Bevacizumab or PARP-Inhibitors Maintenance Therapy for Platinum-Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis
Abstract
1. Introduction
2. Results
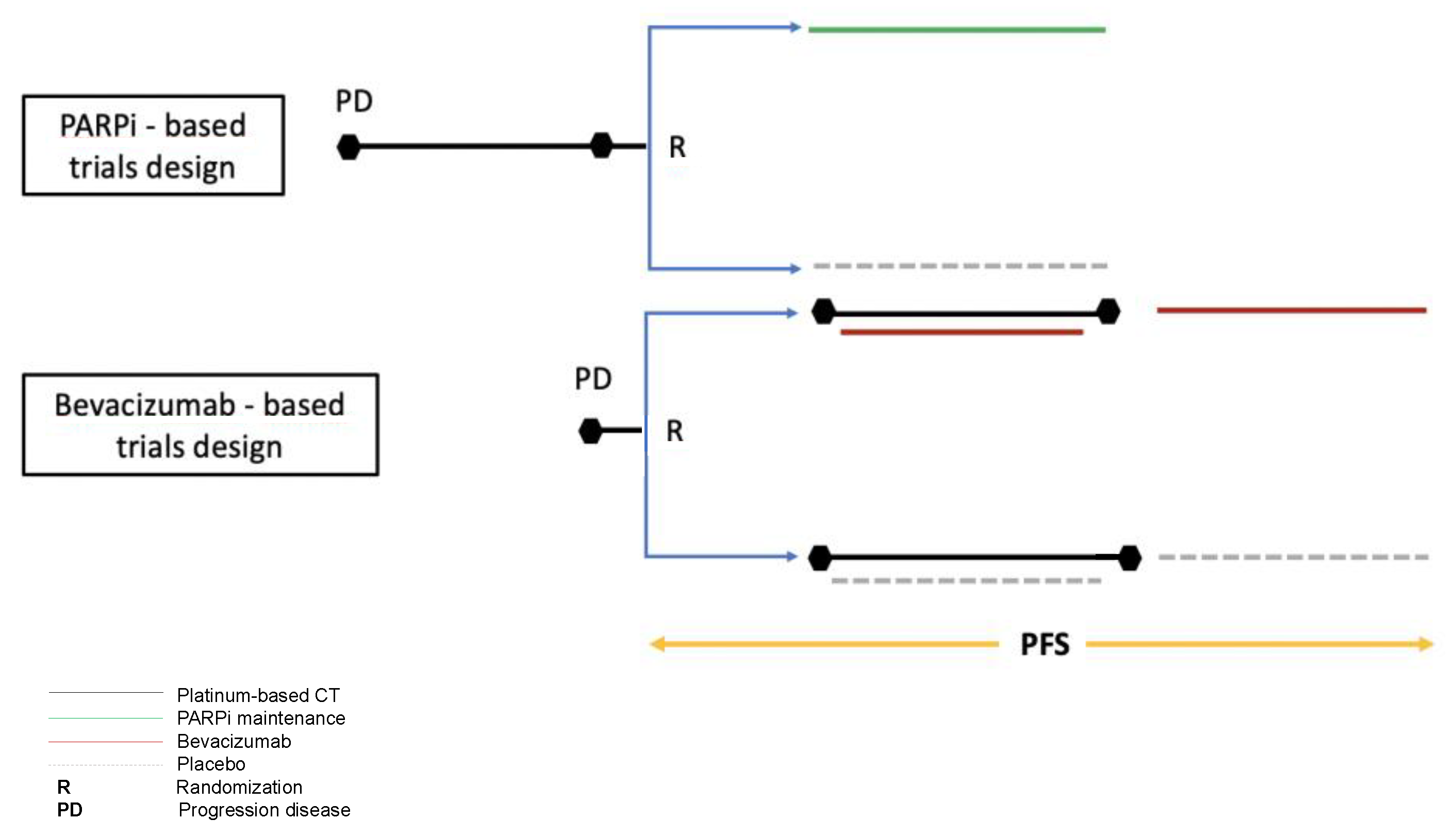
3. Discussion
4. Materials and Methods
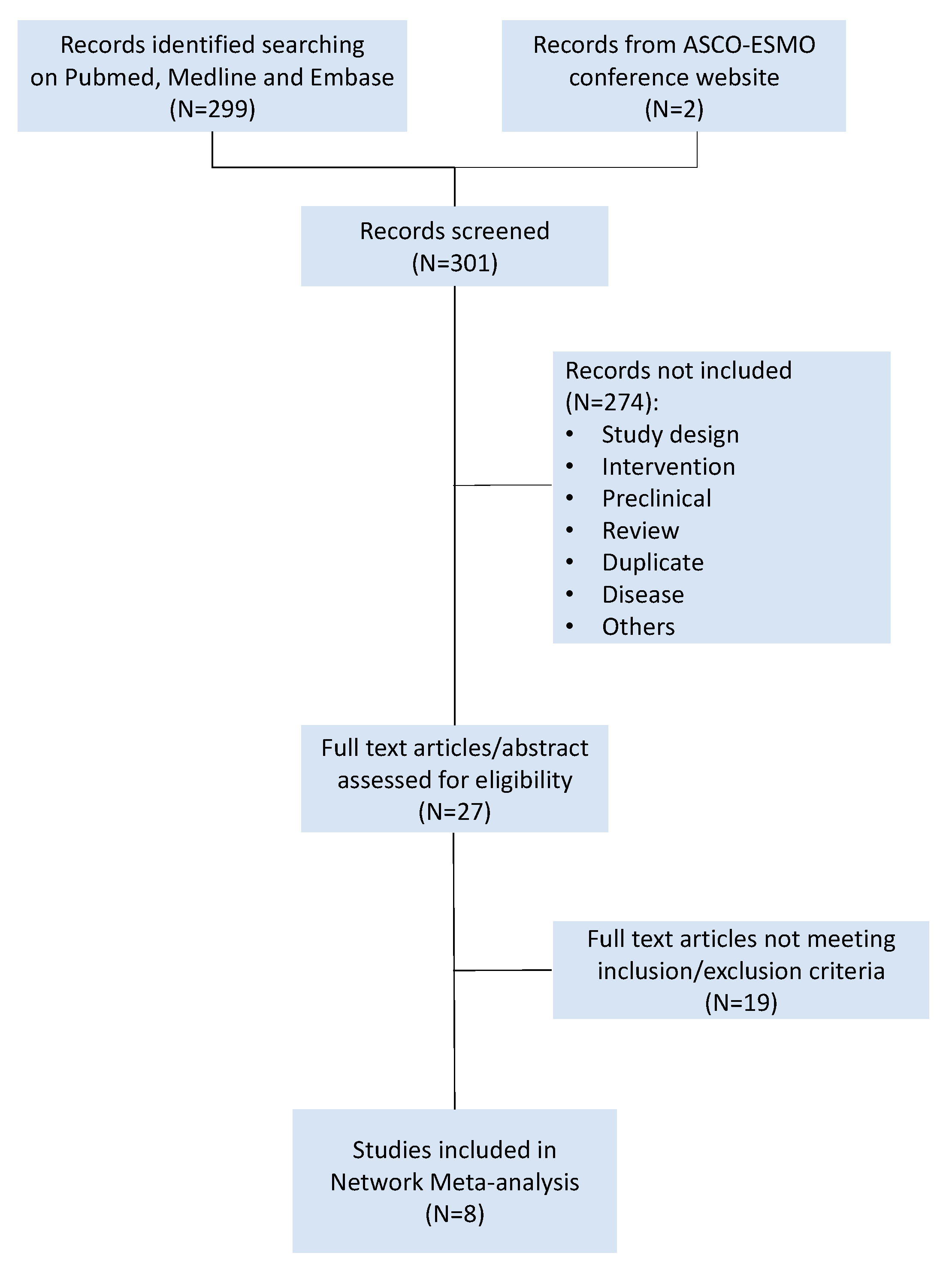
4.1. Search Strategy and Study Selection
4.2. Data Collection and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Ethics approval and consent to participate
Abbreviations
| NMA | Network meta-analysis |
| PS rOC | Platinum-sensitive recurrent ovarian cancer |
| PARPi | Poly (ADP-ribose) polymerase inhibitors |
| PFS | Progression-free survival |
| BRCAwt | BRCA wild type |
| AC | All comers |
| BRCAm | BRCA mutated |
| SUCRA | Surface under the cumulative ranking value |
| EOC | Epithelial ovarian cancer |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Raja, F.A.; Fotopoulou, C.; Gonzalez-Martin, A.; Colombo, N.; Sessa, C.; ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi24–vi32. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.K.; Pujade-Lauraine, E.; Aoki, D.; Mirza, M.R.; Lorusso, D.; Oza, A.M.; du Bois, A.; Vergote, I.; Reuss, A.; Bacon, M.; et al. Fifth Ovarian Cancer Consensus Conference of the Gynecologic Cancer InterGroup: Recurrent disease. Ann. Oncol. 2017, 28, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.-G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef]
- Pignata, S.; Lorusso, D.; Joly, F.; Gallo, C.; Colombo, N.; Sessa, C.; Bamias, A.; Pisano, C.; Selle, F.; Zaccarelli, E.; et al. Chemotherapy plus or minus bevacizumab for platinum-sensitive ovarian cancer patients recurring after a bevacizumab containing first line treatment: The randomized phase 3 trial MITO16B-MaNGO OV2B-ENGOT OV17. JCO 2018, 36, 5506. [Google Scholar] [CrossRef]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N. Engl. J. Med. 2012, 366, 1382–1392. [Google Scholar] [CrossRef]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef]
- Oza, A.M.; Cibula, D.; Benzaquen, A.O.; Poole, C.; Mathijssen, R.H.J.; Sonke, G.S.; Colombo, N.; Špaček, J.; Vuylsteke, P.; Hirte, H.; et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: A randomised phase 2 trial. Lancet Oncol. 2015, 16, 87–97. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Tinker, A.V.; Oaknin, A.; Shapira-Frommer, R.; McNeish, I.A.; Swisher, E.M.; Ray-Coquard, I.; Bell-McGuinn, K.; Coleman, R.L.; O’Malley, D.M.; et al. Antitumor activity and safety of the PARP inhibitor rucaparib in patients with high-grade ovarian carcinoma and a germline or somatic BRCA1 or BRCA2 mutation: Integrated analysis of data from Study 10 and ARIEL2. Gynecol. Oncol. 2017, 147, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.; Fleming, G.F.; Hendrickson, A.E.W.; Azodi, M.; DiSilvestro, P.; Oza, A.M.; et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019, 20, 636–648. [Google Scholar] [CrossRef]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Abson, C.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Ryan, J.E.; Sheriff, M.; Rassy, E.; Pavlidis, N. Veliparib in ovarian cancer: A new synthetically lethal therapeutic approach. Investig. New Drugs 2020, 38, 181–193. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, H.; Wan, T.; Zhang, C.; Tong, C.; Liu, J. Comparison of PARPis with Angiogenesis Inhibitors and Chemotherapy for Maintenance in Ovarian Cancer: A Network Meta-Analysis. Adv. Ther. 2019, 36, 3368–3380. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-avastin-ii/0092_en.pdf (accessed on 25 May 2020).
- 933OCarboplatin/Pegylated Liposomal Doxorubicin/Bevacizumab (CD-BEV) vs. Carboplatin/Gemcitabine/Bevacizumab (CG-BEV) in Patients with Recurrent Ovarian Cancer: A Prospective Randomized Phase III ENGOT/GCIG-Intergroup Study (AGO Study Group, AGO-Austria, ANZGOG, GINECO, SGCTG)|Annals of Oncology|Oxford Academic. Available online: https://academic.oup.com/annonc/article/29/suppl_8/mdy285.142/5141721 (accessed on 24 December 2019).
- Lorusso, D.; Fontanella, C.; Maltese, G.; Lepori, S.; Tripodi, E.; Bogani, G.; Raspagliesi, F. The safety of antiangiogenic agents and PARP inhibitors in platinum-sensitive recurrent ovarian cancer. Expert Opin. Drug Saf. 2017, 16, 687–696. [Google Scholar] [CrossRef]
- Mullen, M.M.; Kuroki, L.M.; Thaker, P.H. Novel treatment options in platinum-sensitive recurrent ovarian cancer: A review. Gynecol. Oncol. 2019, 152, 416–425. [Google Scholar] [CrossRef]
- Hopkins, T.A.; Ainsworth, W.B.; Ellis, P.A.; Donawho, C.K.; DiGiammarino, E.L.; Panchal, S.C.; Abraham, V.C.; Algire, M.A.; Shi, Y.; Olson, A.M.; et al. PARP1 Trapping by PARP Inhibitors Drives Cytotoxicity in Both Cancer Cells and Healthy Bone Marrow. Mol. Cancer Res. 2019, 17, 409–419. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [PubMed]
- Wilcoxen, K.M.; Becker, M.; Neff, C.; Abkevich, V.; Jones, J.T.; Hou, X.; Wang, Y.; Hartman, A.-R.; AlHilli, M.M.; Gutin, A.; et al. Use of homologous recombination deficiency (HRD) score to enrich for niraparib sensitive high grade ovarian tumors. JCO 2015, 33, 5532. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Vidula, N.; Rich, T.A.; Sartor, O.; Yen, J.; Hardin, A.; Nance, T.; Lilly, M.B.; Nezami, M.A.; Patel, S.P.; Carneiro, B.A.; et al. Routine Plasma-Based Genotyping to Comprehensively Detect Germline, Somatic, and Reversion BRCA Mutations among Patients with Advanced Solid Tumors. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Karathanasi, A.; Sadauskaite, A.; Rassy, E.; Pavlidis, N. Combined Strategies with Poly (ADP-Ribose) Polymerase (PARP) Inhibitors for the Treatment of Ovarian Cancer: A Literature Review. Diagnostics 2019, 9, 87. [Google Scholar] [CrossRef]
- Rücker, G. Network meta-analysis, electrical networks and graph theory. Res. Synth. Methods 2012, 3, 312–324. [Google Scholar] [CrossRef]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef]
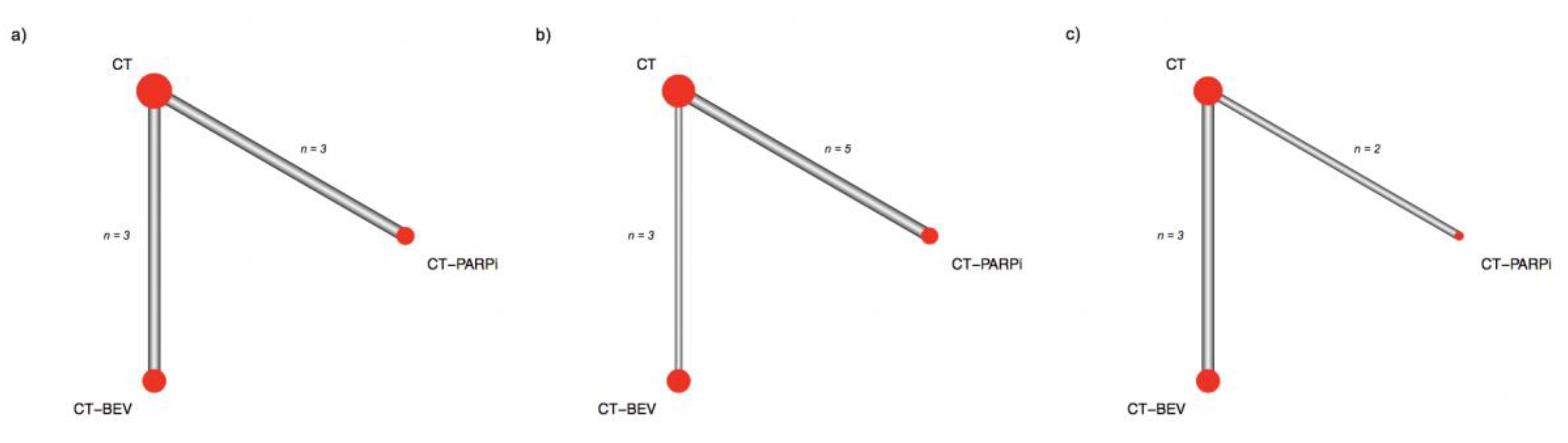
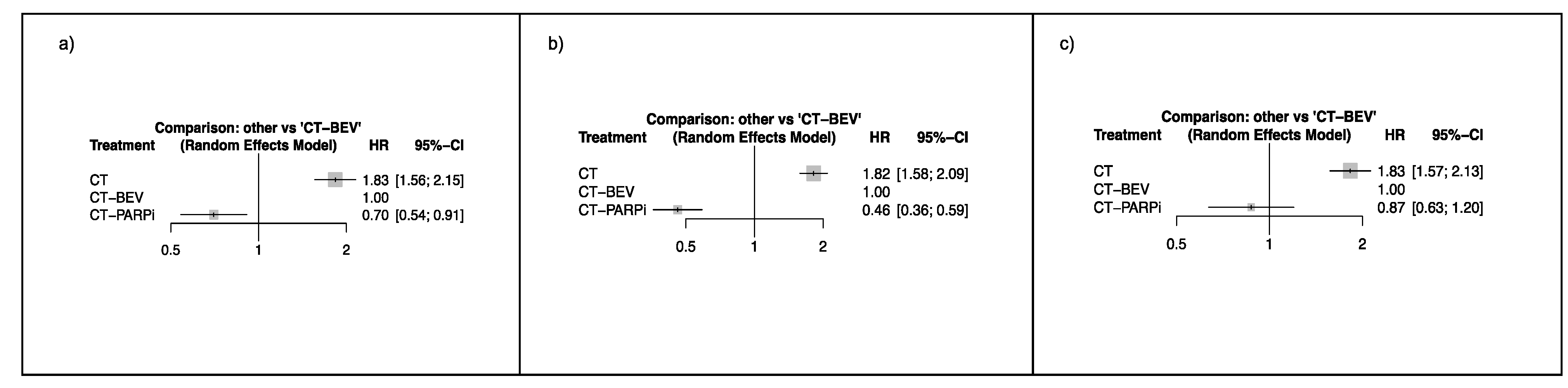
| Authors | Design | Population | Primary Endpoint | N. Patients Randomized | Treatment Arms | HR for PFS | CI (95%) | p Value |
|---|---|---|---|---|---|---|---|---|
| Coleman R. et al. (2017) GOG 0213 | Phase III | Recurrent EOC, PFI ≥ 6 m, prior anti-VEGF allowed | OS PFS (secondary) | A: 337 B: 337 | A: Carboplatin AUC5 + Paclitaxel 175 mg/m2 q21 ×6 cycles B: Carboplatin AUC5 + Paclitaxel 175 mg/m2 + bevacizumab 15 mg/kg q21 × 6 cycles followed by bevacizumab 15 mg/kg q21 maintenance until PD | 0.628 for B | 0.534–0.739 | 0.0001 |
| Aghajanian C et al. (2012) OCEANS | Phase III | Recurrent EOC, PFI ≥ 6 m, prior anti-VEGF not allowed | PFS | A: 242 B: 242 | A: Carboplatin AUC4 d1 + Gemcitabine 1000 mg/m2 d1-8 q21 × 6 cycles B: Carboplatin AUC4 d1 + Gemcitabine 1000 mg/m2 d1-8 + bevacizumab 15 mg/kg d1 q21 × 6 cycles followed by bevacizumab 15 mg/kg q21 maintenance until PD | 0.484 for B | 0.388–0.605 | 0.0001 |
| Pignata S. et al. ASCO 2018 MITO-16 | Phase III | Recurrent EOC, PFI ≥ 6 m, treated with anti-VEGF in 1°line | PFS | A: 203 B: 202 | A: Carboplatin + Paclitaxel/Gemcitabine/PLD q21 × 6 cycles. B: Carboplatin + Paclitaxel/Gemcitabine/PLD + bevacizumab 15 mg/kg q21 x 6 cycles followed by bevacizumab 15 mg/kg q21 maintenance until PD | 0.51 for B | 0.41–0.64 | 0.001 |
| Ledermann J. et al. (2012) STUDY-19 | Phase II | Recurrent HGSOC, PFI ≥ 6 m, treated with a median of 2 platinum-based regimens All comers | PFS | A: 129 B: 136 | A: Placebo B: Olaparib 400 mg twice daily until PD | 0.35 for B | 0.25–0.49 | 0.001 |
| Ledermann J. et al. (2014) STUDY-19 | Phase II | Recurrent HGSOC, PFI ≥ 6 m, treated with a median of 2 platinum-based regimens BRCAm | PFS | A: 62 B: 74 | A: Placebo B: Olaparib 400 mg twice daily until PD | 0.18 for B | 0.10–0.31 | 0.0001 |
| Ledermann J. et al. (2014) STUDY-19 | Phase II | Recurrent HGSOC, PFI ≥ 6 m, treated with a median of 2 platinum-based regimens BRCAwt. | PFS | A: 61 B: 57 | A: Placebo B: Olaparib 400 mg twice daily until PD | 0.54 for B | 0.34–0.85 | 0.0075 |
| Oza M. et al. (2015) | Phase II | Recurrent EOC, PFI ≥ 6 m. All comers | PFS | A: 81 B: 81 | A: Carboplatin AUC5 + Paclitaxel 175 mg/m2 q21 × 6 cycles B: Carboplatin AUC5 + Paclitaxel 175 mg/m2 q21 + olaparib 200 mg d1-10 q21 × 6 cycles » Olaparib 400 mg twice daily until PD | 0.51 for B | 0,34–0,77 | 0.0012 |
| Oza M. et al. (2015) | Phase II | Recurrent EOC, PFI ≥ 6m BRCAm | PFS | A: 21 B: 20 | A: Carboplatin AUC5 + Paclitaxel 175mg/m2 q21 × 6 cycles B: Carboplatin AUC5 + Paclitaxel 175mg/m2 q21 + olaparib 200 mg d1-10 q21 × 6 cycles » Olaparib 400 mg twice daily until PD | 0.21 for B | 0.08–0.55 | 0.0015 |
| Mirza M.R. et al. (2016) NOVA | Phase III | Recurrent HGSOC, PFI ≥ 6m, at least 2 platinum-based regimens | PFS | A: 65 B: 138 | gBRCAm A: Placebo B: Niraparib 300 mg twice daily until PD | 0.27 for B | 0.173–0.410 | 0.0001 |
| Mirza M.R. et al. (2016) NOVA | Phase III | Recurrent HGSOC, PFI ≥ 6m, at least 2 platinum-based regimens | PFS | A: 116 B: 234 | not-gBRCAm A: Placebo B: Niraparib 300 mg twice daily until PD | 0.45 for B | 0.338–0.607 | 0.0001 |
| Coleman R et al. (2017) ARIEL-3 | Phase III | Recurrent HGS or endometrioid OC PFI ≥ 6m. at least 2 platinum-based regimens All comers | PFS | A: 189 B: 375 | A: Placebo B: Rucaparib 600 mg | 0.36 (ITT population) | 0.30–0.45 | 0.0001 |
| Coleman R et al. (2017) ARIEL-3 | Phase III | Recurrent HGS or endometrioid OC, PFI ≥ 6m, at least 2 platinum-based regimens BRCAm | PFS | A: 66 B: 130 | A: Placebo B: Rucaparib 600 mg | 0.23 | 0.16–0.34 | 0.0001 |
| Pujade-Lauraine E et al. (2017) SOLO-2 | Phase III | Recurrent HGS or endometrioid OC with g/sBRCA 1/2 m, PFI ≥ 6 m, at least 2 platinum-based regimens | PFS | A: 99 B: 196 | A: Placebo B: Olaparib 300 mg in two 150 mg tablets, twice daily, until PD | 0.30 for B | 0.22–0.41 | 0.0001 |
| Treatment Efficacy | ||
|---|---|---|
| Treatment | SUCRA | Rank |
| PARPi | 90% | 1 |
| BEV | 60% | 2 |
| CT | 0% | 3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoletti, M.; Pelizzari, G.; Gerratana, L.; Bortot, L.; Lombardi, D.; Nicoloso, M.; Scalone, S.; Giorda, G.; Baldassarre, G.; Sorio, R.; et al. Bevacizumab or PARP-Inhibitors Maintenance Therapy for Platinum-Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3805. https://doi.org/10.3390/ijms21113805
Bartoletti M, Pelizzari G, Gerratana L, Bortot L, Lombardi D, Nicoloso M, Scalone S, Giorda G, Baldassarre G, Sorio R, et al. Bevacizumab or PARP-Inhibitors Maintenance Therapy for Platinum-Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis. International Journal of Molecular Sciences. 2020; 21(11):3805. https://doi.org/10.3390/ijms21113805
Chicago/Turabian StyleBartoletti, Michele, Giacomo Pelizzari, Lorenzo Gerratana, Lucia Bortot, Davide Lombardi, Milena Nicoloso, Simona Scalone, Giorgio Giorda, Gustavo Baldassarre, Roberto Sorio, and et al. 2020. "Bevacizumab or PARP-Inhibitors Maintenance Therapy for Platinum-Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis" International Journal of Molecular Sciences 21, no. 11: 3805. https://doi.org/10.3390/ijms21113805
APA StyleBartoletti, M., Pelizzari, G., Gerratana, L., Bortot, L., Lombardi, D., Nicoloso, M., Scalone, S., Giorda, G., Baldassarre, G., Sorio, R., & Puglisi, F. (2020). Bevacizumab or PARP-Inhibitors Maintenance Therapy for Platinum-Sensitive Recurrent Ovarian Cancer: A Network Meta-Analysis. International Journal of Molecular Sciences, 21(11), 3805. https://doi.org/10.3390/ijms21113805







