New Insights on Vitamin K Metabolism in Senegalese sole (Solea senegalensis) Based on Ontogenetic and Tissue-Specific Vitamin K Epoxide Reductase Molecular Data
Abstract
1. Introduction
2. Results
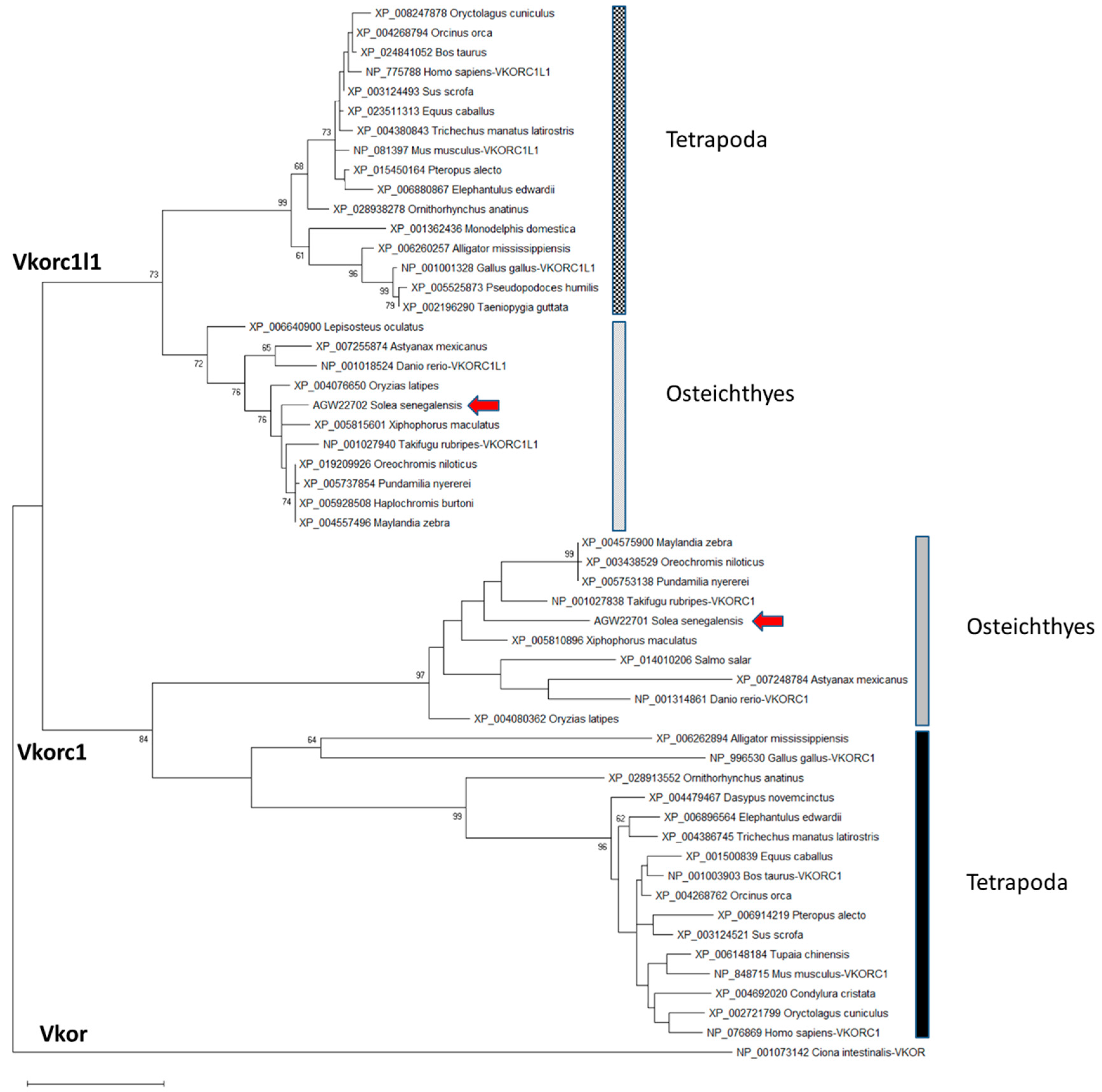
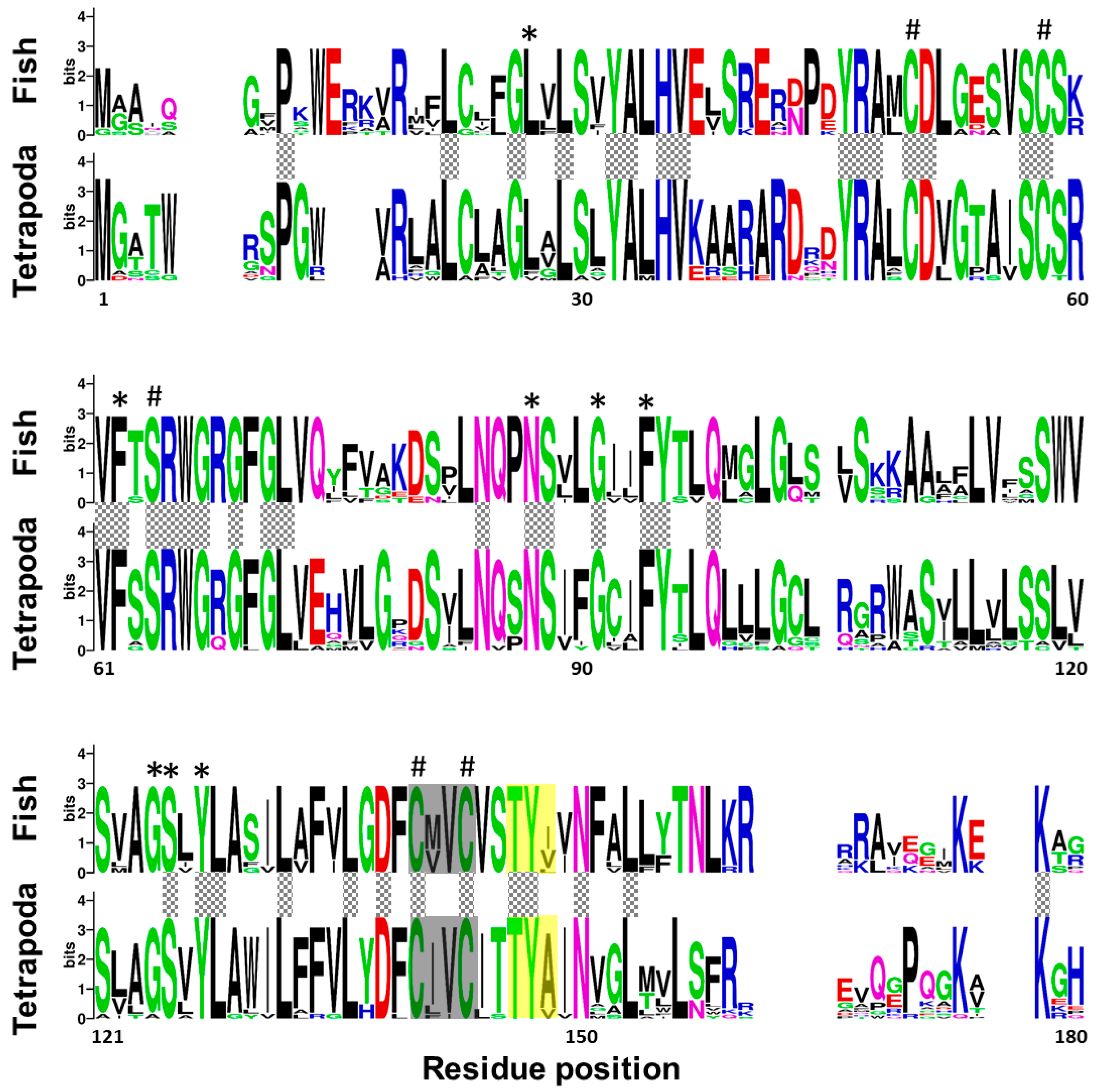
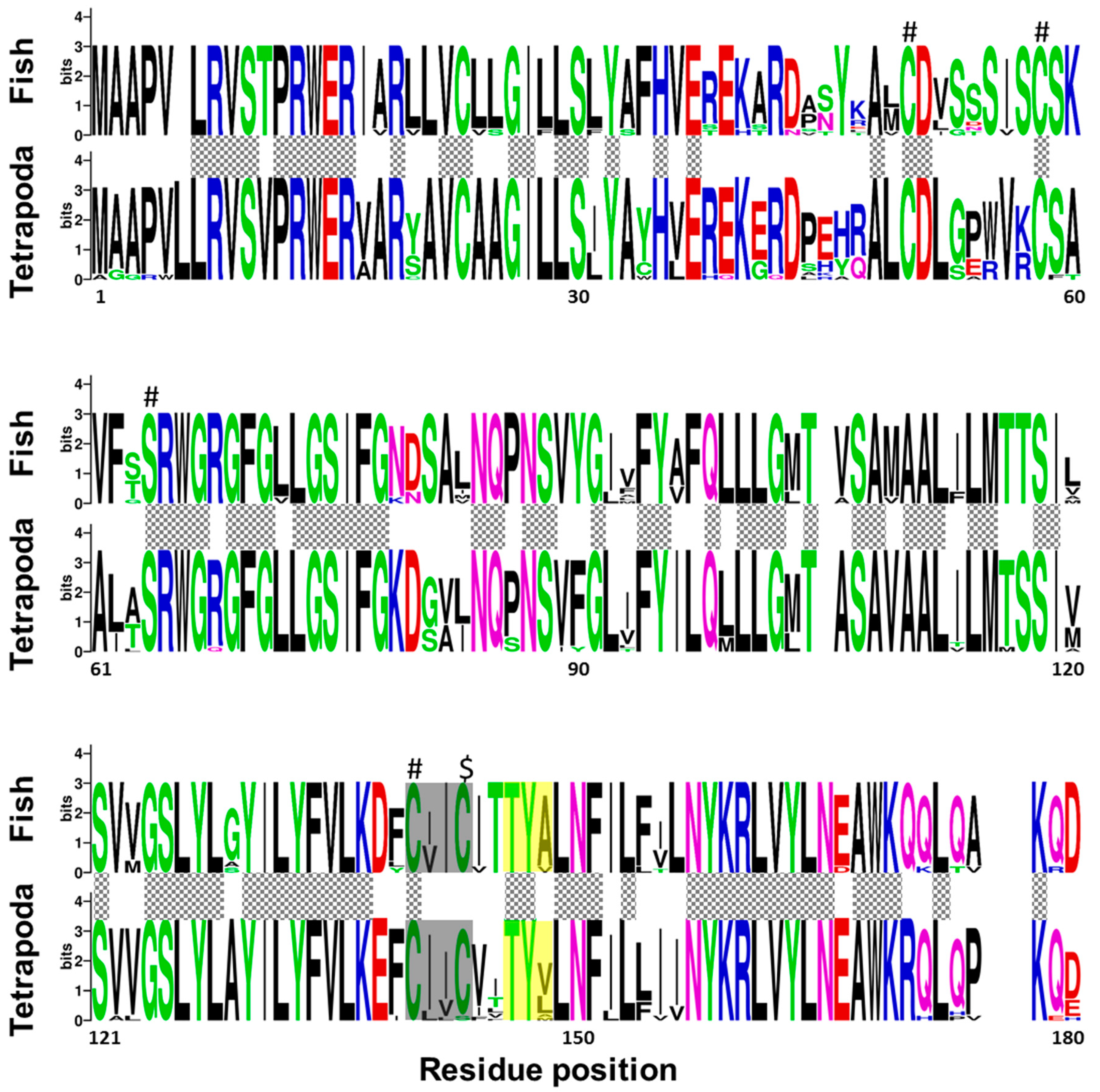
2.1. Senegalese Sole vkorc1 and vkorc1l1 Full-Length cDNA Sequences and Common Protein Sequence Features with Vertebrate Vkors
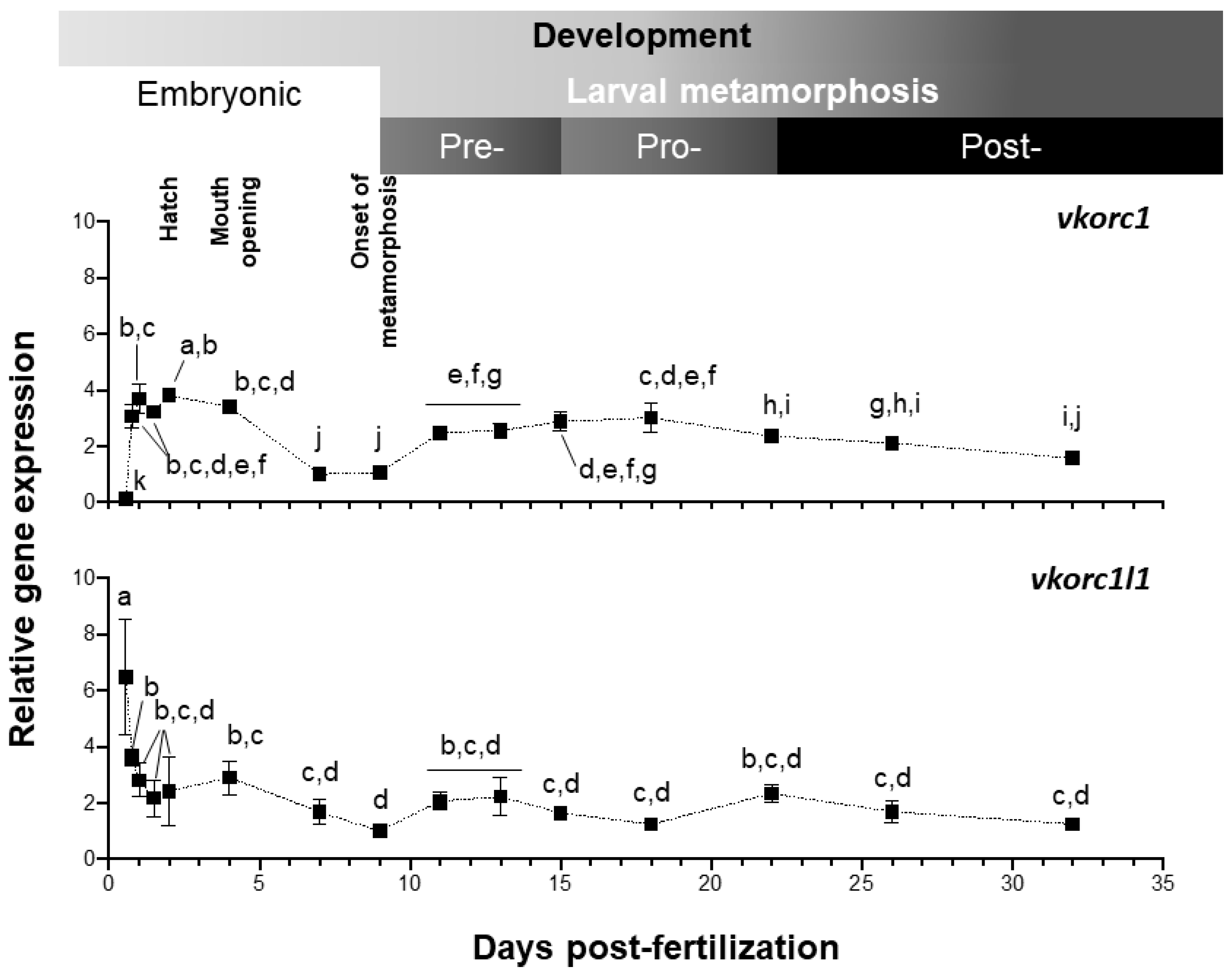
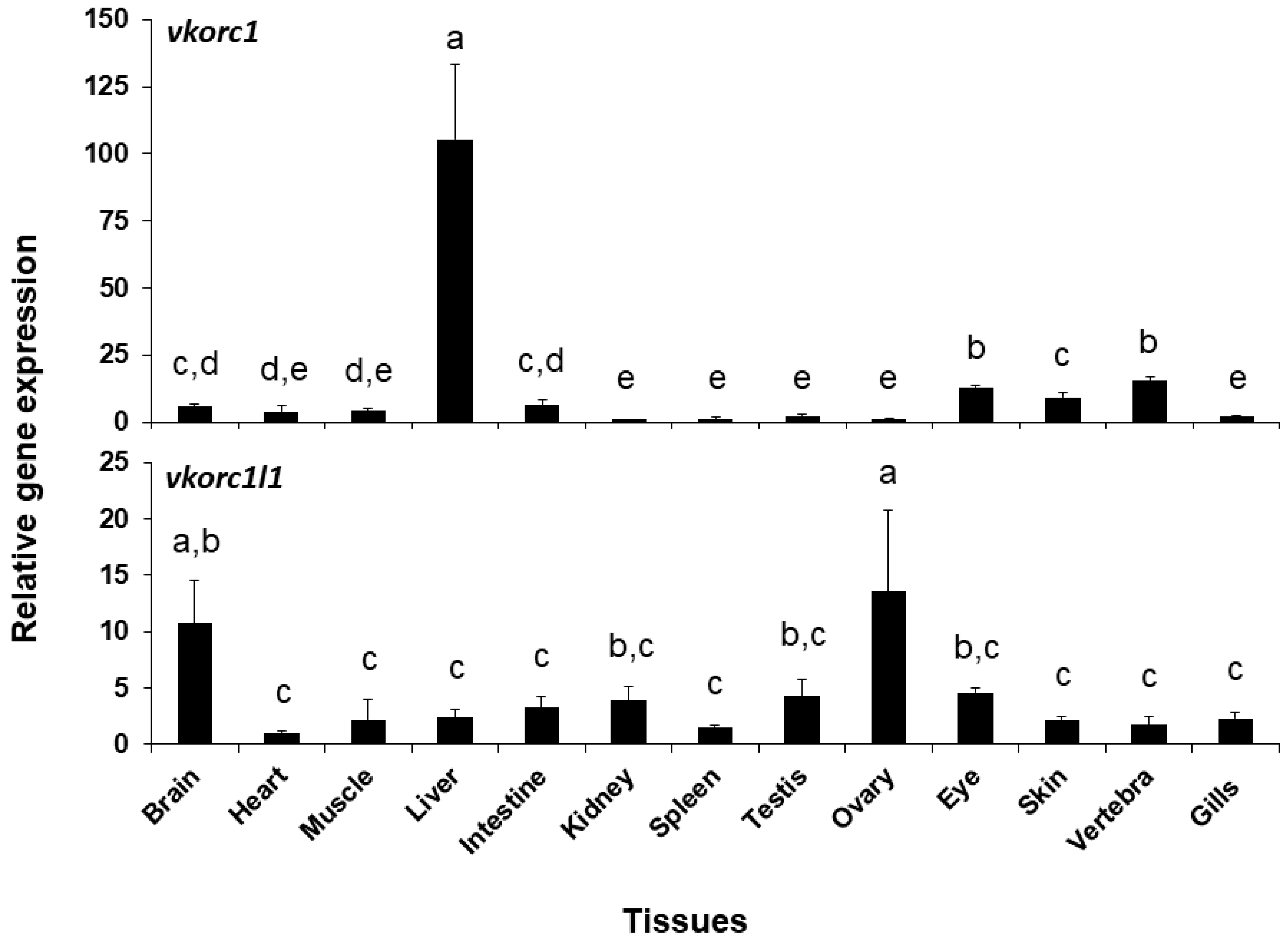
2.2. Expression of ssvkorc1 and ssvkorc1l1 During Development and in Adult Tissues
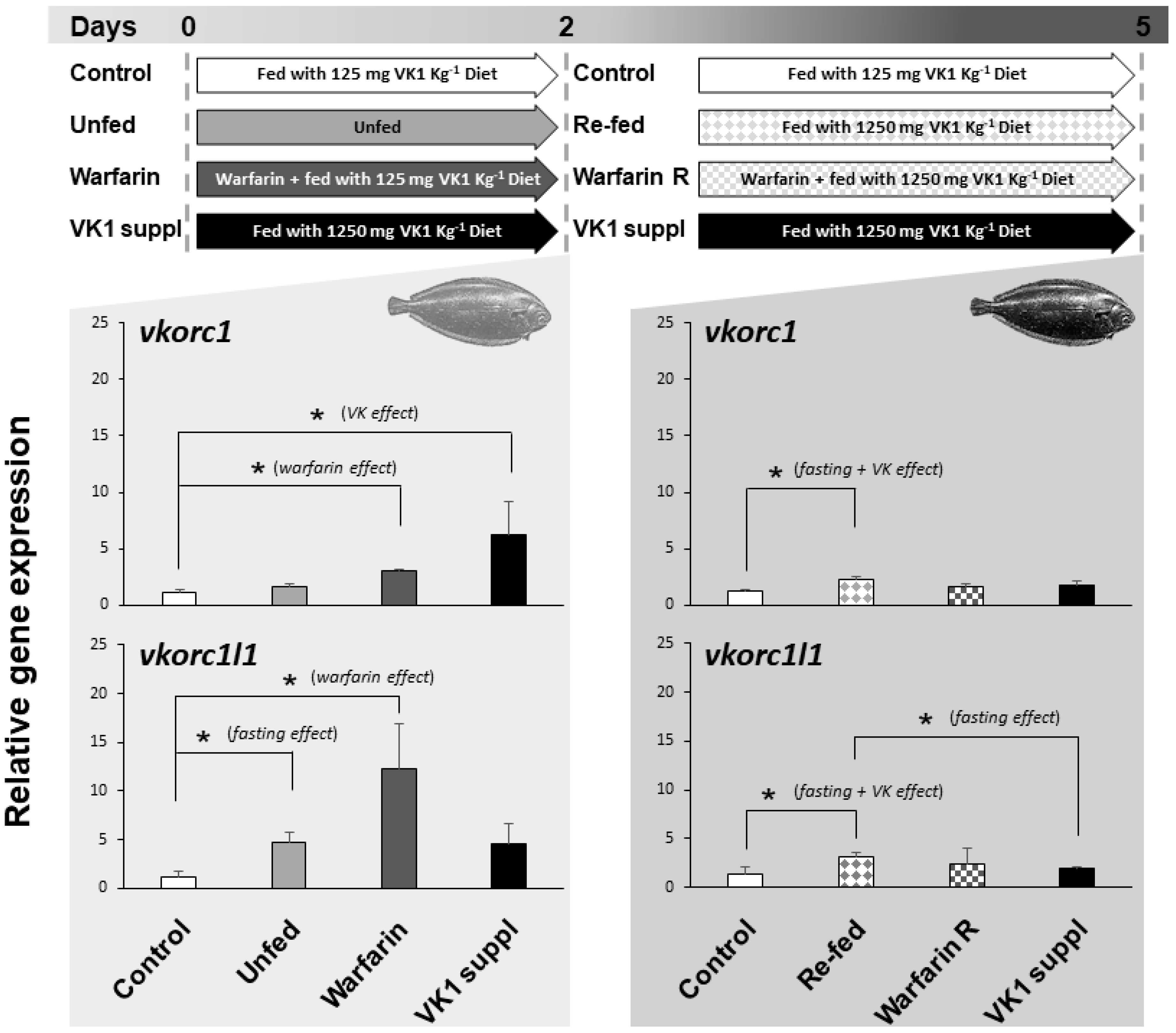
2.3. Expression of ssvkorc1 and sskorc1l1 under Different Physiological Conditions
3. Discussion
3.1. ssvkorc1 and ssvkorc1l1 are Orthologous to Vertebrate Vkors
3.2. ssvkorc1 and ssvkorc1l1 Distinct Gene Expression Patterns May Reflect Their Functional Specialization
3.3. Expression of ssvkorc1 and ssvkorc1l1 is Differentially Regulated under Relevant Physiological Conditions
4. Materials and Methods
4.1. Ethics Statement
4.2. Rearing and Sampling Senegalese Sole Larvae and Juveniles
4.3. Maintenance of Adult Senegalese Sole and Tissues Sampling
4.4. Exposure of Senegalese Sole Juveniles to Different Physiological Conditions
4.5. RNA Isolation and Construction of Senegalese sole cDNA Library
4.6. cDNA Partial and Full-Length Amplification through 3′- and 5′-RACE
4.7. Sequences Collection and Phylogenetic Reconstruction
4.8. Multiple sequence Alignment and Construction of Sequence Logos
4.9. RNA Extraction and qPCR Amplification
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BGP | Bone gla protein |
| BLAST | Basic Local Alignment Search Tool |
| bp | base pairs |
| cDNA | Complementary Deoxyribonucleic acid |
| DW | dry weight |
| GCCX | γ-glutamyl carboxylase (Ggcx) |
| hpf | hours post-fertilization |
| JTT | Jones-Taylor-Thornton |
| MEGA | Molecular Evolutionary Genetics Analysis |
| MGP | Matrix gla protein |
| MK-4 | Menaquinone 4 |
| MK-7 | Menaquinone 7 |
| M-MLV | Moloney Murine Leukemia Virus |
| M-MLV | Moloney Murine Leukemia Virus |
| MUSCLE | MUltiple Sequence Comparison by Log-Expectation |
| no | number |
| PCR | Polymerase Chain Reaction |
| PSI-BLAST | Position Specific Iterated Basic Local Alignment Search Tool |
| PXR | Pregnane X receptor |
| qPCR | Quantitative polymerase chain reaction |
| RACE | Rapid Amplification of cDNA Ends |
| RNA | Ribonucleic acid |
| SNPs | Single nucleotide polymorphisms |
| UBQ | Ubiquitin |
| UBUAD1 | UbiA prenyltransferase domain-containing protein 1 |
| VK1 | Vitamin K1 - phylloquinone |
| VK2 | Vitamin K2 - menaquinone |
| VK3 | Vitamin K3 - menadione |
| VK | Vitamin K |
| VKDPs | Vitamin K dependent proteins |
| VKOR | Vitamin K epoxide reductase |
| VKORC1 | Vitamin K epoxide reductase complex subunit 1 |
| VKORC1L1 | Vitamin K epoxide reductase complex subunit 1 like protein 1 |
| VKORS | Vitamin K epoxide reductases |
References
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; Food and Agriculture Organization of the United Nation: Rome, Italy, 2018. [Google Scholar]
- Transforming our World: The 2030 Agenda for Sustainable Development. Sustainable Development Knowledge Platform. (n.d.). Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld/publication (accessed on 5 April 2020).
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M. Microalgal Biotechnology; IntechOpen: Halifax, NS, Canada, 2018; pp. 151–175. [Google Scholar]
- Romanelli, C.; Cooper, H.D.; de Souza Dias, B.F. The integration of biodiversity into One Health. Rev. Sci. Tech. Off. Int. Epiz. 2014, 33, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.K.; Smith, L.M.; Fulford, R.S.; de Jesus Crespo, R. Ecosystem Services and Global Ecology; IntechOpen: London, UK, 2018; pp. 145–168. [Google Scholar]
- Halver, J.E.; Hardy, R.W. Fish Nutrition, 3rd ed.; Academic Press: San Diego, CA, USA, 2003; pp. 1–824. [Google Scholar]
- Lall, S.P.; Dumas, A. Feed and Feeding Practices in Aquaculture; Woodhead Publishing: Oxford, UK, 2015; pp. 53–109. [Google Scholar]
- Holt, G.J. Larval Fish Nutrition; Wiley-Blackwell: Oxford, UK, 2011; pp. 1–435. [Google Scholar]
- Fernández, I.; Gavaia, P.; Darias, M.J.; Gisbert, E. Emerging Issues in Fish Larvae Research; Springer International Publishing: Cham, Switzerland, 2018; pp. 159–208. [Google Scholar]
- Krossøy, C.; Waagbø, R.; Ørnsrud, R. Vitamin K in fish nutrition. Aquac. Nutr. 2011, 17, 585–594. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Shearer, M.J.; Okano, T. Key Pathways and Regulators of Vitamin K Function and Intermediary Metabolism. Annu. Rev. Nutr. 2018, 38, 127–151. [Google Scholar] [CrossRef]
- Fernández, I.; Fernandes, J.M.O.; Roberto, V.P.; Kopp, M.; Oliveira, C.; Riesco, M.F.; Dias, J.; Cox, C.J.; Leonor Cancela, M.; Cabrita, E.; et al. Circulating small non-coding RNAs provide new insights into vitamin K nutrition and reproductive physiology in teleost fish. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 39–51. [Google Scholar] [CrossRef]
- Richard, N.; Fernández, I.; Wulff, T.; Hamre, K.; Cancela, L.; Conceicao, L.E.; Gavaia, P.J. Dietary supplementation with vitamin k affects transcriptome and proteome of Senegalese sole, improving larval performance and quality. Mar. Biotechnol. (N.Y.) 2014, 16, 522–537. [Google Scholar] [CrossRef]
- Fernández, I.; Santos, A.; Cancela, M.L.; Laize, V.; Gavaia, P.J. Warfarin, a potential pollutant in aquatic environment acting through Pxr signaling pathway and gamma-glutamyl carboxylation of vitamin K-dependent proteins. Environ. Pollut. 2014, 194, 86–95. [Google Scholar] [CrossRef]
- Cardeira, J.; Gavaia, P.J.; Fernández, I.; Cengiz, I.F.; Moreira-Silva, J.; Oliveira, J.M.; Reis, R.L.; Cancela, M.L.; Laize, V. Quantitative assessment of the regenerative and mineralogenic performances of the zebrafish caudal fin. Sci. Rep. 2016, 6, 39191. [Google Scholar] [CrossRef]
- Granadeiro, L.; Dirks, R.P.; Ortiz-Delgado, J.B.; Gavaia, P.J.; Sarasquete, C.; Laize, V.; Cancela, M.L.; Fernández, I. Warfarin-exposed zebrafish embryos resembles human warfarin embryopathy in a dose and developmental-time dependent manner—From molecular mechanisms to environmental concerns. Ecotoxicol. Environ. Saf. 2019, 181, 559–571. [Google Scholar] [CrossRef]
- Marques, C.; Roberto, V.P.; Granadeiro, L.; Trindade, M.; Gavaia, P.J.; Laize, V.; Cancela, M.L.; Fernández, I. The xenobiotic sensor PXR in a marine flatfish species (Solea senegalensis): Gene expression patterns and its regulation under different physiological conditions. Mar. Environ. Res. 2017, 130, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Sun, A.; Zhou, C.; Grun, F.; Errandi, J.; Romero, K.; Pham, H.; Inoue, S.; Mallick, S.; Lin, M.; et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J. Biol. Chem. 2003, 278, 43919–43927. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Casey, S.C.; Ito, M.; Urano, T.; Horie, K.; Ouchi, Y.; Kirchner, S.; Blumberg, B.; Inoue, S. Pregnane X receptor knockout mice display osteopenia with reduced bone formation and enhanced bone resorption. J. Endocrinol. 2010, 207, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Marinova, M.; Müller-Reible, C.; Watzka, M. Vitamin K; Academic Press: San Diego, CA, USA, 2008; pp. 35–62. [Google Scholar]
- Westhofen, P.; Watzka, M.; Marinova, M.; Hass, M.; Kirfel, G.; Muller, J.; Bevans, C.G.; Muller, C.R.; Oldenburg, J. Human vitamin K 2,3-epoxide reductase complex subunit 1-like 1 (VKORC1L1) mediates vitamin K-dependent intracellular antioxidant function. J. Biol. Chem. 2011, 286, 15085–15094. [Google Scholar] [CrossRef] [PubMed]
- Hammed, A.; Matagrin, B.; Spohn, G.; Prouillac, C.; Benoit, E.; Lattard, V. VKORC1L1, an enzyme rescuing the vitamin K 2,3-epoxide reductase activity in some extrahepatic tissues during anticoagulation therapy. J. Biol. Chem. 2013, 288, 28733–28742. [Google Scholar] [CrossRef]
- Rishavy, M.A.; Hallgren, K.W.; Wilson, L.A.; Usubalieva, A.; Runge, K.W.; Berkner, K.L. The vitamin K oxidoreductase is a multimer that efficiently reduces vitamin K epoxide to hydroquinone to allow vitamin K-dependent protein carboxylation. J. Biol. Chem. 2013, 288, 31556–31566. [Google Scholar] [CrossRef]
- Oldenburg, J.; Watzka, M.; Bevans, C.G. VKORC1 and VKORC1L1: Why do Vertebrates Have Two Vitamin K 2,3-Epoxide Reductases? Nutrients 2015, 7, 6250–6280. [Google Scholar] [CrossRef]
- Lacombe, J.; Rishavy, M.A.; Berkner, K.L.; Ferron, M. VKOR paralog VKORC1L1 supports vitamin K-dependent protein carboxylation in vivo. JCI Insight 2018, 3, 1–11. [Google Scholar] [CrossRef]
- Lacombe, J.; Ferron, M. VKORC1L1, An Enzyme Mediating the Effect of Vitamin K in Liver and Extrahepatic Tissues. Nutrients 2018, 10, 970. [Google Scholar] [CrossRef]
- Campinho, M.A.; Silva, N.; Martins, G.G.; Anjos, L.; Florindo, C.; Roman-Padilla, J.; Garcia-Cegarra, A.; Louro, B.; Manchado, M.; Power, D.M. A thyroid hormone regulated asymmetric responsive centre is correlated with eye migration during flatfish metamorphosis. Sci. Rep. 2018, 8, 12267. [Google Scholar] [CrossRef]
- Louro, B.; Marques, J.P.; Manchado, M.; Power, D.M.; Campinho, M.A. Sole head transcriptomics reveals a coordinated developmental program during metamorphosis. Genomics 2020, 112, 592–602. [Google Scholar] [CrossRef]
- Gonçalves, C.; Martins, M.; Diniz, M.S.; Costa, M.H.; Caeiro, S.; Costa, P.M. May sediment contamination be xenoestrogenic to benthic fish? A case study with Solea senegalensis. Mar. Environ. Res. 2014, 99, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Solé, M.; Mananos, E.; Blazquez, M. Vitellogenin, sex steroid levels and gonadal biomarkers in wild Solea solea and Solea senegalensis from NW Mediterranean fishing grounds. Mar. Environ. Res. 2016, 117, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Aragão, C.; Cabrita, E.; Conceição, L.E.C.; Constenla, M.; Costas, B.; Dias, J.; Duncan, N.; Engrola, S.; Estevez, A.; et al. New developments and biological insights into the farming of Solea senegalensis reinforcing its aquaculture potential. Rev. Aquac. 2016, 8, 227–263. [Google Scholar] [CrossRef]
- Muñoz-Cueto, J.A.; Mañanós-Sánchez, E.; Sánchez-Vázquez, F.J. The Biology of Sole; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–404. [Google Scholar]
- Anguis, V.; Cañavate, J.P. Spawning of captive Senegal sole (Solea senegalensis) under a naturally fluctuating temperature regime. Aquaculture 2005, 243, 133–145. [Google Scholar] [CrossRef]
- Carazo, I.; Chereguini, O.; Martín, I.; Huntingford, F.; Duncan, N. Reproductive ethogram and mate selection in captive wild Senegalese sole (Solea senegalensis). Span. J. Agric. Res. 2016, 14, 6. [Google Scholar] [CrossRef]
- Chauvigne, F.; Fatsini, E.; Duncan, N.; Olle, J.; Zanuy, S.; Gomez, A.; Cerda, J. Plasma levels of follicle-stimulating and luteinizing hormones during the reproductive cycle of wild and cultured Senegalese sole (Solea senegalensis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 191, 35–43. [Google Scholar] [CrossRef]
- Riesco, M.F.; Valcarce, D.G.; Martinez-Vazquez, J.M.; Martin, I.; Calderon-Garcia, A.A.; Gonzalez-Nunez, V.; Robles, V. Male reproductive dysfunction in Solea senegalensis: New insights into an unsolved question. Reprod. Fertil. Dev. 2019, 31, 1104–1115. [Google Scholar] [CrossRef]
- Gavaia, P.J.; Dinis, M.T.; Cancela, M.L. Osteological development and abnormalities of the vertebral column and caudal skeleton in larval and juvenile stages of hatchery-reared Senegal sole (Solea senegalensis). Aquaculture 2002, 211, 305–323. [Google Scholar] [CrossRef]
- Fernández, I.; Pimentel, M.S.; Ortiz-Delgado, J.B.; Hontoria, F.; Sarasquete, C.; Estévez, A.; Zambonino-Infante, J.L.; Gisbert, E. Effect of dietary vitamin A on Senegalese sole (Solea senegalensis) skeletogenesis and larval quality. Aquaculture 2009, 295, 250–265. [Google Scholar] [CrossRef]
- Fernández, I.; Gisbert, E. Senegalese sole bone tissue originated from chondral ossification is more sensitive than dermal bone to high vitamin A content in enrichedArtemia. J. Appl. Ichthyol. 2010, 26, 344–349. [Google Scholar] [CrossRef]
- Losada, A.P.; de Azevedo, A.M.; Barreiro, A.; Barreiro, J.D.; Ferreiro, I.; Riaza, A.; Quiroga, M.I.; Vázquez, S. Skeletal malformations in Senegalese sole (Solea senegalensis Kaup, 1858): Gross morphology and radiographic correlation. J. Appl. Ichthyol. 2014, 30, 804–808. [Google Scholar] [CrossRef]
- De Azevedo, A.M.; Losada, A.P.; Ferreiro, I.; Riaza, A.; Vazquez, S.; Quiroga, M.I. New insight on vertebral anomalies in cultured Senegalese sole (Solea senegalensis, Kaup) at early stages of development. J. Fish Dis. 2017, 40, 987–1000. [Google Scholar] [CrossRef] [PubMed]
- Fernández, I.; Ortiz-Delgado, J.B.; Darias, M.J.; Hontoria, F.; Andree, K.B.; Manchado, M.; Sarasquete, C.; Gisbert, E. Vitamin A Affects Flatfish Development in a Thyroid Hormone Signaling and Metamorphic Stage Dependent Manner. Front. Physiol. 2017, 8, 458. [Google Scholar] [CrossRef]
- Fernández, I.; Granadeiro, L.; Darias, M.J.; Gavaia, P.J.; Andree, K.B.; Gisbert, E. Solea senegalensis skeletal ossification and gene expression patterns during metamorphosis: New clues on the onset of skeletal deformities during larval to juvenile transition. Aquaculture 2018, 496, 153–165. [Google Scholar] [CrossRef]
- Geffen, A.J.; van der Veer, H.W.; Nash, R.D.M. The cost of metamorphosis in flatfishes. J. Sea Res. 2007, 58, 35–45. [Google Scholar] [CrossRef]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Zhu, A.; Sun, H.; Raymond, R.M., Jr.; Furie, B.C.; Furie, B.; Bronstein, M.; Kaufman, R.J.; Westrick, R.; Ginsburg, D. Fatal hemorrhage in mice lacking gamma-glutamyl carboxylase. Blood 2007, 109, 5270–5275. [Google Scholar] [CrossRef]
- Berkner, K.L. Vitamin K; Academic Press: San Diego, CA, USA, 2008; pp. 131–156. [Google Scholar]
- Sidorova, Y.A.; Perepechaeva, M.L.; Pivovarova, E.N.; Markel, A.L.; Lyakhovich, V.V.; Grishanova, A.Y. Menadione Suppresses Benzo(alpha)pyrene-Induced Activation of Cytochromes P450 1A: Insights into a Possible Molecular Mechanism. PLoS ONE 2016, 11, e0155135. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K, an emerging nutrient in brain function. Biofactors 2012, 38, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Ferron, M. The contribution of bone to whole-organism physiology. Nature 2012, 481, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Chouet, J.; Ferland, G.; Feart, C.; Rolland, Y.; Presse, N.; Boucher, K.; Barberger-Gateau, P.; Beauchet, O.; Annweiler, C. Dietary Vitamin K Intake Is Associated with Cognition and Behaviour among Geriatric Patients: The CLIP Study. Nutrients 2015, 7, 6739–6750. [Google Scholar] [CrossRef] [PubMed]
- Soutif-Veillon, A.; Ferland, G.; Rolland, Y.; Presse, N.; Boucher, K.; Feart, C.; Annweiler, C. Increased dietary vitamin K intake is associated with less severe subjective memory complaint among older adults. Maturitas 2016, 93, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Suhara, Y. New Aspects of Vitamin K Research with Synthetic Ligands: Transcriptional Activity via SXR and Neural Differentiation Activity. Int. J. Mol. Sci. 2019, 20, 3006. [Google Scholar] [CrossRef]
- Schurgers, L.J.; Uitto, J.; Reutelingsperger, C.P. Vitamin K-dependent carboxylation of matrix Gla-protein: A crucial switch to control ectopic mineralization. Trends Mol. Med. 2013, 19, 217–226. [Google Scholar] [CrossRef]
- Nowak, J.K.; Grzybowska-Chlebowczyk, U.; Landowski, P.; Szaflarska-Poplawska, A.; Klincewicz, B.; Adamczak, D.; Banasiewicz, T.; Plawski, A.; Walkowiak, J. Prevalence and correlates of vitamin K deficiency in children with inflammatory bowel disease. Sci. Rep. 2014, 4, 4768. [Google Scholar] [CrossRef]
- Scheiber, D.; Veulemans, V.; Horn, P.; Chatrou, M.L.; Potthoff, S.A.; Kelm, M.; Schurgers, L.J.; Westenfeld, R. High-Dose Menaquinone-7 Supplementation Reduces Cardiovascular Calcification in a Murine Model of Extraosseous Calcification. Nutrients 2015, 7, 6991–7011. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Ershadifar, S.; Moghadam, M.M.; Sheibani, N. Vitamins and regulation of angiogenesis: [A, B1, B2, B3, B6, B9, B12, C, D, E, K]. J. Funct. Foods 2017, 38, 180–196. [Google Scholar] [CrossRef]
- Shirakawa, H.; Ohsaki, Y.; Minegishi, Y.; Takumi, N.; Ohinata, K.; Furukawa, Y.; Mizutani, T.; Komai, M. Vitamin K deficiency reduces testosterone production in the testis through down-regulation of the Cyp11a a cholesterol side chain cleavage enzyme in rats. Biochim. Biophys. Acta 2006, 1760, 1482–1488. [Google Scholar] [CrossRef]
- Ito, A.; Shirakawa, H.; Takumi, N.; Minegishi, Y.; Ohashi, A.; Howlader, Z.H.; Ohsaki, Y.; Sato, T.; Goto, T.; Komai, M. Menaquinone-4 enhances testosterone production in rats and testis-derived tumor cells. Lipids Health Dis. 2011, 10, 158. [Google Scholar] [CrossRef] [PubMed]
- Harshman, S.G.; Fu, X.; Karl, J.P.; Barger, K.; Lamon-Fava, S.; Kuliopulos, A.; Greenberg, A.S.; Smith, D.; Shen, X.; Booth, S.L. Tissue Concentrations of Vitamin K and Expression of Key Enzymes of Vitamin K Metabolism Are Influenced by Sex and Diet but Not Housing in C57Bl6 Mice. J. Nutr. 2016, 146, 1521–1527. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, W.; Lin, S.; Xu, W.; Mai, K. Effects of dietary vitamin K on growth performances, blood coagulation time and menaquinone-4 (MK-4) concentration in tissues of juvenile large yellow croakerPseudosciaena crocea. Aquac. Res. 2015, 46, 1269–1275. [Google Scholar] [CrossRef]
- Fernández, I.; Vijayakumar, P.; Marques, C.; Cancela, M.L.; Gavaia, P.J.; Laize, V. Zebrafish Vitamin K epoxide reductases: Expression in vivo, along extracellular matrix mineralization and under phylloquinone and warfarin in vitro exposure. Fish Physiol. Biochem. 2015, 41, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Bevans, C.G.; Krettler, C.; Reinhart, C.; Watzka, M.; Oldenburg, J. Phylogeny of the Vitamin K 2,3-Epoxide Reductase (VKOR) Family and Evolutionary Relationship to the Disulfide Bond Formation Protein B (DsbB) Family. Nutrients 2015, 7, 6224–6249. [Google Scholar] [CrossRef] [PubMed]
- Harshman, S.G.; Saltzman, E.; Booth, S.L. Vitamin K: Dietary intake and requirements in different clinical conditions. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Shea, M.K.; Booth, S.L. Concepts and Controversies in Evaluating Vitamin K Status in Population-Based Studies. Nutrients 2016, 8, 8. [Google Scholar] [CrossRef]
- Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Dietary reference values for vitamin K. EFSA J. 2017, 15, 4780. [Google Scholar] [CrossRef]
- Goodstadt, L.; Ponting, C.P. Vitamin K epoxide reductase: Homology, active site and catalytic mechanism. Trends Biochem. Sci. 2004, 29, 289–292. [Google Scholar] [CrossRef]
- Chatron, N.; Hammed, A.; Benoit, E.; Lattard, V. Structural Insights into Phylloquinone (Vitamin K1), Menaquinone (MK4, MK7), and Menadione (Vitamin K3) Binding to VKORC1. Nutrients 2019, 11, 67. [Google Scholar] [CrossRef]
- Li, T.; Lange, L.A.; Li, X.; Susswein, L.; Bryant, B.; Malone, R.; Lange, E.M.; Huang, T.-Y.; Stafford, D.W.; Evans, J.P. Polymorphisms in the VKORC1 gene are strongly associated with warfarin dosage requirements in patients receiving anticoagulation. J. Med. Genet. 2006, 43, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Czogalla, K.J.; Watzka, M.; Oldenburg, J. Structural Modeling Insights into Human VKORC1 Phenotypes. Nutrients 2015, 7, 6837–6851. [Google Scholar] [CrossRef]
- Rost, S.; Pelz, H.-J.; Menzel, S.; MacNicoll, A.D.; León, V.; Song, K.-J.; Jäkel, T.; Oldenburg, J.; Clemens, R.; Müller, C.R. Novel mutations in the VKORC1 gene of wild rats and mice—A response to 50 years of selection pressure by warfarin? BMC Genet. 2009, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.; Lynch, M.R.; Prescott, V.V.; Clegg, T.; Loughlin, M.; Hannon, B.; Moore, C.; Faulkner, R. VKORC1 sequence variants associated with resistance to anticoagulant rodenticides in Irish populations of Rattus norvegicus and Mus musculus domesticus. Sci. Rep. 2018, 8, 4535. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, S.M.M.; Morita, A.; Ikenaka, Y.; Kawai, Y.K.; Watanabe, K.P.; Ishii, C.; Mizukawa, H.; Yohannes, Y.B.; Saito, K.; Watanabe, Y.; et al. Avian interspecific differences in VKOR activity and inhibition: Insights from amino acid sequence and mRNA expression ratio of VKORC1 and VKORC1L1. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2020, 228, 108635. [Google Scholar] [CrossRef]
- Hall, J.G.; Pauli, R.M.; Wilson, K.M. Maternal and fetal sequelae of anticoagulation during pregnancy. Am. J. Med. 1980, 68, 122–140. [Google Scholar] [CrossRef]
- Hou, J.W. Fetal warfarin syndrome. Chang Gung Med. J. 2004, 27, 691–695. [Google Scholar]
- Mehndiratta, S.; Suneja, A.; Gupta, B.; Bhatt, S. Fetotoxicity of warfarin anticoagulation. Arch. Gynecol. Obstet. 2010, 282, 335–337. [Google Scholar] [CrossRef]
- Pedro Cañavate, J.; Zerolo, R.; Fernández-Díaz, C. Feeding and development of Senegal sole (Solea senegalensis) larvae reared in different photoperiods. Aquaculture 2006, 258, 368–377. [Google Scholar] [CrossRef]
- Caspers, M.; Czogalla, K.J.; Liphardt, K.; Muller, J.; Westhofen, P.; Watzka, M.; Oldenburg, J. Two enzymes catalyze vitamin K 2,3-epoxide reductase activity in mouse: VKORC1 is highly expressed in exocrine tissues while VKORC1L1 is highly expressed in brain. Thromb. Res. 2015, 135, 977–983. [Google Scholar] [CrossRef]
- Sanyaolu, A.O.; Oremosu, A.A.; Osinubi, A.A.; Vermeer, C.; Daramola, A.O. Warfarin-induced vitamin K deficiency affects spermatogenesis in Sprague-Dawley rats. Andrologia 2019, 51, e13416. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, M. The Effect of Parental Vitamin K Deficiency on Bone Structure in Mummichog Fundulus heteroclitus. J. World Aquac. Soc. 2004, 35, 366–371. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double Bonds beyond Coagulation Insights into Differences between Vitamin K1 and K2 in Health and Disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef] [PubMed]
- Gijsbers Birgit, L.M.G.; Jie Kon-Siong, G.; Vermeer, C. Effect of food composition on vitamin K absorption in human volunteers. Br. J. Nutr. 1996, 76, 223–229. [Google Scholar] [CrossRef]
- Koivu-Tikkanen Terhi, J.; Schurgers Leon, J.; Thijssen Henk, H.W.; Vermeer, C. Intestinal, hepatic, and circulating vitamin K levels at low and high intakes of vitamin K in rats. Br. J. Nutr. 2000, 83, 185–190. [Google Scholar] [CrossRef][Green Version]
- Spronk, H.M.; Soute, B.A.; Schurgers, L.J.; Thijssen, H.H.; De Mey, J.G.; Vermeer, C. Tissue-specific utilization of menaquinone-4 results in the prevention of arterial calcification in warfarin-treated rats. J. Vasc. Res. 2003, 40, 531–537. [Google Scholar] [CrossRef]
- Engrola, S.; Conceição, L.E.C.; Gavaia, P.J.; Cancela, M.L.; Dinis, M.T. Effect of pre-weaning feeding regime on weaning performance of Senegalese sole, Solea senegalensis (Kaup, 1858). Isr. J. Aquac. 2005, 57, 10–18. [Google Scholar]
- Bjørklund, G.; Svanberg, E.; Dadar, M.; Card, D.J.; Chirumbolo, S.; Harrington, D.J.; Aaseth, J. The role of matrix gla protein (MGP) in vascular calcification. Curr. Med. Chem. 2020, 27, 1647–1660. [Google Scholar] [CrossRef]
- Boskey Adele, L.; Gadaleta, S.; Gundberg, C.; Doty, S.B.; Ducy, P.; Karsenty, G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 1998, 23, 187–196. [Google Scholar] [CrossRef]
- Perez-Jimenez, A.; Cardenete, G.; Hidalgo, M.C.; Garcia-Alcazar, A.; Abellan, E.; Morales, A.E. Metabolic adjustments of Dentex dentex to prolonged starvation and refeeding. Fish Physiol. Biochem. 2012, 38, 1145–1157. [Google Scholar] [CrossRef]
- Bevans, C.G.; Krettler, C.; Reinhart, C.; Tran, H.; Kossmann, K.; Watzka, M.; Oldenburg, J. Determination of the warfarin inhibition constant Ki for vitamin K 2,3-epoxide reductase complex subunit-1 (VKORC1) using an in vitro DTT-driven assay. Biochim. Biophys. Acta 2013, 1830, 4202–4210. [Google Scholar] [CrossRef]
- Watanabe, K.P.; Saengtienchai, A.; Tanaka, K.D.; Ikenaka, Y.; Ishizuka, M. Comparison of warfarin sensitivity between rat and bird species. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2010, 152, 114–119. [Google Scholar] [CrossRef]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reprod. Toxicol. 2012, 33, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Lao, W.; Gan, J. Enantioselective degradation of warfarin in soils. Chirality 2012, 24, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Regnery, J.; Friesen, A.; Geduhn, A.; Göckener, B.; Kotthoff, M.; Parrhysius, P.; Petersohn, E.; Reifferscheid, G.; Schmolz, E.; Schulz, R.S.; et al. Rating the risks of anticoagulant rodenticides in the aquatic environment: A review. Environ. Chem. Lett. 2018, 17, 215–240. [Google Scholar] [CrossRef]
- Kotthoff, M.; Rudel, H.; Jurling, H.; Severin, K.; Hennecke, S.; Friesen, A.; Koschorreck, J. First evidence of anticoagulant rodenticides in fish and suspended particulate matter: Spatial and temporal distribution in German freshwater aquatic systems. Environ. Sci. Pollut. Res. Int. 2019, 26, 7315–7325. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Notredame, C.; Higgins, D.G.; Heringa, J. T-Coffee: A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000, 302, 205–217. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.M.; Brenner, S.E. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Derveaux, S.; Vandesompele, J.; Hellemans, J. How to do successful gene expression analysis using real-time PCR. Methods 2010, 50, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Infante, C.; Matsuoka, M.P.; Asensio, E.; Canavate, J.P.; Reith, M.; Manchado, M. Selection of housekeeping genes for gene expression studies in larvae from flatfish using real-time PCR. BMC Mol. Biol. 2008, 9, 28. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Component | 5’ to 3’ Nucleotide Sequences | Amplicon Size (bp) | Target Sequence |
|---|---|---|---|---|
| SsF1VKORC1L1-A_deg | Forward | agtgttcrgstcmaggtgggg | - | |
| SsR1VKORC1L1-A_deg | Reverse | aygtggtgaygcagatgaygc | - | |
| SsF2VKORC1L1-A_deg | Forward | ygtggagagggaamadgcbcgg | - | |
| SsR2VKORC1L1-A_deg | Reverse | ratvagrgcvgccatygcac | - | |
| SsF1VKORC1L1-B_deg | Forward | ccmgattacmgggcgmtgtgcg | - | |
| SsR1VKORC1L1-B_deg | Reverse | rcagaccatrcagaartchc | - | |
| SsF2VKORC1L1-B_deg | Forward | tggggacgwggwtttgghytgg | - | |
| SsR2VKORC1L1-B_deg | Reverse | gcytywgamacccaggaggm | - | |
| SsF2VKORmass-marathon | Forward | ccaggtgaaaccacagcgagcccc | - | |
| SsR2VKORmass-marathon | Reverse | tcccccaggtcacacatcgccc | - | |
| SsF1VKORc1l1-marathon | Forward | ccaacagtgtctatgggattgcttt | - | |
| SsR1VKORc1l1-marathon | Reverse | ctgaaaggcataaaaagcaatccca | - | |
| SsF2VKORc1l1-marathon | Forward | acatccatcttgtcggtggtgggt | - | |
| SsR2VKORc1l1-marathon | Reverse | agaggatgtagcccaggtagagtga | - | |
| SsVKORC1l1Fw3-Maraton | Forward | cggtggtgggttcactctacctgggc | - | |
| SsVKORC1l1Rev3-marathon | Reverse | gcccaagagaccaaaacctcgacccca | - | |
| SsVKORC1l1Fw4-marathon | Forward | actactgcgtcatctgcatcaccac | - | |
| SsVKORC1/massFw3-marathon | Forward | tgggggatttctgcgtggtctgcgt | - | |
| SsVKORC1/massRev3-marathon | Reverse | aggctgttgggctggttcagagggt | - | |
| SsVKORC1Fw2-qpcr | Forward | aaaccacagcgagcccctcc | 245 | KC108910 |
| SsVKORC1Rev2-qpcr | Reverse | accgtttcattcatcaacaccacct | ||
| SsVKORC1l1Fw2-qpcr | Forward | tggggttgtttcacgggcga | 91 | KC108911 |
| SsVKORC1l1Rev2-qpcr | Reverse | ggacactctcaggacgggcg |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beato, S.; Marques, C.; Laizé, V.; Gavaia, P.J.; Fernández, I. New Insights on Vitamin K Metabolism in Senegalese sole (Solea senegalensis) Based on Ontogenetic and Tissue-Specific Vitamin K Epoxide Reductase Molecular Data. Int. J. Mol. Sci. 2020, 21, 3489. https://doi.org/10.3390/ijms21103489
Beato S, Marques C, Laizé V, Gavaia PJ, Fernández I. New Insights on Vitamin K Metabolism in Senegalese sole (Solea senegalensis) Based on Ontogenetic and Tissue-Specific Vitamin K Epoxide Reductase Molecular Data. International Journal of Molecular Sciences. 2020; 21(10):3489. https://doi.org/10.3390/ijms21103489
Chicago/Turabian StyleBeato, Silvia, Carlos Marques, Vincent Laizé, Paulo J. Gavaia, and Ignacio Fernández. 2020. "New Insights on Vitamin K Metabolism in Senegalese sole (Solea senegalensis) Based on Ontogenetic and Tissue-Specific Vitamin K Epoxide Reductase Molecular Data" International Journal of Molecular Sciences 21, no. 10: 3489. https://doi.org/10.3390/ijms21103489
APA StyleBeato, S., Marques, C., Laizé, V., Gavaia, P. J., & Fernández, I. (2020). New Insights on Vitamin K Metabolism in Senegalese sole (Solea senegalensis) Based on Ontogenetic and Tissue-Specific Vitamin K Epoxide Reductase Molecular Data. International Journal of Molecular Sciences, 21(10), 3489. https://doi.org/10.3390/ijms21103489








