Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies
Abstract
1. Introduction
2. Results
2.1. Preclinical in Vitro Studies
2.1.1. Human Tissues
2.1.2. Canine Tissues
2.1.3. Rats
2.2. Preclinical in Vivo Studies
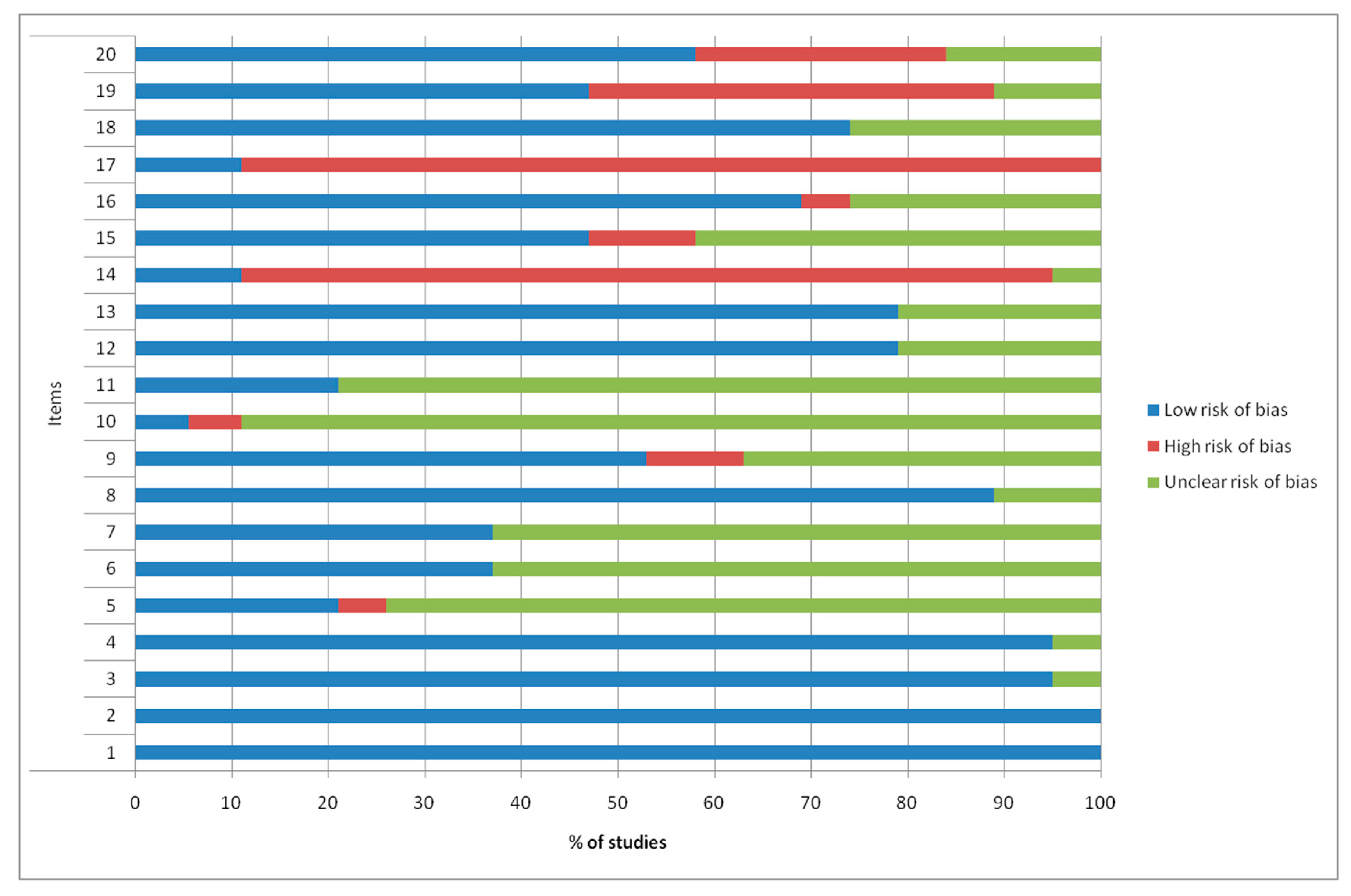
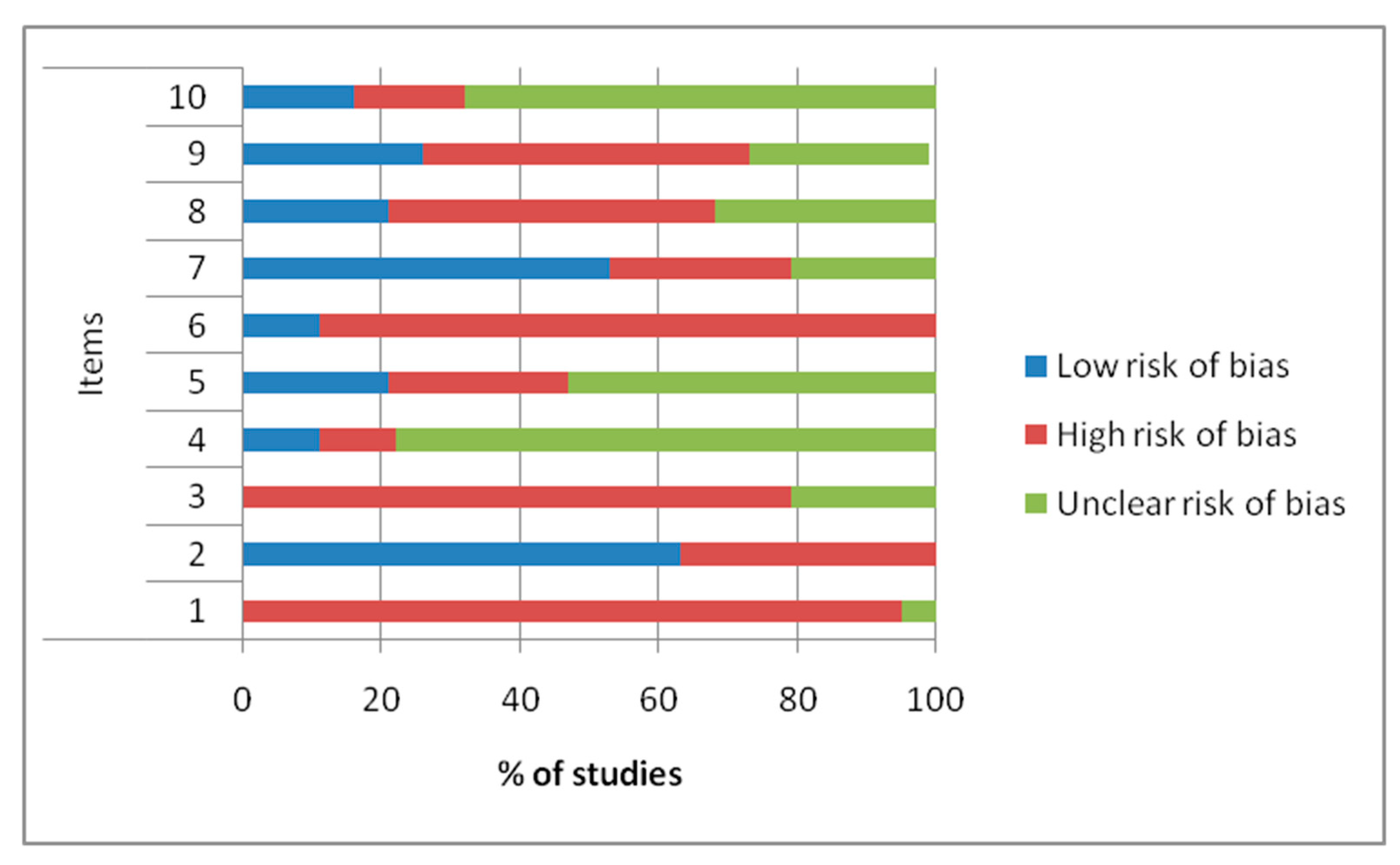
2.2.1. Quality and Risk of Bias Assessments
2.2.2. Mice
2.2.3. Rats
2.2.4. Guinea Pigs
2.2.5. Pigs/Mini-Pigs
2.2.6. Baboons
3. Discussion
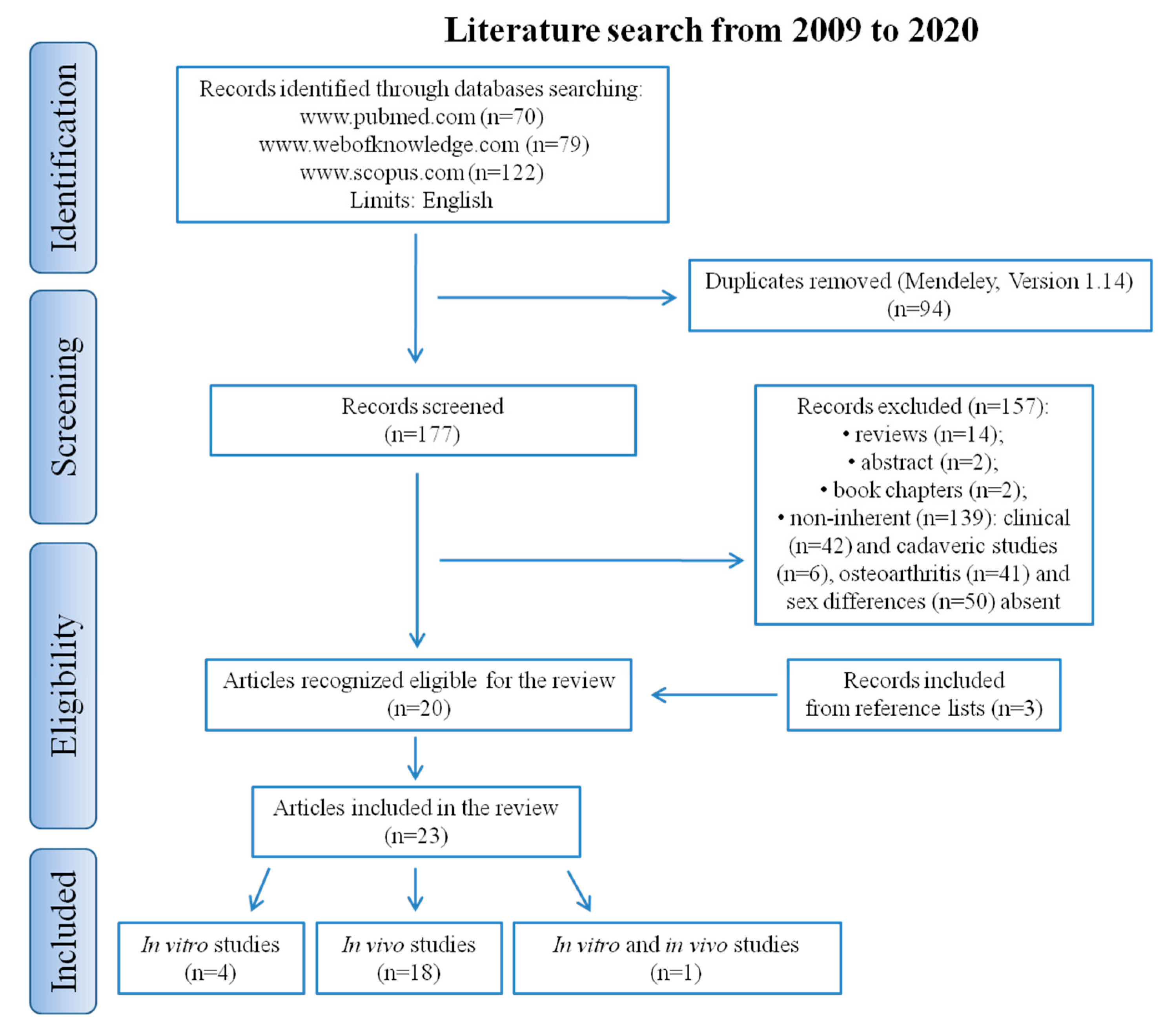
4. Materials and Methods
4.1. Search Strategy
4.2. Data Extraction
4.3. Methodologic Quality Assessment and Risk of Bias
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OA | Osteoarthritis |
| WHO | World Health Organization |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| MMPs | Matrix metalloproteinases |
| NO | Nitric oxide |
| PGE2 | Prostaglandin E2 |
| COX-2 | Cyclooxygenase 2 |
| TKA | Total knee arthroplasty |
| ACR | American College of Rheumatology |
| 3D | Three-dimensional |
| Col | Collagen |
| E2 | 17β-estradiol |
| IHC | Immunohistochemistry |
| ER | Estrogen receptor |
| AR | Androgen receptor |
| FACS | Fluorescence-activated cell sorting |
| TGF | Transforming growth factor |
| FGF-2 | Fibroblast growth factor-2 |
| aCGH | Array-based comparative genomic hybridization |
| FISH | Fluorescent in situ hybridization |
| THR | Total hip replacement |
| DMEM | Dulbecco’s modified Eagle eedium |
| TNAP | Tissue non-specific alkaline phosphatase |
| ELISA | Enzyme-linked immunosorbent assay |
| MCP | Monocyte Chemoattractant Protein |
| RT-PCR | Real-time C-reactive Protein |
| TMJ | Temporomandibular joint |
| FLS | Fibroblast-like synoviocytes |
| sGAGs | Sulphated glycosaminoglycans |
| HGF | Hepatocyte growth factor |
| ALP | Alkaline phosphatase |
| BMD | Bone mineral density |
| μCT | Micro-computed tomography |
| BV/TV | Trabecular bone volume |
| Tb.N | Trabecular number |
| Tb.Th | Trabecular thickness |
| MV | Medullary volume |
| cBV/TV | Cortical bone volume per total tissue volume |
| ADITT | Angular degree of internal tibial torsion |
| CSA | Cross sectional area |
| Frzb | Frizzled-related protein |
| OARSI | Osteoarthritis Research Society International |
| MMT | Medial meniscectomy |
| DMM | Destabilization of the medial meniscus |
| EGFR | Epidermal growth factor receptor |
| NMR | Nuclear magnetic resonance |
| OPLS-DA | Orthogonal partial least squares-discriminant analysis |
| PUFAs | Polyunsaturated fatty acids |
| n-6 | Omega-6 |
| n-3 | Omega-3 |
| GC-FID | Gas chromatography with flame-ionization detection |
| hCRP | Human C-reactive protein |
| CETP | Cholesteryl ester transfer protein |
| MIA | Monosodium iodoacetate |
| IGF1 | Insulin-like growth factor-1 |
| IGFR1 | Insulin-like growth factor type 1 receptor |
| IGFBP3 | Insulin-like growth factor binding protein-3 |
| CFA | Complete Freund’s adjuvant |
| iNOS | Inducible nitric oxide synthase |
| MD | Mineral density |
| ACL | Anterior cruciate ligament |
| ECM | Extracellular matrix |
| PRP | Platelet-rich plasma |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| DNA | Deoxyribonucleic acid |
| OC | Osteocalcin |
| OP | Osteoprotegerin |
| ACAN | Aggrecan |
| VDR | Vitamin D receptor |
| M-CSF | Macrophage colony-stimulating factor |
| COMP | Cartilage oligomeric matrix protein |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| 2D | Two-dimensional |
| MIG | Monokine induced by gamma interferon |
| PDIA3 | Protein disulfide isomerase A3 |
| LIF | Leukemia inhibitory factor |
| MIF | Macrophage migration inhibitory factor |
| GRO-α | Growth-regulated oncogene α |
| ARRIVE | Animals in Research Reporting In Vivo Experiments |
| SYRCLE | Systematic Review Centre for Laboratory animal Experimentation |
| TRAIL | TNF-related apoptosis-inducing ligand |
| NOV | Nephroblastoma overexpressed |
| PCNA | Proliferating cell nuclear antigen |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
References
- Johnson, V.L.; Hunter, D.J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Hawker, G.A. Osteoarthritis is a serious disease. Clin. Exp.Rheumatol. 2019, 37, 3–6. [Google Scholar] [PubMed]
- Reginster, J.L.; Arden, N.K.; Haugen, I.K.; Rannou, F.; Cavalier, E.; Bruyère, O.; Branco, J.; Chapurlat, R.; Collaud Basset, S.; Al-Daghri, N.M.; et al. Guidelines for the conduct of pharmacological clinical trials in hand osteoarthritis: Consensus of a Working Group of the European Society on Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Semin. Arthritis Rheum. 2018, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Doherty, M. EULAR recommendations for knee and hip osteoarthritis: A critique of the methodology. Br. J. Sports Med. 2006, 40, 664–669. [Google Scholar] [CrossRef]
- Mannoni, A.; Briganti, M.P.; Di Bari, M.; Ferrucci, L.; Costanzo, S.; Serni, U.; Masotti, G.; Marchionni, N. Epidemiological profile of symptomatic osteoarthritis in older adults: A population based study in Dicomano, Italy. Ann. Rheum. Dis. 2003, 62, 576–578. [Google Scholar] [CrossRef]
- Dagenais, S.; Garbedian, S.; Wai, E.K. Systematic review of the prevalence of radiographic primary hip osteoarthritis. Clin. Orthop. Relat. Res. 2009, 467, 623–637. [Google Scholar] [CrossRef]
- Reijman, M.; Hazes, J.M.; Pols, H.A.; Bernsen, R.M.; Koes, B.W.; Bierma-Zeinstra, S.M. Role of radiography in predicting progression of osteoarthritis of the hip: Prospective cohort study. BMJ 2005, 330, 1183. [Google Scholar] [CrossRef]
- Neogi, T.; Zhang, Y. Epidemiology of osteoarthritis. Rheum. Dis. Clin. North. Am. 2013, 39, 1–19. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef]
- Reijman, M.; Pols, H.A.; Bergink, A.P.; Hazes, J.M.; Belo, J.N.; Lievense, A.M.; Bierma-Zeinstra, S.M. Body mass index associated with onset and progression of osteoarthritis of the knee but not of the hip: The Rotterdam Study. Ann. Rheum. Dis. 2007, 66, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed. Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M.; Tsuchimochi, K.; Ijiri, K.; Li, Y. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann. Rheum. Dis. 2008, 67, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, F.; Della Bella, E.; Cepollaro, S.; Brogini, S.; Martini, L.; Fini, M. Novel therapeutic targets in osteoarthritis: Narrative review on knock-out genes involved in disease development in mouse animal models. Cytotherapy 2016, 18, 593–612. [Google Scholar] [CrossRef]
- Schulze-Tanzil, G. Intraarticular Ligament Degeneration Is Interrelated with Cartilage and Bone Destruction in Osteoarthritis. Cells 2019, 8, 990. [Google Scholar] [CrossRef]
- Braun, H.J.; Gold, G.E. Diagnosis of osteoarthritis: Imaging. Bone 2012, 51, 278–288. [Google Scholar] [CrossRef]
- Maleki-Fischbach, M.; Jordan, J.M. New developments in osteoarthritis. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis Res. Ther. 2010, 12, 212. [Google Scholar] [CrossRef]
- Kolhe, R.; Hunter, M.; Liu, S.; Jadeja, R.N.; Pundkar, C.; Mondal, A.K.; Mendhe, B.; Drewry, M.; Rojiani, M.V.; Liu, Y.; et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci. Rep. 2017, 7, 2029. [Google Scholar] [CrossRef]
- Phinyomark, A.; Osis, S.T.; Hettinga, B.A.; Kobsar, D.; Ferber, R. Gender differences in gait kinematics for patients with knee osteoarthritis. BMC Musculoskelet. Disord. 2016, 17, 157. [Google Scholar] [CrossRef]
- Boyan, B.D.; Hart, D.; Enoka, R.M.; Nicolella, D.P.; Resnick, E.; Berkley, K.J.; Sluka, K.A.; Kwoh, C.K.; Tosi, L.L.; O’Connor, M.I.; et al. Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol. Sex. Differ. 2013, 4, 3. [Google Scholar] [CrossRef]
- Ouellette, E.A.; Makowski, A.L. How men and women are affected by osteoarthritis of the hand. Orthop. Clin. North. Am. 2006, 37, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Birchfield, P.C. Osteoarthritis overview. Geriatr. Nurs. 2001, 22, 124–130. [Google Scholar] [CrossRef] [PubMed]
- De Klerk, B.M.; Schiphof, D.; Groeneveld, F.P.; Koes, B.W.; van Osch, G.J.; van Meurs, J.B.; Bierma-Zeinstra, S.M. No clear association between female hormonal aspects and osteoarthritis of the hand, hip and knee: A systematic review. Rheumatology 2009, 48, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.M. Factors that influence outcome in experimental osteoarthritis. Osteoarthr. Cartil. 2017, 25, 369–375. [Google Scholar] [CrossRef]
- Koelling, S.; Miosge, N. Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthritis Rheum. 2010, 62, 1077–1087. [Google Scholar] [CrossRef]
- Pan, Q.; O’Connor, M.I.; Coutts, R.D.; Hyzy, S.L.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Characterization of osteoarthritic human knees indicates potential sex differences. Biol. Sex. Differ. 2016, 7, 27. [Google Scholar] [CrossRef]
- Stumm, M.; Boger, E.; Gaissmaier, C.G.; Oßwald, C.; Blankenburg, M.; Wegner, R.D.; Mollenhauer, J.A. Genomic chondrocyte culture profiling by array-CGH, interphase-FISH and RT-PCR. Osteoarthr. Cartil. 2012, 20, 1039–1045. [Google Scholar] [CrossRef][Green Version]
- Meeson, R.L.; Perpétuo, I.P.; Parsons, K.; Orriss, I.R.; Shah, M.; Pitsillides, A.A.; Doube, M. The in vitro behaviour of canine osteoblasts derived from different bone types. BMC Vet. Res. 2019, 15, 114. [Google Scholar] [CrossRef]
- Xue, X.T.; Zhang, T.; Cui, S.J.; He, D.Q.; Wang, X.D.; Yang, R.L.; Liu, D.W.; Liu, Y.; Gan, Y.H.; Kou, X.X.; et al. Sexual dimorphism of estrogen-sensitized synoviocytes contributes to gender difference in temporomandibular joint osteoarthritis. Oral. Dis. 2018, 24, 1503–1513. [Google Scholar] [CrossRef]
- Bouderlique, T.; Vuppalapati, K.K.; Newton, P.T.; Li, L.; Barenius, B.; Chagin, A.S. Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann. Rheum. Dis. 2016, 75, 627–631. [Google Scholar] [CrossRef]
- Cai, A.; Hutchison, E.; Hudson, J.; Kawashima, Y.; Komori, N.; Singh, A.; Brush, R.S.; Anderson, R.E.; Sonntag, W.E.; Matsumoto, H.; et al. Metabolic enrichment of omega-3 polyunsaturated fatty acids does not reduce the onset of idiopathic knee osteoarthritis in mice. Osteoarthr. Cartil. 2014, 22, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Skelly, J.D.; Ayers, D.C.; Song, J. Age-dependent Changes in the Articular Cartilage and Subchondral Bone of C57BL/6 Mice after Surgical Destabilization of Medial Meniscus. Sci. Rep. 2017, 7, 42294. [Google Scholar] [CrossRef]
- Javaheri, B.; Razi, H.; Piles, M.; de Souza, R.; Chang, Y.M.; Maric-Mur, I.; Hopkinson, M.; Lee, P.D.; Pitsillides, A.A. Sexually dimorphic tibia shape is linked to natural osteoarthritis in STR/Ort mice. Osteoarthr. Cartil. 2018, 26, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Kozijn, A.E.; Gierman, L.M.; van der Ham, F.; Mulder, P.; Morrison, M.C.; Kühnast, S.; van der Heijden, R.A.; Stavro, P.M.; van Koppen, A.; Pieterman, E.J.; et al. Variable cartilage degradation in mice with diet-induced metabolic dysfunction: Food for thought. Osteoarthr. Cartil. 2018, 26, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Lories, R.J.; Peeters, J.; Szlufcik, K.; Hespel, P.; Luyten, F.P. Deletion of frizzled-related protein reduces voluntary running exercise performance in mice. Osteoarthr. Cartil. 2009, 17, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Malfait, A.M.; Ritchie, J.; Gil, A.S.; Austin, J.S.; Hartke, J.; Qin, W.; Tortorella, M.D.; Mogil, J.S. ADAMTS-5 deficient mice do not develop mechanical allodynia associated with osteoarthritis following medial meniscal destabilization. Osteoarthr. Cartil. 2010, 18, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Mickiewicz, B.; Shin, S.Y.; Pozzi, A.; Vogel, H.J.; Clark, A.L. Serum Metabolite Profiles Are Altered by Erlotinib Treatment and the Integrin α1-Null Genotype but Not by Post-Traumatic Osteoarthritis. J. Proteome. Res. 2016, 15, 815–825. [Google Scholar] [CrossRef]
- Roddy, K.A.; Boulter, C.A. Targeted mutation of NOV/CCN3 in mice disrupts joint homeostasis and causes osteoarthritis-like disease. Osteoarthr. Cartil. 2015, 23, 607–615. [Google Scholar] [CrossRef][Green Version]
- Uchida, K.; Urabe, K.; Naruse, K.; Kozai, Y.; Onuma, K.; Mikuni-Takagaki, Y.; Kashima, I.; Ueno, M.; Sakai, R.; Itoman, M.; et al. Differential age-related bone architecture changes between female and male STR/Ort mice. Exp. Anim. 2012, 61, 59–66. [Google Scholar] [CrossRef][Green Version]
- Temp, J.; Labuz, D.; Negrete, R.; Sunkara, V.; Machelska, H. Pain and knee damage in male and female mice inthe medial meniscal transection-induced osteoarthritis. Osteoarthr. Cartil. 2020, 28, 475–485. [Google Scholar] [CrossRef]
- Ro, J.Y.; Zhang, Y.; Tricou, C.; Yang, D.; da Silva, J.T.; Zhang, R. Age and Sex Differences in Acute and Osteoarthritis-Like Pain Responses in Rats. J.Gerontol. A Biol. Sci. Med. Sci. 2019, 186. [Google Scholar] [CrossRef] [PubMed]
- Sannajust, S.; Imbert, I.; Eaton, V.; Henderson, T.; Liaw, L.; May, M.; Barbe, M.F.; King, T. Females have greater susceptibility to develop ongoing pain and central sensitization in a rat model of temporomandibular joint pain. Pain 2019, 160, 2036–2049. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Sun, L.; Liu, L.; Jiao, K.; Wang, M. Differential expression of IGF1, IGFR1 and IGFBP3 in mandibular condylar cartilage between male and female rats applied with malocclusion. J. Oral. Rehabil. 2012, 39, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Scannell, B.P.; Honeycutt, P.R.; Mauerhan, D.R.; Norton, J.; Hanley, E.N., Jr. Cartilage Degeneration, Subchondral Mineral and Meniscal Mineral Densities in Hartley and Strain 13 Guinea Pigs. Open Rheumatol. J. 2015, 9, 65–70. [Google Scholar] [CrossRef]
- Kiapour, A.M.; Fleming, B.C.; Proffen, B.L.; Murray, M.M. Sex Influences the Biomechanical Outcomes of Anterior Cruciate Ligament Reconstruction in a Preclinical Large Animal Model. Am. J. Sports Med. 2015, 43, 1623–1631. [Google Scholar] [CrossRef]
- Kiapour, A.M.; Fleming, B.C.; Murray, M.M. Biomechanical Outcomes of Bridge-enhanced Anterior Cruciate Ligament Repair Are Influenced by Sex in a Preclinical Model. Clin. Orthop. Relat. Res. 2015, 473, 2599–2608. [Google Scholar] [CrossRef][Green Version]
- Macrini, T.E.; Coan, H.B.; Levine, S.M.; Lerma, T.; Saks, C.D.; Araujo, D.J.; Bredbenner, T.L.; Coutts, R.D.; Nicolella, D.P.; Havill, L.M. Reproductive status and sex show strong effects on knee OA in a baboon model. Osteoarthr. Cartil. 2013, 21, 839–848. [Google Scholar] [CrossRef][Green Version]
- Henrotin, Y.; Lambert, C.; Richette, P. Importance of synovitis in osteoarthritis: Evidence for the use of glycosaminoglycans against synovial inflammation. Semin. Arthritis Rheum. 2014, 43, 579–587. [Google Scholar] [CrossRef]
- Migliore, A.; Procopio, S. Effectiveness and utility of hyaluronic acid in osteoarthritis. Clin. Cases Miner. Bone Metab. 2015, 12, 31–33. [Google Scholar] [CrossRef]
- Thysen, S.; Luyten, F.P.; Lories, R.J.U. Targets, models and challenges in osteoarthritis research. Dis. Models Mech. 2015, 8, 17–30. [Google Scholar] [CrossRef]
- Maglio, M.; Brogini, S.; Pagani, S.; Giavaresi, G.; Tschon, M. Current Trends in the Evaluation of Osteochondral Lesion Treatments: Histology, Histomorphometry, and Biomechanics in Preclinical Models. Biomed. Res. Int. 2019, 2019, 4040236. [Google Scholar] [CrossRef] [PubMed]
- Vincent, T.L.; Williams, R.O.; Maciewicz, R.; Silman, A.; Garside, P. Arthritis Research UK animal models working group. Mapping pathogenesis of arthritis through small animal models. Rheumatology 2012, 51, 1931–1941. [Google Scholar] [CrossRef] [PubMed]
- Kuyinu, E.L.; Narayanan, G.; Nair, L.S.; Laurencin, C.T. Animal models of osteoarthritis: Classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Seifirad, S.; Haghpanah, V. Inappropriate modeling of chronic and complex disorders: How to reconsider the approach in the context of predictive, preventive and personalized medicine, and translational medicine. EPMA J. 2019, 10, 195–209. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Animals 2013, 4, 35–44. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef]
- Maturo, M.G.; Soligo, M.; Gibson, G.; Manni, L.; Nardini, C. The greater inflammatory pathway-high clinical potential by innovative predictive, preventive, and personalized medical approach. EPMA J. 2019, 11, 1–16. [Google Scholar] [CrossRef]
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Carè, A.; Bellini, T. "Bridging the Gap" Everything that Could Have Been Avoided If We Had Applied Gender Medicine, Pharmacogenetics and Personalized Medicine in the Gender-Omics and Sex-Omics Era. Int. J. Mol. Sci. 2019, 21, 296. [Google Scholar] [CrossRef]
| Cell Source | Cell Phenotype | OA Diagnosis | Culture Conditions | Analysis and Experimental Times | Main Results | References |
|---|---|---|---|---|---|---|
| Cartilage and synovial fluid from 372 late stage OA patients (64% were female) undergoing TKA | Chondrogenic progenitor cells | The patients met the ACR classification criteriafor knee OA | 50,000 cells into 3D alginate bead with human fibronectin or bovine Col IV or Col II or Matrigel with or without E2 or testosterone supplementation | Measurement in synovial fluids (E2 and testosterone). IHC and immunoblotting (ERα, ERβ, and AR). Microarray analysis and RT-qPCR (ESR1, ESR2, AR, Sox9, Col I, Col II, and Runx2). FACS analyses | ↑testosterone in male synovial fluid vs. female. Microarrays show that 4.9% genes exhibited expression activities different between sexes. ↓ESR1 and ESR2 after testosterone in females. ↑ESR1 and ESR2 after testosterone in males. ↑AR after E2 and testosterone in males. ↑Sox9 and Col I after E2 in females and after testosterone in males. ↓Col II after E2 and testosterone in males. ↓Runx2 after E2 in males | Koelling et al. 2010 [25] |
| Osteochondral tissue, meniscus, synovial membrane, and synovial fluid from 20 patients (10 males and 10 females) undergoing TKA | Chondrocytes (from 6 males and 6 females) and osteoblasts | Radiographic diagnosis (Kellgren–Lawrence grading scale) | Chondrocytes and osteoblasts were harvested from both minimally or maximally eroded zones with and without 1α,25(OH)2D3 or E2 | Histology on meniscus, synovium, bone, and cartilage. Measurements on synovial fluid (1α,25(OH)2D3, MMPs, sGAGs, TGF-β1,TGF-β2, and TGF-β3). RT-qPCR on cultures (VDR, PDIA3, ESR1, ERα36, IL1A, IL1B, IL6, IL8, IL10, WNT3A, WNT5A, CTNNB, DKK1, DKK2, ACAN, Col II, and COMP) (12 h). ALP in chondrocyte culture and OC and OP in osteoblast culture (24 h) | ↓pain thresholds in females at baseline. In synovial fluid: ↑MMP-1, MMP-7, MMP-9, MMP-13, HGF, SCF, SCGF-β, sGAGs, TGF-β1 and TGF-β2 in males vs. females. ↑IL2α, IL3, IL12p40, IL16, IL18, TNF-β, TRAIL, LIF, M-CSF, MIF, GRO-α, MCP-3, and MIG in females vs. males. In chondrocyte culture: ↓VDR and PDIA3 and ↑ESR1 in females vs. males. ↑IL1A, IL6, and IL8ESR1 in females vs. males. ↑WNT5A, DKK1, and DKK2 in males vs. females. E2 induced ↑ALP, ACAN, COL2A1, and COMP in females vs. males. In osteoblast culture: ↑ESR1 and ERα36 in females vs. males. ↑VDR in males vs.females. E2 induced ↑ALP and OC and ↓OP in females vs. males. 1α,25(OH)2D3 induced ↑ALP in males vs. females | Pan et al.2016 [26] |
| Articular cartilage from 8 patients (3 males and 5 females) undergoing TKA | Chondrocytes | Not reported | Chondrocyte cultures with or without proliferative stimulus (FGF-2) | DNA extraction, aCGH, FISH and qRT-PCR (Col I, Col II, ACAN, and IL1-β) at day 0, 4, 14, and 19 | Cell expansion and growth factor addition did not cause any autosomal imbalances or loss of DNA. Cell expansion from females did not cause sex chromosome abnormalities while in males a loss of Y-chromosome sequences was observed (aCGH and FISH). No changes in gene expression between males and females | Stumm et al. 2012 [27] |
| Subchondral, trabecular, and cortical bone from femoral head of 11 dogs (7 males and 4 females) undergoing THR | Osteoblasts | Clinical and radiographic diagnosis | Osteoblast culture from subchondral, trabecular and cortical bone in mineralizing medium (DMEM plus β-glycerophosphate and ascorbate) | Cell number and viability (6 days). TNAP activity (1 day). Mineralization analysis (40 days) | No differences in any of the analysis parameters irrespective of the bone types between males and female | Meeson et al. 2019 [28] |
| Synovial membrane of 8 male and 8 female Sprague-Dawley rats (8-weeks-old) | FLSs | TMJ-OA chemically induced by Freund’s adjuvant combined with MIA | FLSs treated with or without TNF-α for 6 h; FLSs (lower chamber) in co-culture system with macrophage cell line (NR8383, in the upper chamber) | ELISA and Western blot (MCP-1). RT-PCR (CD68, MCP-1, iNOS, and IL-1β expression). Macrophage migration assay in the co-culture system (24 h) | ↑iNOS, IL-1β, MCP-1 expression, macrophages number, and migration in female FLSs vs. males. No difference in the mRNA expression of iNOS, IL-1β, MCP-1, and in the migration assay from male and female FLSs without treatment of TNF-α. ↑iNOS, IL-1β, and MCP-1 in female FLSs treated with TNF-α vs. male FLSs. Female FLSs recruited more macrophages vs. male FLSs upon stimulation with TNF-α | Xue et al. 2018 [29] (in vitro) |
| Animal Species and Age | OA Model and Site | Aim | Follow-up and Evaluations | Main Results | References |
|---|---|---|---|---|---|
| 18 male and 20 female STR/Ort mice (5, 10, 15, 20, and 35weeks-old) | Spontaneous OA (naturally occurring). Knee | To investigate and compared age- and sex-related BMD and bone architecture | 35 weeks. μCT (cancellous and cortical BMD, trabecular BV/TV, Tb.N, Tb.Th, MV, and cBV/TV). ADITT | ↑OA changes, ADITT and MV in males vs. females. ↑cancellous BDM, trabecular BV/TV, Tb.N, Tb.Th, and cBV/TV in females vs. males. =cortical BDM in males and females | Uchida et al. 2012 [39] |
| 5 OA-prone males and 5 non-prone females STR/Ort mice (8–10weeks-old: pre-OA; 18–20weeks-old: OA onset; 40+ weeks-old: advanced OA) | Spontaneous OA (naturally occurring). Knee | To evaluate tibial bone phenotype | 10, 20, and 40 weeks. μCT (trabecular bone mass, number and thickness, CSA, BMD, BV/TV, ellipticity, curvature, and shape). Gait analysis. Histological score (articular cartilage lesion severity scoreon toluidine blue-stained samples, OA severity) | ↑trabecular bone mass, number and thickness, CSA, BMD, and BV/TV in females vs. males. ↑gait asymmetry, OA severity, ellipticity, tibia curvature and shape deviations in males vs. females | Javaheri et al. 2018 [33] |
| 4 male and 7 female mice lacking Frzb gene (Frzb-/-), susceptible gene for OA (7weeks-old) | Spontaneous OA (genetic modifications). Knee | To study the effect of Frzb deletion on voluntary running wheel exercise performance and OA development | 6–12 months. Histological score (OARSI score on safranin-o/hematoxylin-stained samples, OA severity: cartilage damage, synovitis, and osteophyte formation). Immunofluorescence (anti-type-I and anti-type IIa myosin antibodies: muscle fiber composition of soleus and extensor digitorumlongus) | ↑running performancein females vs. males. =OA severity and muscle composition in males and females | Lories et al. 2009 [35] |
| 28 male and 28 female mice carrying a targeted mutation in Nov (Novdel3-/-) that causes joint degeneration (2, 6, and 12 months-old) | Spontaneous OA (genetic modifications). Knee | To determine the effect of NOV expression on the anatomy of the knee joint | 2, 6, and 12 months. X-ray (anatomy ofknee joint). Histological score (OARSI score on hematoxylin/eosin, toluidine blue, safranin o-stained samples, OA severity: osteophytes, proteoglycans, fibrillation, erosion, fibrocartilage-like tissue, subchondral sclerosis, cartilage cell density, and thickness). IHC (PCNA, PARP p85, Col I, and Col X) | At 2 months: ↑cartilage cell density in males vs. females. At 6 months: ↑Col I, Col X, and OA severity in males vs. females. ↑PARP p85 in females vs. males. ↓PCNA in females vs. males. ↓cartilage cell density in males and females. At 12 months: ↑Col I, Col X, PCNA, cartilage thickness, and OA severity (with abnormal bone, enlarged epicondyles and meniscus) in males vs. females. ↓cartilage cell density in males and females | Roddy et al. 2015 [38] |
| 12 male and 20 female mice Atg5cKO (lacking Atg5 autophagic gene in their chondrocytes) (n = 6 of 2 months-old; 5 males and 10 females of 6months-old; 7 males and 10 females of 12 months-old; n = 8 of 1month-old and n = 5 of 2months-old) | Spontaneous OA (genetic modifications). Surgically-induced OA (post traumatic): partial MMT. Knee | To evaluate OA development in mice without autophagy in their chondrocytes | 2, 6, and 12 months (spontaneous). 1–2 months (post traumatic). Histological score (OARSI score on safranin-O-stained samples: fibrillation, loss of proteoglycan, and cartilage degradation) | At 2 months (spontaneous): no sign of joint abnormality in males and females. At 6 months (spontaneous): first signs of fibrillation and loss of proteoglycan in males. No sign of OA development in females. At 12 months (spontaneous): substantial OA in males. First signs of cartilage degradation in females. ↑OARSI score in males vs. females. At 2 months (post-traumatic): ↑OA severity in males vs. females | Bouderlique et al. 2016 [30] |
| 35 male (2, 1017, and 20 months-old) and 15 female (10–19 months-old) wild-type C57BL/6 mice | Surgically-induced OA: DMM. Knee | To evaluate subchondral bone plate sclerosis and articular cartilage changes | 2 months. Histological scores (OARSI score on safranin-o/fast green-stained samples, OA severity: cartilage degeneration). μCT (subchondral bone plate thickness and BV/TV of osteophytes) | ↑OA severity and subchondral bone plate thickening in males (12–19+ months) and in females (21 months). ↑BV/TV of osteophytes in males (19+ months) and in females (18 months). ↑OA severity and subchondral bone plate thickening in males vs. females (4.5 and 12 months). =BV/TV of osteophytes in males and females (12 months) | Huang et al. 2017 [32] |
| Male and female CD-1, wild-type, and ADAMTS-5KO mice (lacking Adamts5 gene) on congenic C57BL/6J background (8-12 weeks-old) | Surgically-induced OA: DMM. Knee | To characterize pain-related behavior during OA | 8 weeks. Histology (Toluidine blue: cartilage damage). von Frey testing (mechanical allodynia) | CD-1 mice: =OA-like lesions and allodynia in male and female. Wild-type mice: ↑OA changes and allodynia in male vs. female. ADAMTS-5 mice: no OA changes and allodynia in males and female | Malfait et al. 2010 [36] |
| 50 male and 50 female α1integrin-deficient BALB/c mice (13±1 weeks-old) | Surgically-induced OA: DMM. Knee | To determine metabolite profiles of serum of mice treated with EGFR inhibitor erlotinib | 8 and 12 weeks. µCT (OA signs: cartilage degeneration, bone volume, and subchondral bone thickness). NMR spectroscopy and OPLS-DA statistical model (metabolic profiles: dimethyl sulfone, glutamine, serotonin, 3-hydroxyisovalerate, phenylalanine) | ↓OA signs in females vs.males. ↑OPLS-DA model for females vs. males (relationship between metabolic data and erlotinib treatment). ↑influence and interconnectedness of metabolites unique to males vs. to those unique to females. Glutamine identified as a potential biomarker for resistance to EGFR tyrosine kinase inhibitors. ↑dimethyl sulfone and glutamine in males and females. ↓serotonin, 3-hydroxyisovalerate and phenylalanine in males and females. No clear association between OA and metabolite profiles. Erlotinib treatment and genotype influence metabolite profile, while DMM does not | Mickiewicz et al. 2016 [37] |
| 12 male and 12 female C57BL/6J mice (8–10weeks-old) | Surgically-induced OA: MMT. Knee | To investigate sex effects on pain and knee damage | 12 weeks. Histological score (OARSI score on toluidine blue-stained samples: cartilage damage and osteophytes), mechanical and heat sensitivity (von Frey filaments and Hargreaves test), limb use and locomotor activity | ↑cartilage damage in males vs. females. =osteophyte formation in males and females. Heat sensitivity in males and females, but more delayed in females vs. males. ↑mechanical sensitivity and locomotor activity in females vs. males. ↓load on limb in males vs. females | Temp et al. 2020 [40] |
| 17 male and 10 female C57BL/6 mice with fat-1 transgene, which convert dietary n-6 to n-3 PUFAs endogenously, are fed an n-6 PUFA enriched diet (9–14 months-old) | Metabolically-induced OA: n-6 PUFAs enriched diet. Knee | To evaluate effect of fat-1 transgene expression on inflammation and idiopathicdevelopment of OA in cartilage, bone and synovium | 14 months. Serum analyses: GC-FID (PUFAs) and ELISA (IL6 and TNF-α). μCT (subchondral cortical and trabecular bone: thickness, mineral density, bone spacing and BV/TV). Histological score (Mankin score, OA severity: cartilage degeneration, osteophytes, synovial thickness and extension) | ↓n-6:n-3 PUFA ratio in males vs. females. ↓IL-6 and TNF-α in males and females. =subchondral cortical and trabecular bone parameters and OA severity in males and females | Cai et al. 2014 [31] |
| 39 male and 39 female genetically modified mice hCRP, LDLr-/- and ApoE*3Leiden.CETP, based on C57BL/6J background (8–14 weeks-old) | Metabolically-induced OA: high-caloric diet (16 or 45 kcal% energy from fat). Knee | To examine impact of metabolic dysfunctionon osteophyteformation, synovial inflammation, and cartilagedegradation | 38 weeks. ELISA (metabolic parameters: bodyweight, cholesterol, glucose and insulin levels). Histological score (OARSI score on hematoxylin, fast green and safranin-o -stained samples, OA severity: cartilage degradation, osteophyte formation, and synovitis) | Metabolic dysfunction in all strains. ↑OA severity, bodyweight, glucose, and insulin levelsin males vs. female (hCRP and ApoE*3Leiden.CETP). ↑cholesterol and synovitis in females vs. males (ApoE*3Leiden.CETP) | Kozijn et al. 2018 [34] |
| 92 male and 98 female Fischer rats (3–6 months-old, young; 20–24 months-old, aged) | Chemically-induced-OA: MIA. Knee | To investigate age and sex differences in acute and chronic pain | 4 weeks. Thermal, mechanical, and nociceptive sensitivity and hyperalgesia | ↓thermal threshold in females vs. males and =between young and old. =mechanical threshold and nociceptive sensitivity in males and females, and in young and old. ↑hyperalgesia old vs. young and in females vs. males | Ro et al. 2019 [41] |
| 46 male and 28 female Sprague-Dawley rats | Chemically-induced OA: unilateral MIA. TMJ | To characterize sex differences in development of ongoing pain and central sensitization | 16 days. Histological score (Mankin score on toluidine blue and hematoxylin/eosin-stained samples, OA severity: cartilage loss, subchondral bone changes, osteophyte, and synovitis). Analysis of pain (meal period, meal number, and tactile sensitivity) | =pathological scores and OA severity in males and females. ↑ongoing pain and tactile hypersensitivity in females vs. males. ↓meal period in males and females. ↑meal number in males and females | Sannajust et al. 2019 [42] |
| 10 male and 10 female Sprague-Dawley rats (8weeks-old) | Surgically-induced OA: malocclusion created by moving the first mesial molarsand the third molars distally. TMJ | To investigate expression differences of IGF1, IGFR1 and IGFBP3 in mandibular condylar cartilage | 2 and 4 weeks. Histology (hematoxylin/eosin, OA-like changes: cartilage morphology and thickness). IHC and RT-PCR (Col II, IGF1, IGFR1, and IGFBP3 expression) | ↑OA-like changes in females vs. males. ↓cartilage thickness, IGF1, IGFR1, IGFBP3, and Col II in females vs. males | Yu et al. 2012 [43] |
| 5 male and 5 female Sprague-Dawley rats (8weeks-old) | Chemically-induced OA: CFA and MIA. TMJ | To evaluate sex difference in synovial inflammation, cartilage destruction, and subchondral bone deterioration | 2 weeks. Histological score (score of synovial inflammation, cartilage destruction, and subchondral bone remodeling on hematoxylin/eosin and toluidine blue-stained samples, OA severity: synovitis, cartilage damage, subchondral bone sclerosis, and deterioration). μCT (BV/TV). IHC (CD68, MCP-1, iNOS and IL-1β expression) | ↑OA severity, iNOS, IL-1β, MCP-1, and CD68 expression in females vs. males. ↓BV/TV in females vs. males | Xue et al. 2018 [29] (in vivo) |
| 5 male and 5 female Hartley guinea pigs, 10 female strain 13 guinea pigs (2months-old) | Spontaneous OA (naturally occurring). Knee | To determine the association of cartilagedegeneration with subchondral BMD and meniscal MD | 12 months. Histological score (Mankin score on safranin-o/fast green-stained samples: cartilage degeneration). X-ray densitometry (subchondral BMD and meniscal MD). Atomic absorption spectrophotometry (meniscal calcium content) | ↑cartilage degeneration, subchondral BMD, and meniscal MD in males vs. females. =meniscal calcium content in males and females. ↑cartilage degeneration and subchondral BMD in females strain 13 vs. females Hartley. =meniscal MD in females Hartley and strain 13 | Sun et al. 2015 [44] |
| 8 males and 9 females Yorkshire pigs (7months-old) | Surgically-induced OA: bilateral transection and bridge-enhanced ACL repair with an ECM-based bioactive scaffold using absorbable or non-absorbable sutures. Knee | To evaluate role of sex on the biomechanical outcomes of bridge-enhanced ACL repair | 15 weeks. Biomechanical testing (linear stiffness, yield and maximum loads, energy to failure, and knee laxity) | Absorbable suture: ↓linear stiffness, yield load, and maximum load in females vs. males. =energy to failure and knee laxity in males and female. Non-absorbable suture: =biomechanical outcomes in males and females | Kiapour et al. 2015 [46] |
| 23 males and 18 females Yucatan mini-pigs (15±1months-old) | Surgically-induced OA: unilateral transection and ACL reconstruction using bone-patellar tendon-bone allografts with or without additional bio-enhancement (ECM-based scaffold loaded with autologous PRP). Knee | To evaluate sex differences in ACL reconstruction outcomes with regards to graft structural properties, knee laxity, and cartilage damage | 15 weeks. Physical examination (weight and range of motion). Biomechanical testing (yield load, linear stiffness, maximum loads, and knee laxity). Histological score (Ligament Maturity Index on hematoxylin/eosin-stained samples: cellularity, collagen, and vascularity). Macroscopic score (India ink: cartilage damage) | =weight and range of motion (from pre-injury to post-injury), cellularity and collagen in males and females. ↓yield load, linear stiffness, and maximum load in females vs. males. ↑vascularity in males vs. females. ↑cartilage damage (in conventional reconstruction without collagen-platelet composite) and knee laxity in females vs. males | Kiapour et al. 2015 [45] |
| 153 male and 153 female baboons Papiohamadryas ssp. (4.5–33 years-old) | Spontaneous OA (naturally occurring). Knee | To quantify occurrence and severity of OA | - Macroscopic score (modified Outerbridge score, OA severity: cartilage degradation and osteophytes) | ↑development OA earlier and mild in males vs. females. ↑OA advanced and severe in females vs. males. =osteophytes in males and females | Macrini et al. 2013 [47] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contartese, D.; Tschon, M.; De Mattei, M.; Fini, M. Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies. Int. J. Mol. Sci. 2020, 21, 3696. https://doi.org/10.3390/ijms21103696
Contartese D, Tschon M, De Mattei M, Fini M. Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies. International Journal of Molecular Sciences. 2020; 21(10):3696. https://doi.org/10.3390/ijms21103696
Chicago/Turabian StyleContartese, Deyanira, Matilde Tschon, Monica De Mattei, and Milena Fini. 2020. "Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies" International Journal of Molecular Sciences 21, no. 10: 3696. https://doi.org/10.3390/ijms21103696
APA StyleContartese, D., Tschon, M., De Mattei, M., & Fini, M. (2020). Sex Specific Determinants in Osteoarthritis: A Systematic Review of Preclinical Studies. International Journal of Molecular Sciences, 21(10), 3696. https://doi.org/10.3390/ijms21103696








