Sex Differences in Proatherogenic Cytokine Levels
Abstract
1. Introduction
2. Results
2.1. General Characteristics of the Population
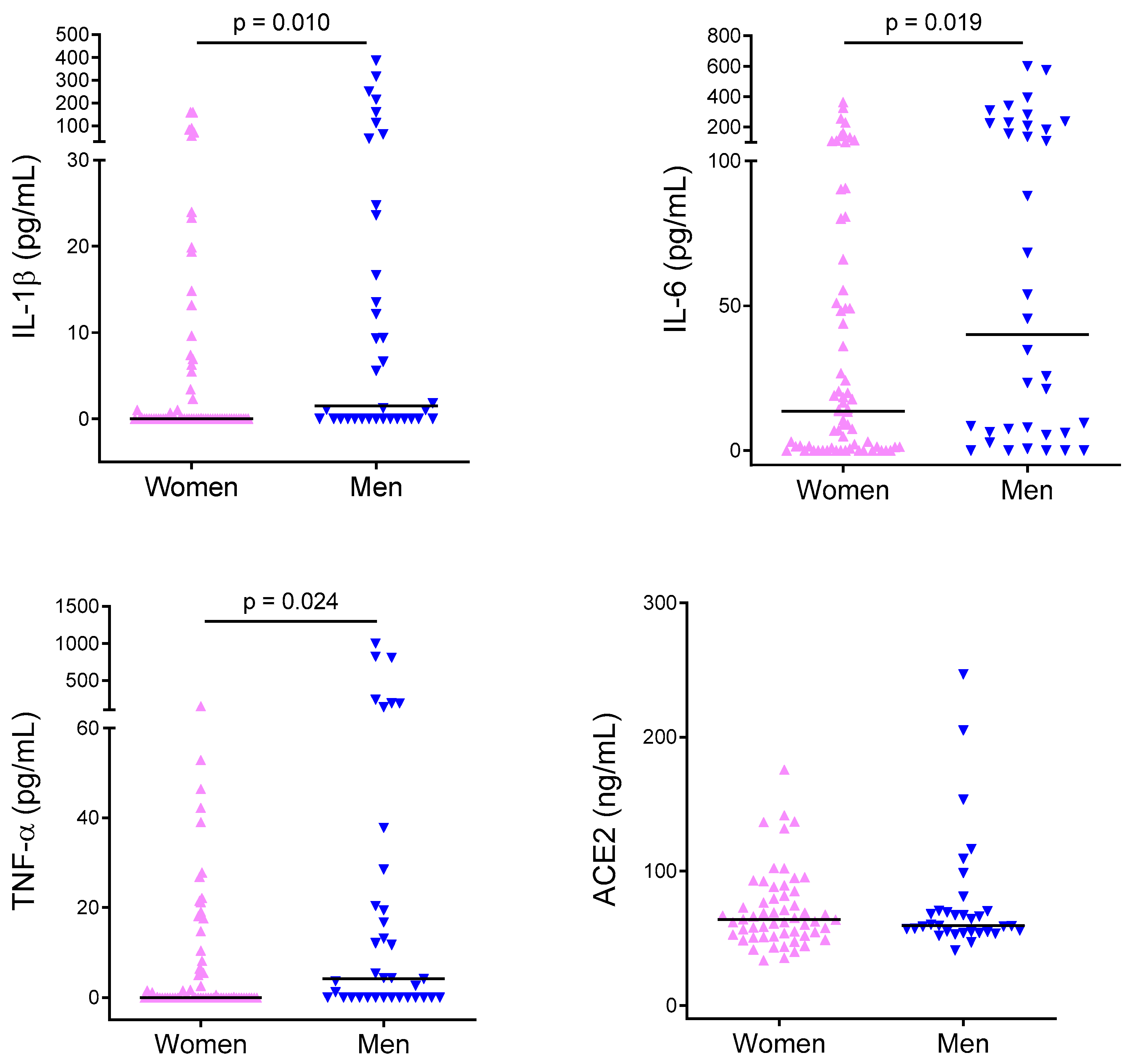
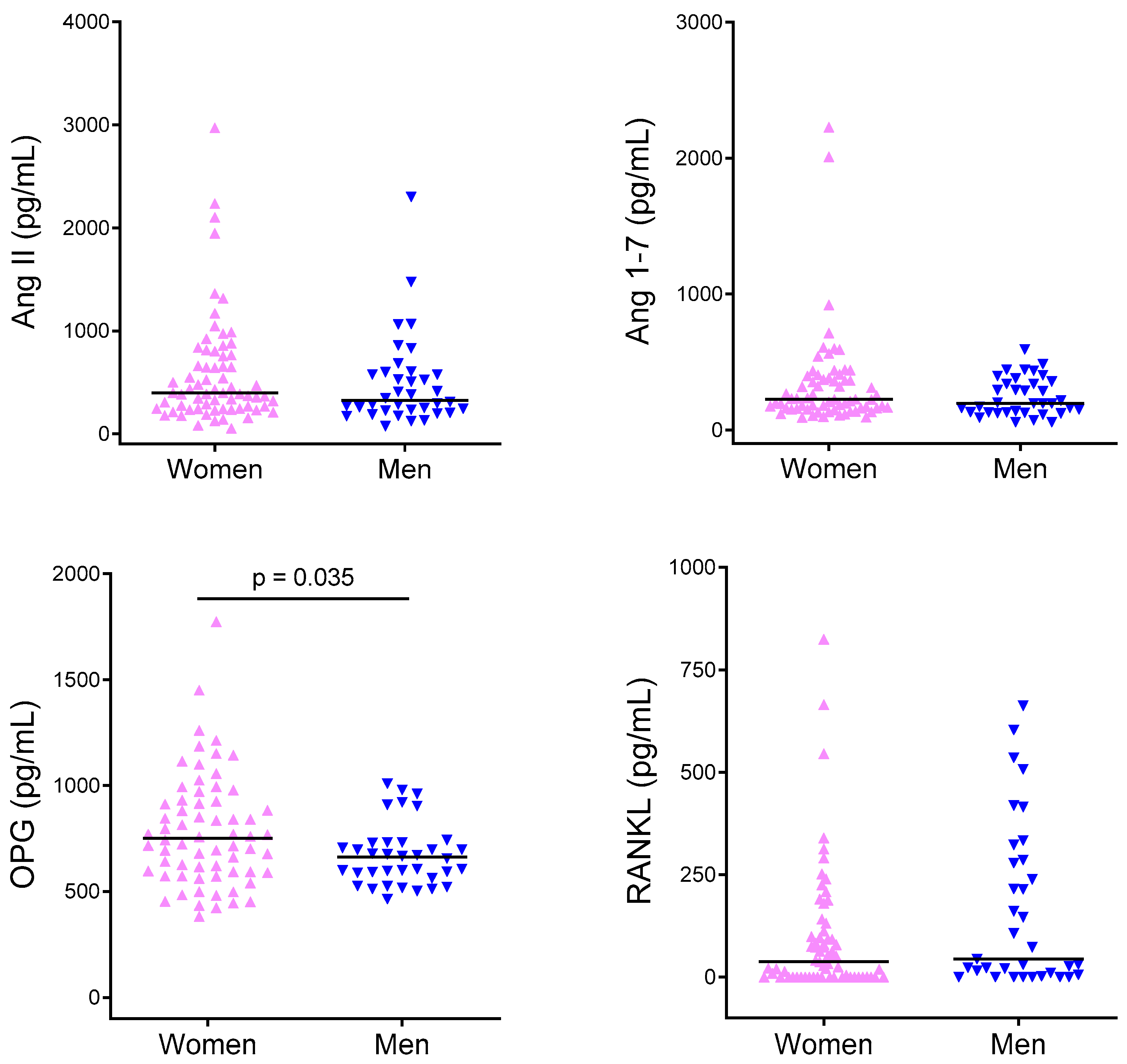
2.2. Cytokine Levels
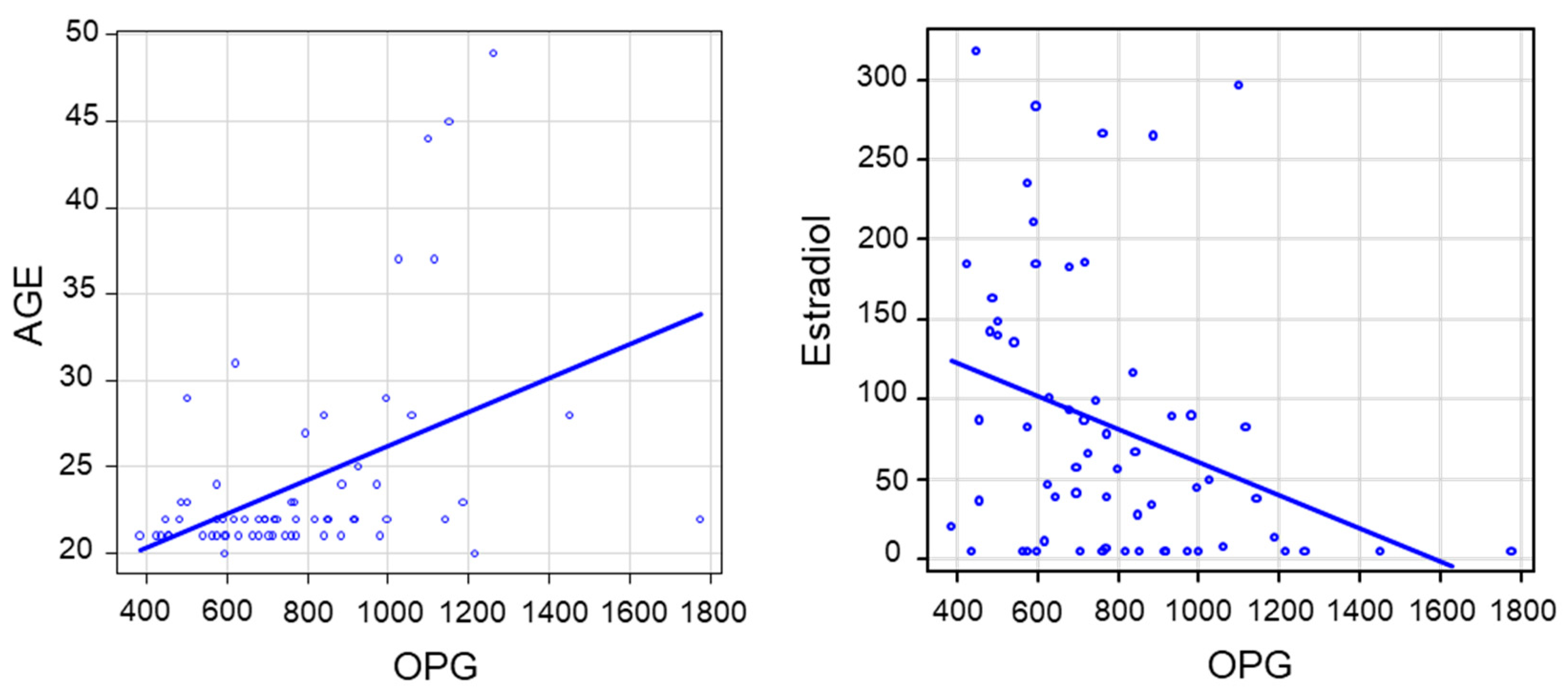
2.3. Linear Correlations between Variables
2.4. Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Population
4.2. Biochemical Characteristics and Cytokine Measurement
4.3. Statistics
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ang | Angiotensin |
| BMI | Body mass index |
| DBP | Diastolic blood pressure |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| IL | Interleukin |
| LPS | lipopolysaccharide |
| MACE | Major adverse cardiovascular events |
| OPG | Osteoprotegerin |
| PBMC | Peripheral blood mononuclear cells |
| RAS | Renin angiotensin system |
| SBP | Systolic blood pressure |
| TNF | Tumor necrosis factor |
References
- Pennell, L.M.; Galligan, C.L.; Fish, E.N. Sex affects immunity. J. Autoimmun. 2012, 38, J282–J291. [Google Scholar] [CrossRef] [PubMed]
- Beeson, P.B. Age and sex associations of 40 autoimmune diseases. Am. J. Med. 1994, 96, 457–462. [Google Scholar] [CrossRef]
- Butterworth, M.; McClellan, B.; Allansmith, M. Influence of sex in immunoglobulin levels. Nature 1967, 214, 1224–1225. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Wenham, C.; Smith, J.; Morgan, R.; Gender and COVID-19 Working Group. COVID-19: The gendered impacts of the outbreak. Lancet 2020, 395, 846–848. [Google Scholar] [CrossRef]
- Bischof, E.; Wolfe, J.; Klein, S.L. Clinical trials for COVID-19 should include sex as a variable. J. Clin. Investig. 2020. [Google Scholar] [CrossRef]
- Ter Horst, R.; Jaeger, M.; Smeekens, S.P.; Oosting, M.; Swertz, M.A.; Li, Y.; Kumar, V.; Diavatopoulos, D.A.; Jansen, A.F.M.; Lemmers, H.; et al. Host and environmental factors influencing individual human cytokine responses. Cell 2016, 167, 1111–1124. [Google Scholar] [CrossRef]
- Giron-Gonzalez, J.A.; Moral, F.J.; Elvira, J.; Garcia-Gil, D.; Guerrero, F.; Gavilan, I.; Escobar, L. Consistent production of a higher TH1:TH2 cytokine ratio by stimulated T cells in men compared with women. Eur. J. Endocrinol. 2000, 143, 31–36. [Google Scholar] [CrossRef]
- Asai, K.; Hiki, N.; Mimura, Y.; Ogawa, T.; Unou, K.; Kaminishi, M. Gender differences in cytokine secretion by human peripheral blood mononuclear cells: Role of estrogen in modulating LPS-induced cytokine secretion in an ex vivo septic model. Shock 2001, 16, 340–343. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis--an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-dose methotrexate for the prevention of atherosclerotic events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef]
- Mallat, Z.; Taleb, S.; Ait-Oufella, H.; Tedgui, A. The role of adaptive T cell immunity in atherosclerosis. J. Lipid Res. 2009, 50, S364–S369. [Google Scholar] [CrossRef]
- Raaz-Schrauder, D.; Schrauder, M.G.; Stumpf, C.; Lewczuk, P.; Kilian, T.; Dietel, B.; Garlichs, C.D.; Schlundt, C.; Achenbach, S.; Klinghammer, L. Plasma levels of sRANKL and OPG are associated with atherogenic cytokines in patients with intermediate cardiovascular risk. Heart Vessels 2017, 32, 1304–1313. [Google Scholar] [CrossRef]
- Thomas, M.C.; Pickering, R.J.; Tsorotes, D.; Koitka, A.; Sheehy, K.; Bernardi, S.; Toffoli, B.; Nguyen-Huu, T.P.; Head, G.A.; Fu, Y.; et al. Genetic Ace2 deficiency accentuates vascular inflammation and atherosclerosis in the ApoE knockout mouse. Circ. Res. 2010, 107, 888–897. [Google Scholar] [CrossRef]
- Bernardi, S.; Michelli, A.; Zuolo, G.; Candido, R.; Fabris, B. Update on RAAS Modulation for the Treatment of Diabetic Cardiovascular Disease. J. Diabetes Res. 2016, 2016, 8917578. [Google Scholar] [CrossRef]
- Kiechl, S.; Schett, G.; Wenning, G.; Redlich, K.; Oberhollenzer, M.; Mayr, A.; Santer, P.; Smolen, J.; Poewe, W.; Willeit, J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 2004, 109, 2175–2180. [Google Scholar] [CrossRef]
- Bernardi, S.; Bossi, F.; Toffoli, B.; Fabris, B. Roles and clinical applications of OPG and TRAIL as biomarkers in cardiovascular disease. Biomed Res. Int. 2016, 2016, 1752854. [Google Scholar] [CrossRef]
- Bouman, A.; Schipper, M.; Heineman, M.J.; Faas, M.M. Gender difference in the non-specific and specific immune response in humans. Am. J. Reprod. Immunol. 2004, 52, 19–26. [Google Scholar] [CrossRef]
- Beenakker, K.G.M.; Westendorp, R.G.J.; de Craen, A.J.M.; Chen, S.; Raz, Y.; Ballieux, B.; Nelissen, R.; Later, A.F.L.; Huizinga, T.W.; Slagboom, P.E.; et al. Men have a stronger monocyte-derived cytokine production response upon stimulation with the gram-negative stimulus lipopolysaccharide than women: A pooled analysis including 15 study populations. J. Innate Immun. 2020, 12, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Bouman, A.; Heineman, M.J.; Faas, M.M. Sex hormones and the immune response in humans. Hum. Reprod. Update 2005, 11, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, N.V.; Wong, S.K.; Wan Hasan, W.N.; Jolly, J.J.; Nur-Farhana, M.F.; Ima-Nirwana, S.; Chin, K.Y. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 2019, 22, 129–140. [Google Scholar] [CrossRef]
- Arnold, A.P. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm. Behav. 2009, 55, 570–578. [Google Scholar] [CrossRef]
- Towfighi, A.; Zheng, L.; Ovbiagele, B. Sex-specific trends in midlife coronary heart disease risk and prevalence. Arch. Intern. Med. 2009, 169, 1762–1766. [Google Scholar] [CrossRef]
- Ridker, P.M. Anticytokine Agents: Targeting interleukin signaling pathways for the treatment of atherothrombosis. Circ. Res. 2019, 124, 437–450. [Google Scholar] [CrossRef]
- Elhage, R.; Maret, A.; Pieraggi, M.T.; Thiers, J.C.; Arnal, J.F.; Bayard, F. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation 1998, 97, 242–244. [Google Scholar] [CrossRef]
- Devlin, C.M.; Kuriakose, G.; Hirsch, E.; Tabas, I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc. Natl. Acad. Sci. USA 2002, 99, 6280–6285. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Stampfer, M.J.; Hennekens, C.H. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation 2000, 101, 1767–1772. [Google Scholar] [CrossRef]
- Lindmark, E.; Diderholm, E.; Wallentin, L.; Siegbahn, A. Relationship between interleukin 6 and mortality in patients with unstable coronary artery disease: Effects of an early invasive or noninvasive strategy. JAMA 2001, 286, 2107–2113. [Google Scholar] [CrossRef]
- Collaboration, I.R.G.C.E.R.F.; Sarwar, N.; Butterworth, A.S.; Freitag, D.F.; Gregson, J.; Willeit, P.; Gorman, D.N.; Gao, P.; Saleheen, D.; Rendon, A.; et al. Interleukin-6 receptor pathways in coronary heart disease: A collaborative meta-analysis of 82 studies. Lancet 2012, 379, 1205–1213. [Google Scholar] [CrossRef]
- Semb, A.G.; Ueland, T.; Aukrust, P.; Wareham, N.J.; Luben, R.; Gullestad, L.; Kastelein, J.J.; Khaw, K.T.; Boekholdt, S.M. Osteoprotegerin and soluble receptor activator of nuclear factor-kappaB ligand and risk for coronary events: A nested case-control approach in the prospective EPIC-Norfolk population study 1993-2003. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 975–980. [Google Scholar] [CrossRef]
- Omland, T.; Ueland, T.; Jansson, A.M.; Persson, A.; Karlsson, T.; Smith, C.; Herlitz, J.; Aukrust, P.; Hartford, M.; Caidahl, K. Circulating osteoprotegerin levels and long-term prognosis in patients with acute coronary syndromes. J. Am. Coll. Cardiol. 2008, 51, 627–633. [Google Scholar] [CrossRef]
- Lieb, W.; Gona, P.; Larson, M.G.; Massaro, J.M.; Lipinska, I.; Keaney, J.F., Jr.; Rong, J.; Corey, D.; Hoffmann, U.; Fox, C.S.; et al. Biomarkers of the osteoprotegerin pathway: Clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1849–1854. [Google Scholar] [CrossRef]
- Bernardi, S.; Voltan, R.; Rimondi, E.; Melloni, E.; Milani, D.; Cervellati, C.; Gemmati, D.; Celeghini, C.; Secchiero, P.; Zauli, G.; et al. TRAIL, OPG, and TWEAK in kidney disease: Biomarkers or therapeutic targets? Clin. Sci. (Lond.) 2019, 133, 1145–1166. [Google Scholar] [CrossRef]
- Khosla, S.; Arrighi, H.M.; Melton, L.J., III; Atkinson, E.J.; O’Fallon, W.M.; Dunstan, C.; Riggs, B.L. Correlates of osteoprotegerin levels in women and men. Osteoporos. Int. 2002, 13, 394–399. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, H.; Zeng, X.; Feng, S. Estrogen stimulates osteoprotegerin expression via the suppression of miR-145 expression in MG-63 cells. Mol. Med. Rep. 2017, 15, 1539–1546. [Google Scholar] [CrossRef]
- Mozzanega, B.; Gizzo, S.; Bernardi, D.; Salmaso, L.; Patrelli, T.S.; Mioni, R.; Finos, L.; Nardelli, G.B. Cyclic variations of bone resorption mediators and markers in the different phases of the menstrual cycle. J. Bone Miner. Metab. 2013, 31, 461–467. [Google Scholar] [CrossRef]
- Sarink, D.; Yang, J.; Johnson, T.; Chang-Claude, J.; Overvad, K.; Olsen, A.; Tjonneland, A.; Fournier, A.; Mancini, F.R.; Kvaskoff, M.; et al. Reproductive and lifestyle factors and circulating sRANKL and OPG concentrations in women: Results from the EPIC cohort. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1746–1754. [Google Scholar] [CrossRef]
- Majetschak, M.; Christensen, B.; Obertacke, U.; Waydhas, C.; Schindler, A.E.; Nast-Kolb, D.; Schade, F.U. Sex differences in posttraumatic cytokine release of endotoxin-stimulated whole blood: Relationship to the development of severe sepsis. J. Trauma 2000, 48, 832–839. [Google Scholar] [CrossRef]
- Schroder, J.; Kahlke, V.; Staubach, K.H.; Zabel, P.; Stuber, F. Gender differences in human sepsis. Arch. Surg. 1998, 133, 1200–1205. [Google Scholar] [CrossRef]
- Meduri, G.U.; Headley, S.; Kohler, G.; Stentz, F.; Tolley, E.; Umberger, R.; Leeper, K. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest 1995, 107, 1062–1073. [Google Scholar] [CrossRef]
- Conti, P.; Younes, A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: Clinical response to viral infection. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Sama, I.E.; Ravera, A.; Santema, B.T.; van Goor, H.; Ter Maaten, J.M.; Cleland, J.G.F.; Rienstra, M.; Friedrich, A.W.; Samani, N.J.; Ng, L.L.; et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur. Heart J. 2020, 41, 1810–1817. [Google Scholar] [CrossRef]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Minerva Anestesiol. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
| Women (n = 68) | Men (n = 36) | p-Value | |
|---|---|---|---|
| Age (years) | 22 (20–49) | 22 (21–37) | 0.997 |
| Smoke (%) | 21% | 15% | 0.49 |
| CVD Family history (%) | 39% | 33% | 0.59 |
| Physical activity (%) | 72% | 70% | 0.84 |
| BMI (weight/height2) | 21 (17–36) | 23.2 (19.8–31.4) | <0.001 |
| SBP (mmHg) | 115 (90–140) | 125 (100–145) | <0.001 |
| DBP (mmHg) | 70 (60–90) | 80 (60–90) | <0.01 |
| Glucose (mg/dL) | 85 (65–100) | 86 (74–123) | 0.06 |
| Total Cholesterol (mg/dL) | 185 (129–254) | 159 (95–249) | <0.05 |
| HDL-C (mg/dL) | 61 (38–94) | 46 (33–64) | <0.001 |
| Triglycerides (mg/dL) | 66 (37–136) | 68 (40–271) | 0.4 |
| LDL-C (mg/dL) | 111 (58–169) | 97 (39–179) | 0.14 |
| Estradiol (pg/mL) | 53 (7.8–319) | 23 (9–45) | <0.05 |
| Testosterone (ng/mL) | 0.3 (0.02–0.6) | 5.7 (2.8–8.2) | <0.001 |
| Variable | IL1-β | IL-6 | TNF-α | |||
|---|---|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age | 0.23 | 0.02 | 0.14 | 0.16 | 0.09 | 0.35 |
| BMI (weight/height2) | 0.15 | 0.12 | 0.13 | 0.21 | 0.19 | 0.07 |
| SBP (mmHg) | 0.17 | 0.09 | 0.17 | 0.09 | 0.12 | 0.25 |
| DBP (mmHg) | 0.14 | 0.17 | 0.15 | 0.13 | 0.09 | 0.36 |
| Glucose | 0.26 | 0.008 | 0.14 | 0.17 | 0.18 | 0.08 |
| Total cholesterol | −0.10 | 0.30 | −0.19 | 0.06 | −0.15 | 0.13 |
| HDL cholesterol | −0.05 | 0.63 | −0.18 | 0.13 | −0.04 | 0.65 |
| LDL cholesterol | −0.12 | 0.22 | −0.15 | 0.13 | −0.18 | 0.08 |
| Triglycerides | −0.01 | 0.89 | −0.03 | 0.79 | −0.09 | 0.35 |
| Estradiol | −0.09 | 0.38 | −0.09 | 0.35 | −0.14 | 0.19 |
| Testosterone | 0.25 | 0.01 | 0.20 | 0.04 | 0.18 | 0.08 |
| T/E2 | 0.28 | 0.005 | 0.24 | 0.02 | 0.27 | 0.007 |
| Women | ||||||
|---|---|---|---|---|---|---|
| Variable | IL1-β | IL-6 | TNF-α | |||
| ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age | 0.11 | 0.37 | 0.04 | 0.74 | 0.04 | 0.75 |
| BMI (weight/height2) | 0.09 | 0.46 | 0.13 | 0.29 | 0.13 | 0.29 |
| SBP (mmHg) | 0.06 | 0.64 | 0.02 | 0.88 | −0.02 | 0.86 |
| DBP (mmHg) | 0.04 | 0.72 | 0.02 | 0.87 | −0.03 | 0.82 |
| Glucose | 0.19 | 0.13 | 0.01 | 0.99 | 0.10 | 0.44 |
| Total cholesterol | −0.02 | 0.88 | −0.05 | 0.71 | −0.11 | 0.39 |
| HDL cholesterol | 0.09 | 0.44 | −0.07 | 0.54 | 0.07 | 0.56 |
| LDL cholesterol | −0.09 | 0.48 | −0.04 | 0.75 | −0.18 | 0.14 |
| Triglycerides | 0.08 | 0.52 | 0.14 | 0.26 | −0.03 | 0.82 |
| Estradiol | −0.01 | 0.91 | −0.07 | 0.57 | −0.10 | 0.45 |
| Testosterone | 0.01 | 0.99 | −0.05 | 0.72 | −0.10 | 0.42 |
| E2/T | −0.06 | 0.64 | −0.08 | 0.51 | −0.09 | 0.49 |
| T/E2 | 0.02 | 0.87 | 0.05 | 0.67 | 0.05 | 0.68 |
| Men | ||||||
| Variable | IL1-β | IL-6 | TNF-α | |||
| ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age | 0.45 | 0.007 | 0.34 | 0.05 | 0.16 | 0.36 |
| BMI | 0.04 | 0.82 | −0.14 | 0.46 | 0.02 | 0.90 |
| SBP | 0.04 | 0.80 | 0.17 | 0.35 | 0.05 | 0.79 |
| DBP | 0.11 | 0.53 | 0.18 | 0.32 | 0.11 | 0.56 |
| Glucose | 0.28 | 0.11 | 0.31 | 0.07 | 0.19 | 0.29 |
| Total cholesterol | −0.07 | 0.67 | −0.23 | 0.19 | −0.05 | 0.77 |
| HDL cholesterol | 0.10 | 0.57 | 0.08 | 0.67 | 0.18 | 0.30 |
| LDL cholesterol | −0.08 | 0.64 | −0.20 | 0.25 | −0.07 | 0.69 |
| Triglycerides | −0.16 | 0.36 | −0.31 | 0.07 | −0.25 | 0.15 |
| Estradiol | −0.09 | 0.58 | 0.03 | 0.85 | −0.16 | 0.37 |
| Testosterone | −0.02 | 0.89 | 0.19 | 0.27 | 0.02 | 0.89 |
| T/E2 | 0.09 | 0.60 | 0.09 | 0.61 | 0.21 | 0.23 |
| Variable | Entire Cohort | Women | Men | |||
|---|---|---|---|---|---|---|
| ρ | p-Value | ρ | p-Value | ρ | p-Value | |
| Age | 0.35 | 0.0004 | 0.42 | 0.0004 | 0.24 | 0.17 |
| BMI | −0.21 | 0.03 | −0.12 | 0.31 | −0.19 | 0.30 |
| SBP | −0.22 | 0.03 | −0.26 | 0.03 | 0.01 | 0.94 |
| DBP | −0.32 | 0.001 | −0.30 | 0.01 | −0.33 | 0.06 |
| Glucose | 0.06 | 0.53 | 0.03 | 0.79 | 0.22 | 0.21 |
| Total cholesterol | 0.03 | 0.77 | 0.08 | 0.52 | −0.23 | 0.19 |
| HDL cholesterol | 0.15 | 0.14 | 0.08 | 0.50 | 0.03 | 0.85 |
| LDL cholesterol | −0.04 | 0.72 | −0.01 | 0.97 | −0.19 | 0.26 |
| Triglycerides | −0.02 | 0.80 | 0.13 | 0.30 | −0.28 | 0.11 |
| Estradiol | −0.21 | 0.04 | −0.36 | 0.004 | 0.008 | 0.96 |
| Testosterone | −0.31 | 0.001 | −0.37 | 0.002 | 0.06 | 0.72 |
| T/E2 | −0.05 | 0.58 | −0.01 | 0.91 | 0.09 | 0.59 |
| Dependent Variable IL-1β | |||
|---|---|---|---|
| Predictive Variables | β-Estimate | Standard Error | p-Value |
| Sex | 27.14 | 13.52 | 0.09 |
| Age | 2.75 | 1.24 | 0.03 |
| BMI | 0.27 | 1.91 | 0.88 |
| SBP | 0.15 | 0.54 | 0.78 |
| Glucose | 1.27 | 0.78 | 0.11 |
| Dependent Variable IL-6 | |||
| Predictive Variables | β-Estimate | Standard Error | p-Value |
| Sex | 61.41 | 26.88 | 0.02 |
| Age | 5.61 | 2.51 | 0.03 |
| BMI | 0.46 | 3.89 | 0.90 |
| SBP | 0.69 | 1.11 | 0.53 |
| Glucose | 2.18 | 1.67 | 1.94 |
| Dependent Variable TNF-α | |||
| Predictive Variables | β-Estimate | Standard Error | p-Value |
| Sex | 70.39 | 32.11 | 0.03 |
| Age | 8.91 | 3.24 | 0.007 |
| BMI | −0.04 | 4.65 | 0.99 |
| SBP (mmHg) | 0.14 | 1.35 | 0.91 |
| Glucose (mg/dL) | 0.78 | 1.99 | 0.69 |
| Dependent Variable OPG | |||
| Predictive Variables | β-Estimate | Standard Error | p-Value |
| Sex | −75.07 | 54.96 | 0.17 |
| Age | 14.77 | 5.55 | 0.009 |
| BMI | −7.10 | 7.96 | 0.37 |
| SBP | −2.22 | 2.31 | 0.34 |
| Glucose | 2.75 | 3.41 | 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardi, S.; Toffoli, B.; Tonon, F.; Francica, M.; Campagnolo, E.; Ferretti, T.; Comar, S.; Giudici, F.; Stenner, E.; Fabris, B. Sex Differences in Proatherogenic Cytokine Levels. Int. J. Mol. Sci. 2020, 21, 3861. https://doi.org/10.3390/ijms21113861
Bernardi S, Toffoli B, Tonon F, Francica M, Campagnolo E, Ferretti T, Comar S, Giudici F, Stenner E, Fabris B. Sex Differences in Proatherogenic Cytokine Levels. International Journal of Molecular Sciences. 2020; 21(11):3861. https://doi.org/10.3390/ijms21113861
Chicago/Turabian StyleBernardi, Stella, Barbara Toffoli, Federica Tonon, Morena Francica, Elena Campagnolo, Tommaso Ferretti, Sarah Comar, Fabiola Giudici, Elisabetta Stenner, and Bruno Fabris. 2020. "Sex Differences in Proatherogenic Cytokine Levels" International Journal of Molecular Sciences 21, no. 11: 3861. https://doi.org/10.3390/ijms21113861
APA StyleBernardi, S., Toffoli, B., Tonon, F., Francica, M., Campagnolo, E., Ferretti, T., Comar, S., Giudici, F., Stenner, E., & Fabris, B. (2020). Sex Differences in Proatherogenic Cytokine Levels. International Journal of Molecular Sciences, 21(11), 3861. https://doi.org/10.3390/ijms21113861






