Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo
Abstract
1. Recent Breakthroughs in Immunotherapy of Cancer
2. Response Markers in the Tumor Microenvironment (TME)
3. Preclinical Data from Rodent Animal Models of Cancer and Exercise
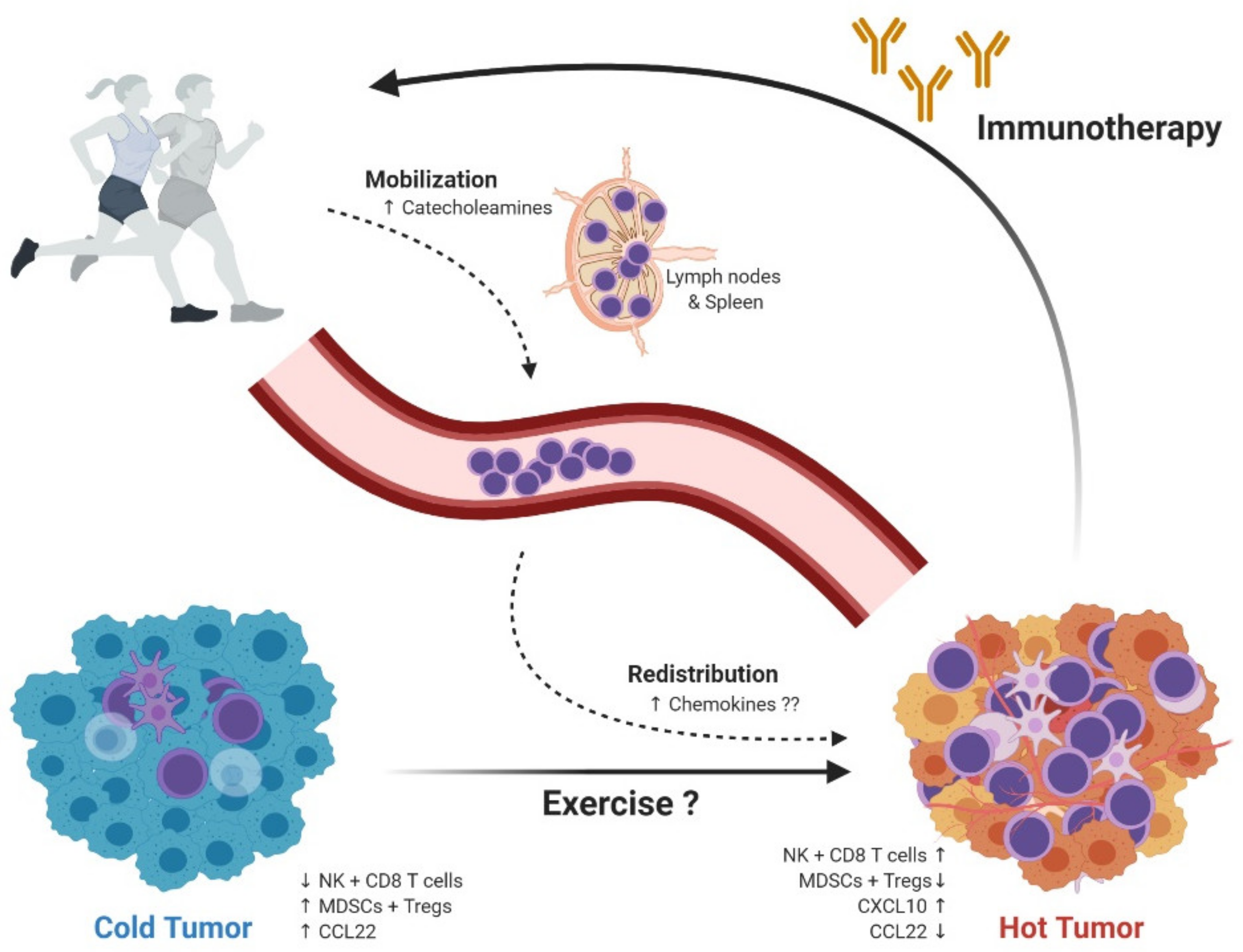
4. Mechanisms Behind Exercise-Induced Tumor Growth Control
5. Exercise Oncology; Focus on the Immune System; From Mouse to Man
6. Exercise Oncology in the Clinic; Current Status and Future Prospects
7. Exercise Oncology in the Clinic; HI AIM
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Zitvogel, L.; Eggermont, A.; Marabelle, A. PD-Loma: A cancer entity with a shared sensitivity to the PD-1/PD-L1 pathway blockade. Br. J. Cancer 2019, 120, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and Novel Combinations in the Treatment of Melanoma-An Update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Schenker, M.; Lee, K.H.; Provencio, M.; Nishio, M.; Lesniewski-Kmak, K.; Sangha, R.; Ahmed, S.; Raimbourg, J.; Feeney, K.; et al. Nivolumab plus ipilimumab versus chemotherapy as first-line treatment in advanced non–small-cell lung cancer with high tumour mutational burden: Patient-reported outcomes results from the randomised, open-label, phase III CheckMate 227 trial. Eur. J. Cancer 2019, 116, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, M.D.; Paz-Ares, L.; Caro, R.B.; Zurawski, B.; Kim, S.-W.; Costa, E.C.; Park, K.; Alexandru, A.; Lupinacci, L.; Jimenez, E.D.L.M.; et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Flies, D.B. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat. Rev. Immunol. 2013, 13, 227–242. [Google Scholar] [CrossRef]
- Tang, J.; Shalabi, A.; Hubbard-Lucey, V.M. Comprehensive analysis of the clinical immuno-oncology landscape. Ann. Oncol. 2018, 29, 84–91. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Goodman, A.M.; Sokol, E.S.; Frampton, G.M.; Lippman, S.M.; Kurzrock, R. Microsatellite-Stable Tumors with High Mutational Burden Benefit from Immunotherapy. Cancer Immunol. Res. 2019, 7, 1570–1573. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Du, C.; Wu, Y.; Xia, D.; Lv, W.; Hu, J. The Predictive Value of Tumor Mutation Burden on Efficacy of Immune Checkpoint Inhibitors in Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Bao, R.; Gajewski, T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015, 523, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.H.; Elder, D.E.; Guerry, D.; Braitman, L.E.; Trock, B.J.; Schultz, D.; Synnestvedt, M.; Halpern, A.C. Model predicting survival in stage I melanoma based on tumor progression. J. Natl. Cancer Inst. 1989, 81, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Angell, H.K.; Bruni, D.; Barrett, J.C.; Herbst, R.; Galon, J. The Immunoscore: Colon Cancer and Beyond. Clin. Cancer Res. 2020, 26, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Coleno-Costes, A.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Zinzindohoué, F.; Bruneval, P.; Cugnenc, P.-H.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- De Guillebon, E.; Dardenne, A.; Saldmann, A.; Séguier, S.; Tran, T.; Paolini, L.; Lebbe, C.; Tartour, E. Beyond the concept of cold and hot tumors for the development of novel predictive biomarkers and the rational design of immunotherapy combination. Int. J. Cancer 2020. [Google Scholar] [CrossRef]
- Perea, F.; Bernal, M.; Carretero, J.; Torres, C.; Bayarri, C.; Gómez-Morales, M.; Garrido, F.; Ruiz-Cabello, F.; Sánchez-Palencia, A. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int. J. Cancer 2017, 140, 888–899. [Google Scholar] [CrossRef]
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment—The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346. [Google Scholar] [CrossRef]
- Parry, T.L.; Hayward, R. Exercise Protects against Cancer-induced Cardiac Cachexia. Med. Sci. Sports Exerc. 2018, 50, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Hojman, P.; Fjelbye, J.; Zerahn, B.; Christensen, J.F.; Dethlefsen, C.; Lonkvist, C.K.; Brandt, C.; Gissel, H.; Pedersen, B.K.; Gehl, J. Voluntary exercise prevents cisplatin-induced muscle wasting during chemotherapy in mice. PLoS ONE 2014, 9, e109030. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, K.; Ballarò, R.; Bover, Q.; Pin, F.; Beltrà, M.; Penna, F.; Costelli, P. Combined Exercise Training Positively Affects Muscle Wasting in Tumor-Bearing Mice. Med. Sci. Sports Exerc. 2019, 51, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Christensen, J.F.; Hojman, P. Effects of exercise on tumor physiology and metabolism. Cancer J. 2015, 21, 111–116. [Google Scholar] [CrossRef]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef]
- Gershbein, L.L.; Benuck, I.; Shurrager, P.S. Influence of stress of lesion growth and on survival of animals bearing parenteral and intracerebral leukemia L1210 and Walker tumors. Oncology 1974, 30, 429–435. [Google Scholar] [CrossRef]
- De Lima, C.; Alves, L.E.; Iagher, F.; Machado, A.F.; Bonatto, S.J.R.; Kuczera, D.; De Souza, C.F.; Pequito, D.C.; Muritiba, A.L.; Nunes, E.; et al. Anaerobic exercise reduces tumor growth, cancer cachexia and increases macrophage and lymphocyte response in Walker 256 tumor-bearing rats. Eur. J. Appl. Physiol. 2008, 104, 957–964. [Google Scholar] [CrossRef]
- De Lima, C.; Alves, L.; Iagher, F.; Machado, A.F.; Kryczyk, M.; Yamazaki, R.K.; Brito, G.A.P.; Nunes, E.; Naliwaiko, K.; Fernandes, L.C. Tumor growth reduction in walker 256 tumorbearing rats performing anaerobic exercise: Participation of Bcl-2, Bax, apoptosis, and peroxidation. Appl. Physiol. Nutr. Metab. 2011, 36, 533–538. [Google Scholar] [CrossRef]
- Moreira, V.M.; Almeida, D.; Franco, C.C.D.S.; Gomes, R.M.; Palma-Rigo, K.; Prates, K.V.; Tófolo, L.P.; Malta, A.; Francisco, F.A.; Pavanello, A.; et al. Moderate exercise training since adolescence reduces Walker 256 tumour growth in adult rats. J. Physiol. 2019, 597, 3905–3925. [Google Scholar] [CrossRef]
- Rincón-Castanedo, C.; Morales, J.S.; Martín-Ruiz, A.; Valenzuela, P.L.; Ramírez, M.; Santos-Lozano, A.; Lucia, A.; Fiuza-Luces, C. Physical exercise effects on metastasis: A systematic review and meta-analysis in animal cancer models. Cancer Metastasis Rev. 2020, 39, 91–114. [Google Scholar] [CrossRef]
- DeMarzo, M.M.P.; Garcia, S.B. Exhaustive physical exercise increases the number of colonic preneoplastic lesions in untrained rats treated with a chemical carcinogen. Cancer Lett. 2004, 216, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, L.; Ji, B.; Li, L.; Qi, Z.; Ding, S. Endurance training but not high-intensity interval training reduces liver carcinogenesis in mice with hepatocellular carcinogen diethylnitrosamine. Exp. Gerontol. 2020, 133, 110853. [Google Scholar] [CrossRef] [PubMed]
- LaVoy, E.C.; Hussain, M.; Reed, J.; Kunz, H.; Pistillo, M.; Bigley, A.B.; Simpson, R.J. T-cell redeployment and intracellular cytokine expression following exercise: Effects of exercise intensity and cytomegalovirus infection. Physiol. Rep. 2017, 5, e13070. [Google Scholar] [CrossRef] [PubMed]
- Hagar, A.; Wang, Z.; Koyama, S.; Aponte-Serrano, J.O.; Melo, L.; Vargas, S.; Carpenter, R.; Foley, J. Endurance training slows breast tumor growth in mice by suppressing Treg cells recruitment to tumors. BMC Cancer 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Pedersen, L.; Idorn, M.; Olofsson, G.H.; Lauenborg, B.; Nookaew, I.; Hansen, R.H.; Johannesen, H.H.; Becker, J.C.; Pedersen, K.S.; Dethlefsen, C.; et al. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016, 23, 554–562. [Google Scholar] [CrossRef]
- Ryen, S.V.D.D.T.; Deldicque, L. The regulation of the metastatic cascade by physical activity: A narrative review. Cancers 2020, 12, 153. [Google Scholar] [CrossRef]
- Hoffman-Goetz, L.; MacNeil, B.; Arumugam, Y.; Simpson, J. Differential effects of exercise and housing condition on murine natural killer cell activity and tumor growth. Int. J. Sports Med. 1992, 13, 167–171. [Google Scholar] [CrossRef]
- Buss, L.A.; Ang, A.D.; Hock, B.; Robinson, B.A.; Currie, M.J.; Dachs, G.U. Effect of post-implant exercise on tumour growth rate, perfusion and hypoxia in mice. PLoS ONE 2020, 15, e0229290. [Google Scholar] [CrossRef]
- Tank, A.W.; Wong, D.L. Peripheral and central effects of circulating catecholamines. Compr. Physiol. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, F.; Yang, R.; Zheng, X.; Gao, H.; Zhang, P. Effect of chronic restraint stress on human colorectal carcinoma growth in mice. PLoS ONE 2013, 8, e61435. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 33, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Nissen, M.D.; Sloan, E.K.; Mattarollo, S.R. β-Adrenergic signaling impairs antitumor CD8+ T-cell responses to B-cell lymphoma immunotherapy. Cancer Immunol. Res. 2018, 6, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Li, B.; Li, Z.; Zhang, J.; Yu, J.; Zhang, L.; Xu, Z. Adrenergic modulation of AMPK-dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int. J. Oncol. 2019, 54, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Higgins, K.A.; Park, D.; Lee, G.Y.; Curran, W.J.; Deng, X. Exercise-induced lung cancer regression: Mechanistic findings from a mouse model. Cancer 2014, 120, 3302–3310. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; DeMaria, S. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Idorn, M.; Hojman, P. Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol. Med. 2016, 22, 565–577. [Google Scholar] [CrossRef]
- Schadler, K.L.; Thomas, N.J.; Galie, P.A.; Bhang, D.H.; Roby, K.C.; Addai, P.; Till, J.E.; Sturgeon, K.; Zaslavsky, A.; Chen, C.S.; et al. Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 2016, 7, 65429–65440. [Google Scholar] [CrossRef]
- Bacurau, A.V.N.; Belmonte, M.A.; Navarro, F.; Moraes, M.R.; Pontes, F.L.; Pesquero, J.; Araujo, R.; Bacurau, R.F.P. Effect of a high-intensity exercise training on the metabolism and function of macrophages and lymphocytes of walker 256 tumor bearing rats. Exp. Boil. Med. 2007, 232, 1289–1299. [Google Scholar] [CrossRef]
- Bacuau, R.F.P.; Belmonte, M.A.; Seelaender, M.C.L.; Bacurau, R.F.P.; Coast Rosa, L.F.B.P. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell Biochem. Funct. 2000, 18, 249–258. [Google Scholar] [CrossRef]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef]
- Almeida, P.W.M.; Gomes-Filho, A.; Ferreira, A.J.; Rodrigues, C.E.M.; Dias-Peixoto, M.F.; Russo, R.C.; Teixeira, M.M.; Cassali, G.D.; Ferreira, E.; Santos, I.C.; et al. Swim training suppresses tumor growth in mice. J. Appl. Physiol. 2009, 107, 261–265. [Google Scholar] [CrossRef] [PubMed]
- McClellan, J.L.; Steiner, J.L.; Day, S.D.; Enos, R.; Davis, M.J.; Singh, U.P.; Murphy, E.A. Exercise effects on polyp burden and immune markers in the ApcMin/+ mouse model of intestinal tumorigenesis. Int. J. Oncol. 2014, 45, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Bigley, A.B.; Agha, N.; Hanley, P.J.; Bollard, C.M. Mobilizing Immune Cells with Exercise for Cancer Immunotherapy. Exerc. Sport Sci. Rev. 2017, 45, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. The short-term stress response—Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocr. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Graff, R.M.; Kunz, H.E.; Agha, N.H.; Baker, F.L.; Laughlin, M.; Bigley, A.B.; Markofski, M.M.; LaVoy, E.C.; Katsanis, E.; Bond, R.A.; et al. β2-Adrenergic receptor signaling mediates the preferential mobilization of differentiated subsets of CD8+ T-cells, NK-cells and non-classical monocytes in response to acute exercise in humans. Brain, Behav. Immun. 2018, 74, 143–153. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef]
- Ngo, S.T.; Steyn, F.; McCombe, P.A. Gender differences in autoimmune disease. Front. Neuroendocr. 2014, 35, 347–369. [Google Scholar] [CrossRef]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Timmons, B.W.; Hamadeh, M.; Devries, M.C.; Tarnopolsky, M.A. Influence of gender, menstrual phase, and oral contraceptive use on immunological changes in response to prolonged cycling. J. Appl. Physiol. 2005, 99, 979–985. [Google Scholar] [CrossRef]
- Aiello, A.; Farzaneh, F.; Candore, G.; Malavolta, M.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Malavolta, M.; Accardi, G.; Virruso, C.; Candore, G. Sex, gender and immunosenescence: A key to understand the different lifespan between men and women? Immun. Ageing 2013, 10, 20. [Google Scholar] [CrossRef]
- Hirokawa, K.; Utsuyama, M.; Hayashi, Y.; Kitagawa, M.; Makinodan, T.; Fülöp, T. Slower immune system aging in women versus men in the Japanese population. Immun. Ageing 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Does patient age influence anti-cancer immunity? Semin. Immunopathol. 2018, 41, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Gounant, V.; Lavolé, A.; Quoix, E. Ongoing challenges of using immunotherapy in special populations: Poor performance status patients, elderly patients, and people living with HIV. Lung Cancer 2020, 145, 71–75. [Google Scholar] [CrossRef]
- Rooney, B.V.; Bigley, A.B.; LaVoy, E.C.; Laughlin, M.; Pedlar, C.; Simpson, R.J. Lymphocytes and monocytes egress peripheral blood within minutes after cessation of steady state exercise: A detailed temporal analysis of leukocyte extravasation. Physiol. Behav. 2018, 194, 260–267. [Google Scholar] [CrossRef]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef]
- Piccirillo, R. Exercise-Induced Myokines with Therapeutic Potential for Muscle Wasting. Front. Physiol. 2019, 10, 287. [Google Scholar] [CrossRef]
- Lehmann, J.F.; DeLisa, J.A.; Warren, C.G.; DeLateur, B.J.; Bryant, P.L.; Nicholson, C.G. Cancer rehabilitation: Assessment of need, development, and evaluation of a model of care. Arch. Phys. Med. Rehabilitation 1978, 59, 410–419. [Google Scholar]
- Winningham, M.L.; MacVicar, M.G.; Bondoc, M.; Anderson, J.I.; Minton, J.P. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncol. Nurs. Forum 1989, 16, 683–689. [Google Scholar]
- Cramp, F.; Byron-Daniel, J. Exercise for the management of cancer-related fatigue in adults. Cochrane Database Syst. Rev. 2012, 11, CD006145. [Google Scholar] [CrossRef] [PubMed]
- DiMeo, F.; Fetscher, S.; Lange, W.; Mertelsmann, R.; Keul, J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood 1997, 90, 3390–3394. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Segal, R.J.; McKenzie, D.C.; Dong, H.; Gelmon, K.; Friedenreich, C.M.; Yasui, Y.; Reid, R.D.; Crawford, J.J.; Mackey, J.R. Effects of exercise during adjuvant chemotherapy on breast cancer outcomes. Med. Sci. Sports Exerc. 2014, 46, 1744–1751. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.; Loblaw, A.; Petrella, T. Exercise for people with cancer: A systematic review. Curr. Oncol. 2017, 24, e290–e315. [Google Scholar] [CrossRef]
- Segal, R.; Zwaal, C.; Green, E.; Tomasone, J.; Loblaw, A.; Petrella, T. Exercise for people with cancer: A clinical practice guideline. Curr. Oncol. 2017, 24, 40. [Google Scholar] [CrossRef]
- Moore, S.C.; Lee, I.-M.; Weiderpass, E.; Campbell, P.T.; Sampson, J.N.; Kitahara, C.M.; Keadle, S.; Arem, H.; De Gonzalez, A.B.; Hartge, P.; et al. Association of Leisure-Time Physical Activity With Risk of 26 Types of Cancer in 1.44 Million Adults. JAMA Intern. Med. 2016, 176, 816–825. [Google Scholar] [CrossRef]
- Ballard-Barbash, R.; Friedenreich, C.M.; Courneya, K.S.; Siddiqi, S.M.; McTiernan, A.; Alfano, C.M. Physical activity, biomarkers, and disease outcomes in cancer survivors: A systematic review. J. Natl. Cancer Inst. 2012, 104, 815–840. [Google Scholar] [CrossRef]
- Richman, E.L.; Kenfield, S.A.; Stampfer, M.J.; Paciorek, A.; Carroll, P.R.; Chan, J.M. Physical activity after diagnosis and risk of prostate cancer progression: Data from the cancer of the prostate strategic urologic research endeavor. Cancer Res. 2011, 71, 3889–3895. [Google Scholar] [CrossRef]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical activity and survival after prostate cancer diagnosis in the health professionals follow-up study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef]
- Hvid, T.; Lindegaard, B.; Winding, K.; Iversen, P.; Brasso, K.; Solomon, T.P.J.; Pedersen, B.K.; Hojman, P. Effect of a 2-year home-based endurance training intervention on physiological function and PSA doubling time in prostate cancer patients. Cancer Causes Control. 2016, 27, 165–174. [Google Scholar] [CrossRef]
- De Rezende, L.F.M.; De Sá, T.H.; Markozannes, G.; Rey-López, J.P.; Lee, I.-M.; Tsilidis, K.K.; Ioannidis, J.P.A.; Eluf-Neto, J. Physical activity and cancer: An umbrella review of the literature including 22 major anatomical sites and 770,000 cancer cases. Br. J. Sports Med. 2018, 52, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Newton, R.U.; Kenfield, S.A.; Hart, N.H.; Chan, J.M.; Courneya, K.S.; Catto, J.W.; Finn, S.; Greenwood, R.; Hughes, D.C.; Mucci, L.; et al. Intense Exercise for Survival among Men with Metastatic Castrate-Resistant Prostate Cancer (INTERVAL-GAP4): A multicentre, randomised, controlled phase III study protocol. BMJ Open 2018, 8, e022899. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Vardy, J.L.; O’Callaghan, C.J.; Friedenreich, C.M.; Campbell, K.L.; Prapavessis, H.; Crawford, J.J.; O’Brien, P.; Dhillon, H.M.; Jonker, D.J.; et al. Effects of a Structured Exercise Program on Physical Activity and Fitness in Colon Cancer Survivors: One Year Feasibility Results from the CHALLENGE Trial. Cancer Epidemiol. Biomark. Prev. 2016, 25, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Courneya, K.S.; Booth, C.; Gill, S.; O’Brien, P.; Vardy, J.; Friedenreich, C.; Au, H.; Brundage, M.; Tu, D.; Dhillon, H.; et al. The Colon Health and Life-Long Exercise Change trial: A randomized trial of the National Cancer Institute of Canada Clinical Trials Group. Curr. Oncol. 2008, 15, 271–278. [Google Scholar] [CrossRef]
- Mok, T.S.; Wu, Y.-L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G.; Srimuninnimit, V.; Laktionov, K.P.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Govindan, R.; Anders, R.A.; Antonia, S.J.; Bonerigo, S.; Davies, M.J.; Dubinett, S.; Ferris, A.; Gandhi, L.; Garon, E.B.; et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J. Immunother. Cancer 2018, 6, 1–15. [Google Scholar] [CrossRef]
- Chiang, A.C.; Herbst, R.S. Frontline immunotherapy for NSCLC—The tale of the tail. Nat. Rev. Clin. Oncol. 2020, 17, 73–74. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef]
- Plesca, I.; Tunger, A.; Müller, L.; Wehner, R.; Lai, X.; Grimm, M.-O.; Rutella, S.; Bachmann, M.; Schmitz, M. Characteristics of Tumor-Infiltrating Lymphocytes Prior to and During Immune Checkpoint Inhibitor Therapy. Front. Immunol. 2020, 11, 364. [Google Scholar] [CrossRef]
- Gao, J.; Shi, L.Z.; Zhao, H.; Chen, J.; Xiong, L.; He, Q.; Chen, T.; Roszik, J.; Bernatchez, C.; Woodman, S.E.; et al. Loss of IFN-γ Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell 2016, 167, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.-P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Ferrone, S. HLA Class I Antigen Processing Machinery Defects in Cancer Cells-Frequency, Functional Significance, and Clinical Relevance with Special Emphasis on Their Role in T Cell-Based Immunotherapy of Malignant Disease. Methods Mol. Biol. 2020, 2055, 325–350. [Google Scholar] [CrossRef]
- Rosenthal, R.; The TRACERx Consortium; Cadieux, E.L.; Salgado, R.; Al Bakir, M.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanic, M.; Reading, J.L.; et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Perea, F.; Sánchez-Palencia, A.; Gómez-Morales, M.; Bernal, M.; Concha, Á.; García, M.M.; Gonzalez-Ramirez, A.R.; Kerick, M.; Martin, J.; Garrido, F.; et al. HLA class I loss and PD-L1 expression in lung cancer: Impact on T-cell infiltration and immune escape. Oncotarget 2018, 9, 4120–4133. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmen Olofsson, G.; Jensen, A.W.P.; Idorn, M.; thor Straten, P. Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. Int. J. Mol. Sci. 2020, 21, 3816. https://doi.org/10.3390/ijms21113816
Holmen Olofsson G, Jensen AWP, Idorn M, thor Straten P. Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. International Journal of Molecular Sciences. 2020; 21(11):3816. https://doi.org/10.3390/ijms21113816
Chicago/Turabian StyleHolmen Olofsson, Gitte, Agnete Witness Praest Jensen, Manja Idorn, and Per thor Straten. 2020. "Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo" International Journal of Molecular Sciences 21, no. 11: 3816. https://doi.org/10.3390/ijms21113816
APA StyleHolmen Olofsson, G., Jensen, A. W. P., Idorn, M., & thor Straten, P. (2020). Exercise Oncology and Immuno-Oncology; A (Future) Dynamic Duo. International Journal of Molecular Sciences, 21(11), 3816. https://doi.org/10.3390/ijms21113816




