophys. Chem. 2013, 175, 63–70. [Google Scholar] [CrossRef]
- Lenormand, H.; Amar-Bacoup, F.; Vincent, J.-C. pH effects on the hyaluronan hydrolysis catalysed by hyaluronidase in the presence of proteins. Part III. The electrostatic non-specific hyaluronan–hyaluronidase complex. Carbohydr. Polym. 2011, 86, 1491–1500. [Google Scholar] [CrossRef]
- Koch, C.; Brandstetter, M.; Wechselberger, P.; Lorantfy, B.; Plata, M.R.; Radel, S.; Herwig, C.; Lendl, B. Ultrasound-Enhanced Attenuated Total Reflection Mid-infrared Spectroscopy In-Line Probe: Acquisition of Cell Spectra in a Bioreactor. Anal. Chem. 2015, 87, 2314–2320. [Google Scholar] [CrossRef]
- Koch, C.; Posch, A.E.; Herwig, C.; Lendl, B. Comparison of Fiber Optic and Conduit Attenuated Total Reflection (ATR) Fourier Transform Infrared (FT-IR) Setup for In-Line Fermentation Monitoring. Appl. Spectrosc. 2016, 70, 1965–1973. [Google Scholar] [CrossRef]
- DeLisa, M.P.; Li, J.; Rao, G.; Weigand, W.A.; Bentley, W.E. Monitoring GFP-operon fusion protein expression during high cell density cultivation of Escherichia coli using an on-line optical sensor. Biotechnol. Bioeng. 1999, 65, 54–64. [Google Scholar] [CrossRef]
- Kopp, J.; Zauner, F.B.; Pell, A.; Hausjell, J.; Humer, D.; Ebner, J.; Herwig, C.; Spadiut, O.; Slouka, C.; Pell, R. Development of a widely applicable reversed-phase liquid chromatography method for protein quantification using analytical quality-by-design principles. 2020. under review. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwaighofer, A.; Ablasser, S.; Lux, L.; Kopp, J.; Herwig, C.; Spadiut, O.; Lendl, B.; Slouka, C. Production of Active Recombinant Hyaluronidase Inclusion Bodies from Apis mellifera in E. coli Bl21(DE3) and characterization by FT-IR Spectroscopy. Int. J. Mol. Sci. 2020, 21, 3881. https://doi.org/10.3390/ijms21113881
Schwaighofer A, Ablasser S, Lux L, Kopp J, Herwig C, Spadiut O, Lendl B, Slouka C. Production of Active Recombinant Hyaluronidase Inclusion Bodies from Apis mellifera in E. coli Bl21(DE3) and characterization by FT-IR Spectroscopy. International Journal of Molecular Sciences. 2020; 21(11):3881. https://doi.org/10.3390/ijms21113881
Chicago/Turabian StyleSchwaighofer, Andreas, Sarah Ablasser, Laurin Lux, Julian Kopp, Christoph Herwig, Oliver Spadiut, Bernhard Lendl, and Christoph Slouka. 2020. "Production of Active Recombinant Hyaluronidase Inclusion Bodies from Apis mellifera in E. coli Bl21(DE3) and characterization by FT-IR Spectroscopy" International Journal of Molecular Sciences 21, no. 11: 3881. https://doi.org/10.3390/ijms21113881
APA StyleSchwaighofer, A., Ablasser, S., Lux, L., Kopp, J., Herwig, C., Spadiut, O., Lendl, B., & Slouka, C. (2020). Production of Active Recombinant Hyaluronidase Inclusion Bodies from Apis mellifera in E. coli Bl21(DE3) and characterization by FT-IR Spectroscopy. International Journal of Molecular Sciences, 21(11), 3881. https://doi.org/10.3390/ijms21113881






