Plasma Oxalate as a Predictor of Kidney Function Decline in a Primary Hyperoxaluria Cohort
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics
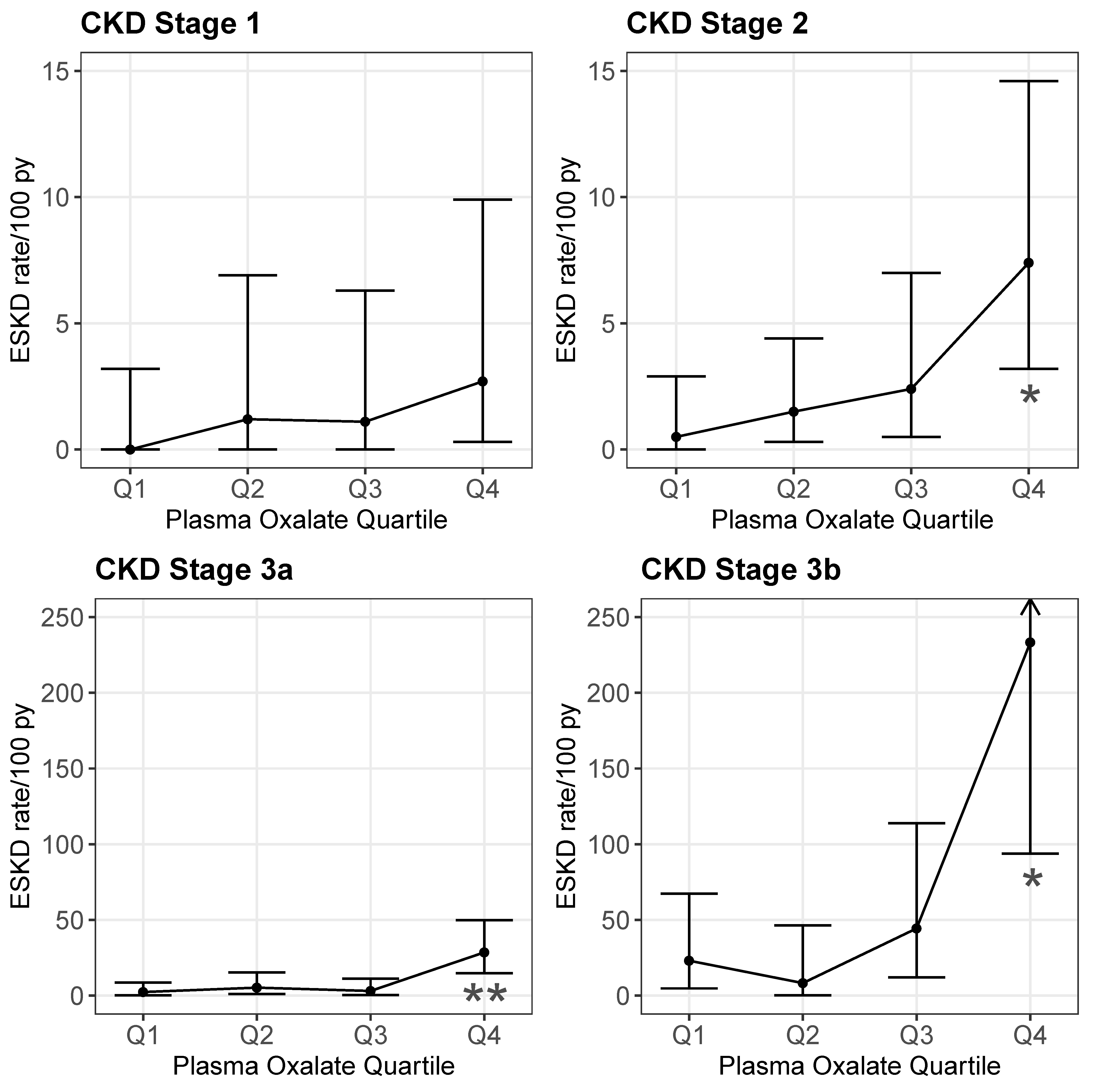
2.2. POx and ESKD
2.3. UOX and ESKD
2.4. ESKD or 40% Sustained Reduction in eGFR
2.5. eGFR Slope
3. Discussion
4. Materials and Methods
4.1. Statistical Methods
Plasma Oxalate
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cochat, P.; Rumsby, G. Primary hyperoxaluria. N. Engl. J. Med. 2013, 369, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Bergstralh, E.J.; Mehta, R.A.; Vaughan, L.E.; Olson, J.B.; Seide, B.M.; Meek, A.M.; Cogal, A.G.; Lieske, J.C.; Milliner, D.S. Predictors of incident ESRD among patients with primary hyperoxaluria presenting prior to kidney failure. Clin. J. Am. Soc. Nephrol. 2016, 11, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Beck, B.B.; Milliner, D.S. The primary hyperoxalurias. Kidney Int. 2009, 75, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Dutta, C.; Avitahl-Curtis, N.; Pursell, N.; Larsson Cohen, M.; Holmes, B.; Diwanji, R.; Zhou, W.; Apponi, L.; Koser, M.; Ying, B.; et al. Inhibition of Glycolate Oxidase with Dicer-substrate siRNA Reduces Calcium Oxalate Deposition in a Mouse Model of Primary Hyperoxaluria Type 1. Mol. Ther. 2016, 24, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, V.O.; Goldfarb, D.S.; Lieske, J.C.; Beara-Lasic, L.; Anglani, F.; Milliner, D.S.; Palsson, R. Hereditary causes of kidney stones and chronic kidney disease. Pediatr. Nephrol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Perinpam, M.; Enders, F.T.; Mara, K.C.; Vaughan, L.E.; Mehta, R.A.; Voskoboev, N.; Milliner, D.S.; Lieske, J.C. Plasma oxalate in relation to eGFR in patients with primary hyperoxaluria, enteric hyperoxaluria and urinary stone disease. Clin. Biochem. 2017, 50, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Elgstoen, K.B.; Johnsen, L.F.; Woldseth, B.; Morkrid, L.; Hartmann, A. Plasma oxalate following kidney transplantation in patients without primary hyperoxaluria. Nephrol. Dial. Transplant. 2010, 25, 2341–2345. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, B.; Ürekli, H.M.; Atta, M.G. Primary and secondary hyperoxaluria: Understanding the enigma. World J. Nephrol. 2015, 4, 235. [Google Scholar] [CrossRef] [PubMed]
- Worcester, E.M.; Evan, A.P.; Coe, F.L.; Lingeman, J.E.; Krambeck, A.; Sommers, A.; Philips, C.L.; Milliner, D. A test of the hypothesis that oxalate secretion produces proximal tubule crystallization in primary hyperoxaluria type I. Am. J. Physiol.-Ren. Physiol. 2013, 305, F1574–F1584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ladwig, P.M.; Liedtke, R.R.; Larson, T.S.; Lieske, J.C. Sensitive spectrophotometric assay for plasma oxalate. Clin. Chem. 2005, 51, 2377–2380. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.; Kemper, M.J.; Hvizd, M.G.; Sailer, D.E.; Langman, C.B. Simultaneous determination of oxalate, citrate and sulfate in children’s plasma with ion chromatography. Kidney Int. 1998, 53, 1348–1352. [Google Scholar] [CrossRef] [PubMed]
- Elgstoen, K.B. Liquid chromatography-tandem mass spectrometry method for routine measurement of oxalic acid in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 873, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Monico, C.G.; Holmes, W.S.; Bergstralh, E.J.; Slezak, J.M.; Rohlinger, A.L.; Olson, J.B.; Milliner, D.S. International registry for primary hyperoxaluria. Am. J. Nephrol. 2005, 25, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.M.; Liedtke, R.R. Modified enzyme-based colorimetric assay of urinary and plasma oxalate with improved sensitivity and no ascorbate interference: Reference values and specimen handling procedures. Clin. Chem. 1991, 37, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Inker, L.A.; Schmid, C.H.; Tighiouart, H.; Eckfeldt, J.H.; Feldman, H.I.; Greene, T.; Kusek, J.W.; Manzi, J.; Van Lente, F.; Zhang, Y.L. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012, 367, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.J.; Munoz, A.; Schneider, M.F.; Mak, R.H.; Kaskel, F.; Warady, B.A.; Furth, S.L. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009, 20, 629–637. [Google Scholar] [CrossRef] [PubMed]
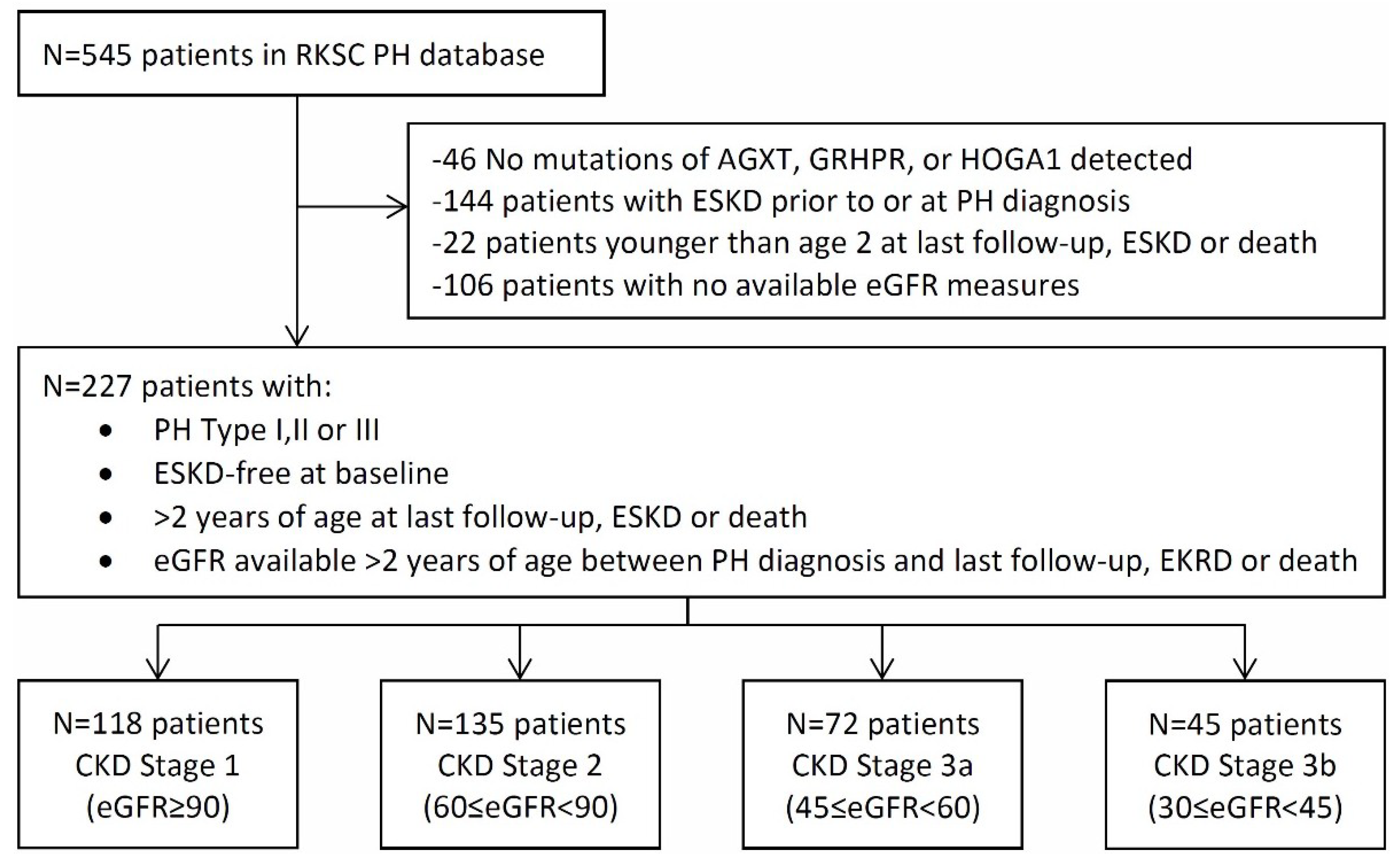
| CKD Stage | ||||
|---|---|---|---|---|
| Stage 1 (≥90) | Stage 2 (60–89) | Stage 3a (45–59) | Stage 3b (30–44) | |
| Characteristics | n = 118 | n = 135 | n = 72 | n = 45 |
| Type of PH, % (n) | ||||
| PH1 | 67 (56.8%) | 99 (73.3%) | 62 (86.1%) | 38 (84.4%) |
| PH2 | 23 (19.5%) | 16 (11.9%) | 6 (8.3%) | 5 (11.1%) |
| PH3 | 28 (23.7%) | 20 (14.8%) | 4 (5.6%) | 2 (4.4%) |
| Sex, % (n) | ||||
| Male | 68 (57.6%) | 76 (56.3%) | 39 (54.2%) | 23 (51.1%) |
| Female | 50 (42.4%) | 59 (43.7%) | 33 (45.8%) | 22 (48.9%) |
| Age at diagnosis, y | 5.4 (2.7, 11.1) | 7.9 (4.0, 23.9) | 10.7 (4.6, 26.4) | 16.0 (7.0, 41.7) |
| Follow-up time, y | 5.3 (2.9, 10.0) | 8.8 (3.1, 15.2) | 6.6 (3.4, 12.8) | 1.8 (1.0, 3.8) |
| Patients with follow-up Pox labs | n = 50 | n = 69 | n = 37 | n = 17 |
| No. follow-up labs | 2.5 (1,5) | 3 (1,6) | 3 (1,5) | 1 (1,3) |
| Oxalate Measure | n | Q1 | Median | Q3 |
|---|---|---|---|---|
| Baseline | ||||
| POx, umol/L | ||||
| CKD stage 1 | 38 | 2.1 | 3.1 | 5.7 |
| CKD stage 2 | 44 | 1.9 | 4.1 | 7.2 |
| CKD stage 3a | 25 | 2.9 | 4.8 | 8.2 |
| CKD stage 3b | 17 | 10.5 | 14.4 | 20.0 |
| Follow-up | ||||
| POx, umol/L | ||||
| CKD stage 1 | 171 | 1.9 | 3.0 | 4.8 |
| CKD stage 2 | 288 | 2.1 | 4.1 | 7.1 |
| CKD stage 3a | 165 | 4.2 | 7.0 | 12.9 |
| CKD stage 3b | 38 | 9.9 | 15.2 | 18.0 |
| CKD Stage | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 (≥90) | Stage 2 (60–89) | Stage 3a (45–59) | Stage 3b (30–44) | |||||||||||||
| Variable | n; E | HR (95% CI) | p | C-Index | n; E | HR (95% CI) | p | C-Index | n; E | HR (95% CI) | p | C-Index | n; E | HR (95% CI) | p | C-Index |
| Demographics | ||||||||||||||||
| PH1 | 118; 9 | 7.45 (0.92–60.2) | 0.06 | 0.677 | 135; 29 | 23.9 (3.06–186) | 0.003 | 0.630 | 72; 34 | 5.74 (0.78–42.1) | 0.085 | 0.544 | 45; 31 | 9.45 (1.28–69.8) | 0.028 | 0.607 |
| Male | 118; 9 | 1.31 (0.32–5.34) | 0.71 | 0.535 | 135; 29 | 1.73 (0.80–3.74) | 0.17 | 0.577 | 72; 34 | 1.10 (0.56–2.18) | 0.78 | 0.517 | 45; 31 | 1.07 (0.51–2.23) | 0.86 | 0.520 |
| Age at diagnosis | 118; 9 | 0.98 (0.90–1.08) | 0.69 | 0.500 | 135; 29 | 1.00 (0.98–1.03) | 0.78 | 0.577 | 72; 34 | 1.01 (0.98–1.03) | 0.70 | 0.550 | 45; 31 | 0.98 (0.96–1.01) | 0.13 | 0.612 |
| Plasma Oxalate | ||||||||||||||||
| POx,umol/L | 38; 2 | NE † | NE † | NE † | 44; 9 | 1.17 (1.01–1.35) | 0.033 | 0.623 | 25; 10 | 1.29 (1.09–1.53) | 0.004 | 0.727 | 17; 14 | 1.24 (1.08–1.42) | 0.003 | 0.810 |
| POx, quartile | 38; 2 | - | - | NE † | 44; 9 | - | - | 0.610 | 25; 10 | - | - | 0.698 | 17; 14 | - | - | 0.791 |
| Q1 | - | REF | REF | - | - | REF | REF | - | - | REF | REF | - | - | REF | REF | - |
| Q2 | - | NE † | NE † | - | - | 0.41 (0.04–4.52) | 0.47 | - | - | 2.40 (0.14–40.0) | 0.54 | - | - | 7.85 (0.82–75.0) | 0.074 | - |
| Q3 | - | NE † | NE † | - | - | 1.68 (0.27–10.52) | 0.58 | - | - | 4.39 (0.49–39.5) | 0.19 | - | - | 10.1 (1.11–91.5) | 0.040 | - |
| Q4 | - | NE † | NE † | - | - | 1.57 (0.25–9.75) | 0.63 | - | - | 13.88 (1.41–136.5) | 0.024 | - | - | 42.1 (3.29–539) | 0.004 | - |
| CKD Stage | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 (≥90) | Stage 2 (60–89) | Stage 3a (45–59) | Stage 3b (30–44) | |||||||||||||
| Follow-up Plasma Oxalate | n; E | HR (95% CI) | p | C-Index | n; E | HR (95% CI) | p | C-Index | n; E | HR (95% CI) | p | C-Index | n; E | HR (95% CI) | p | C-Index |
| POx, umol/L | 171; 4 | 1.12 (1.02–1.24) | 0.018 | 0.854 | 288; 15 | 1.17 (1.08–1.25) | <0.001 | 0.806 | 165; 19 | 1.19 (1.11–1.27) | <0.001 | 0.795 | 38; 15 | 1.12 (1.04–1.21) | 0.003 | 0.729 |
| POx, quartile | 171; 4 | - | - | 0.818 | 288; 15 | - | - | 0.772 | 165; 19 | - | - | 0.757 | 38; 15 | - | - | 0.752 |
| Q1 | - | REF | REF | - | - | REF | REF | - | - | REF | REF | - | - | REF | REF | - |
| Q2 | - | NE † | NE † | - | - | 2.70 (0.28–26.03) | 0.39 | - | - | 2.10 (0.35–12.65) | 0.42 | - | - | 0.39 (0.04–4.40) | 0.45 | - |
| Q3 | - | NE † | NE † | - | - | 3.98 (0.41–38.82) | 0.24 | - | - | 1.28 (0.18–9.17) | 0.80 | - | - | 0.98 (0.19–5.04) | 0.98 | - |
| Q4 | - | NE † | NE † | - | - | 14.21 (1.76–114.7) | 0.013 | - | - | 13.66 (3.02–61.91) | <0.001 | - | - | 5.19 (1.10–24.5) | 0.038 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, R.J.; Vaughan, L.E.; Enders, F.T.; Milliner, D.S.; Lieske, J.C. Plasma Oxalate as a Predictor of Kidney Function Decline in a Primary Hyperoxaluria Cohort. Int. J. Mol. Sci. 2020, 21, 3608. https://doi.org/10.3390/ijms21103608
Shah RJ, Vaughan LE, Enders FT, Milliner DS, Lieske JC. Plasma Oxalate as a Predictor of Kidney Function Decline in a Primary Hyperoxaluria Cohort. International Journal of Molecular Sciences. 2020; 21(10):3608. https://doi.org/10.3390/ijms21103608
Chicago/Turabian StyleShah, Ronak Jagdeep, Lisa E. Vaughan, Felicity T. Enders, Dawn S. Milliner, and John C. Lieske. 2020. "Plasma Oxalate as a Predictor of Kidney Function Decline in a Primary Hyperoxaluria Cohort" International Journal of Molecular Sciences 21, no. 10: 3608. https://doi.org/10.3390/ijms21103608
APA StyleShah, R. J., Vaughan, L. E., Enders, F. T., Milliner, D. S., & Lieske, J. C. (2020). Plasma Oxalate as a Predictor of Kidney Function Decline in a Primary Hyperoxaluria Cohort. International Journal of Molecular Sciences, 21(10), 3608. https://doi.org/10.3390/ijms21103608





