Targeting Actomyosin Contractility Suppresses Malignant Phenotypes of Acute Myeloid Leukemia Cells
Abstract
1. Introduction
2. Results
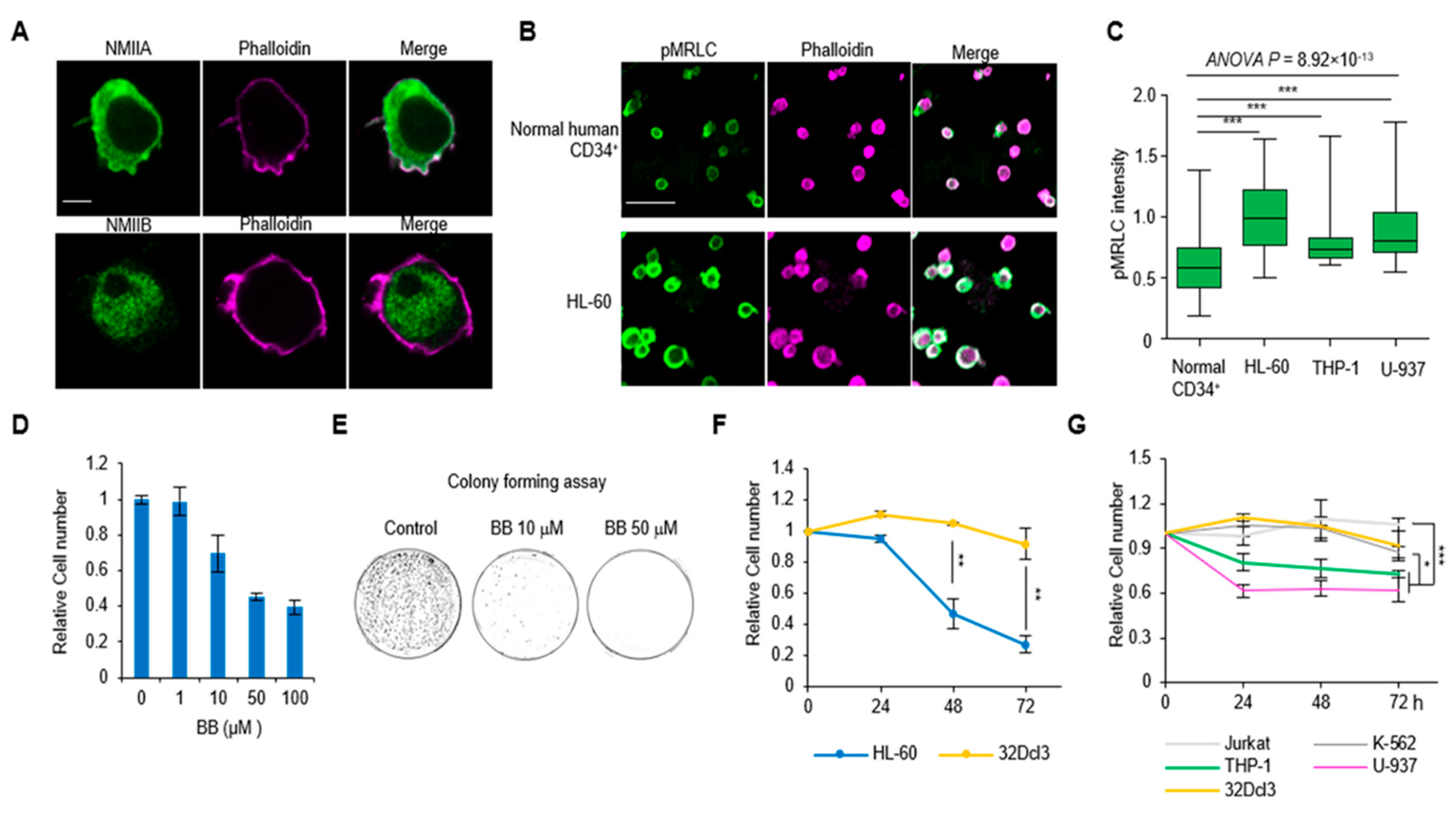
2.1. Hypercontractility of Actomyosin Complex in AML Cells
2.2. Perturbation of Actomyosin Contractility Suppresses the Growth of AML Cells
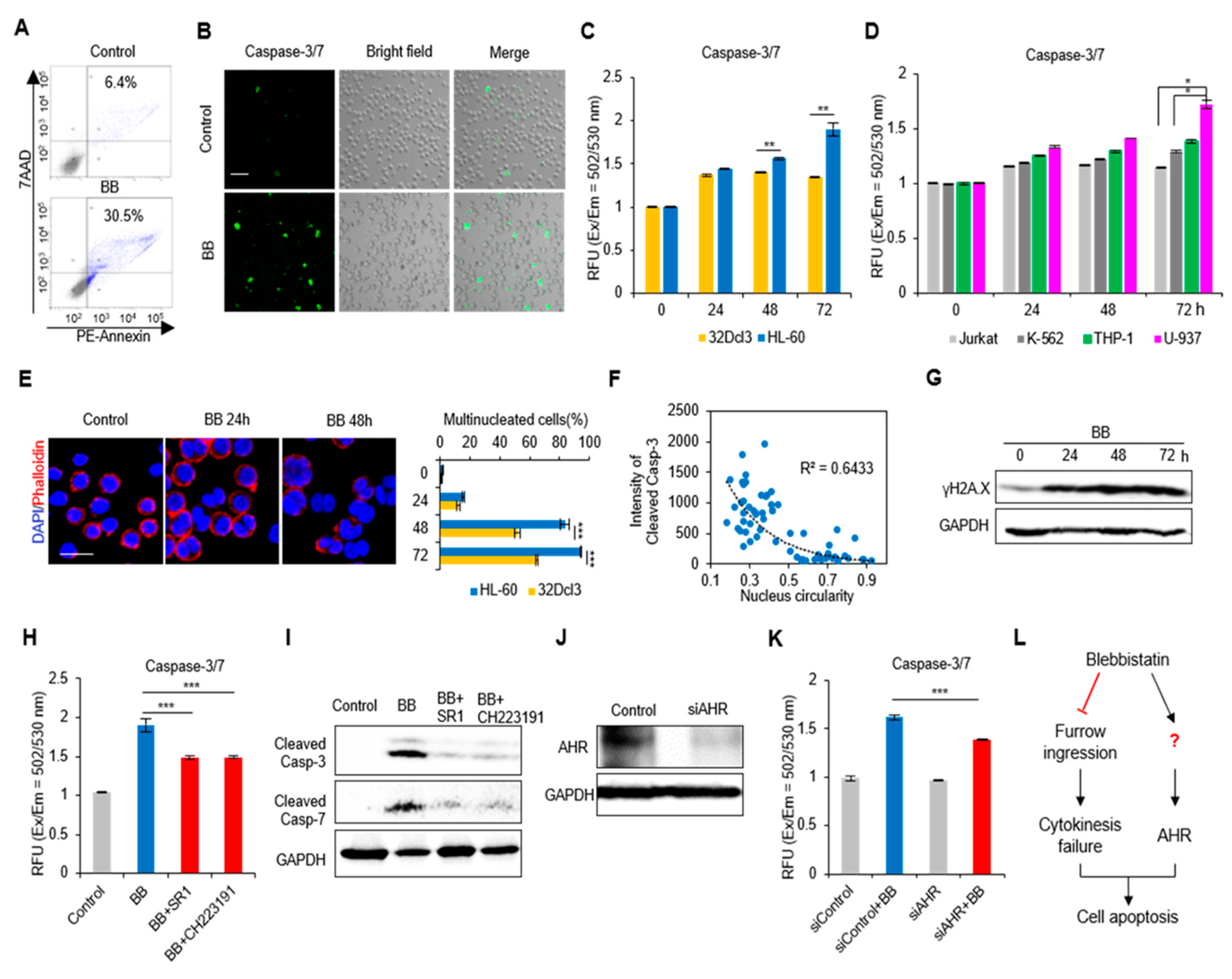
2.3. Perturbation of Actomyosin Contractility Enhances Apoptosis of AML Cells
2.4. Actomyosin Contractility Regulates Transcriptional Activities through the YAP/TAZ Pathway
2.5. Blebbistatin Treatment Suppresses Leukemia Progression with Enhanced Apoptosis
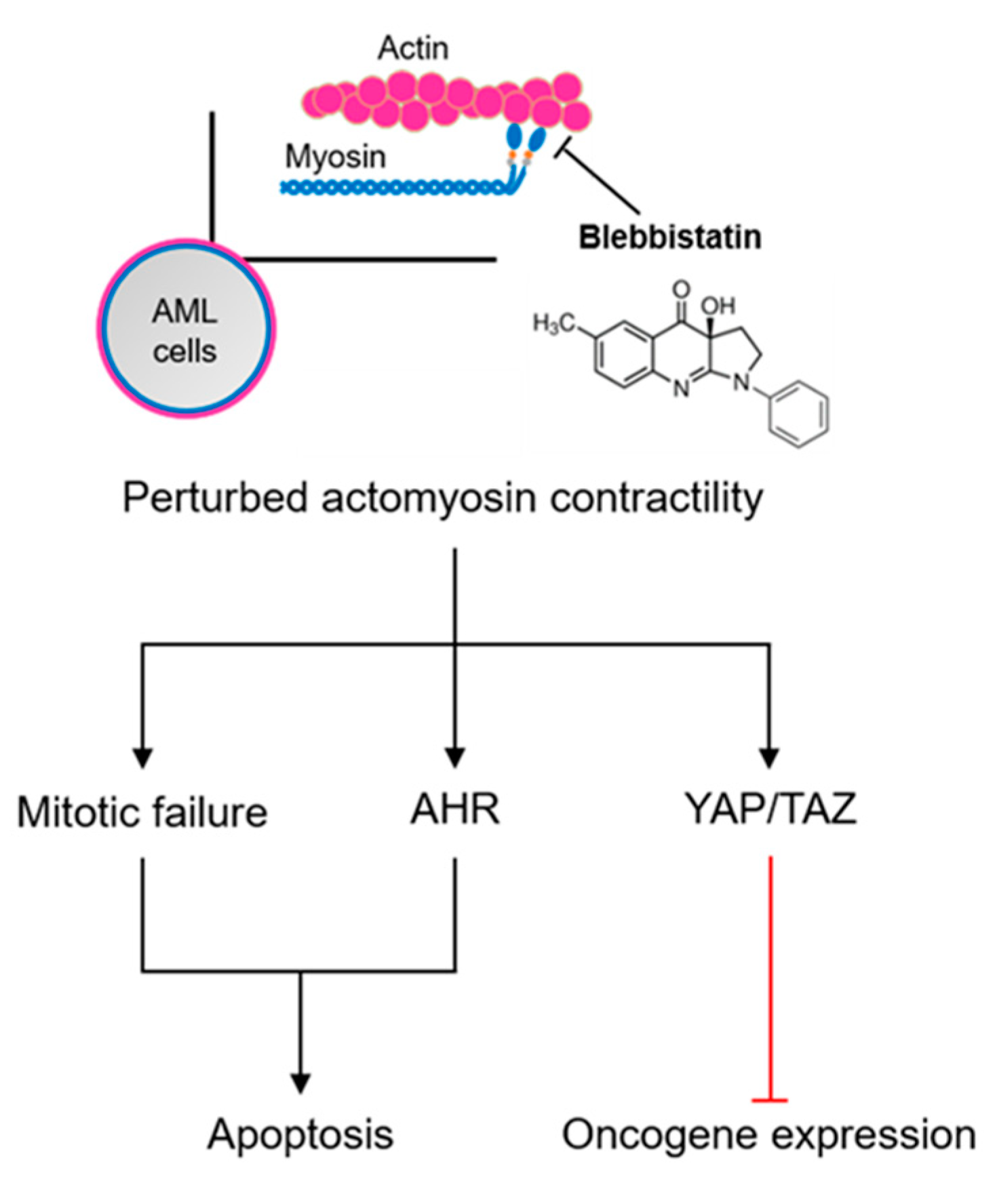
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. Cell Culture and Human Patient Samples
4.3. Cell Number Count
4.4. Colony Forming Assay
4.5. Immunofluorescence
4.6. Western Blotting
4.7. Caspase-3/7 Apoptosis Test
4.8. Flow Cytometry Analysis
4.9. Real-Time PCR and Transcriptome Sequencing
4.10. Small Interfering RNA
4.11. Cell Migration Test
4.12. Mice and Tumor Experiments
4.13. Histologic Analysis
4.14. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Murrell, M.; Oakes, P.W.; Lenz, M.; Gardel, M.L. Forcing cells into shape: The mechanics of actomyosin contractility. Nat. Rev. Mol. Cell Biol. 2015, 16, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Tsankova, A.; Pham, T.T.; Garcia, D.S.; Otte, F.; Cabernard, C. Cell polarity regulates biased myosin activity and dynamics during asymmetric cell division via Drosophila Rho kinase and protein kinase N. Dev. Cell 2017, 42, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Moreau, H.D.; Piel, M.; Voituriez, R.; Lennon-Dumenil, A.M. Integrating physical and molecular insights on immune cell migration. Trends Immunol. 2018, 39, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Zaidel-Bar, R.; Zhenhuan, G.; Luxenburg, C. The contractome—A systems view of actomyosin contractility in non-muscle cells. J. Cell Sci. 2015, 128, 2209–2217. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Harley, B.A. Marrow-inspired matrix cues rapidly affect early fate decisions of hematopoietic stem and progenitor cells. Sci. Adv. 2017, 3, e1600455. [Google Scholar] [CrossRef]
- Shin, J.W.; Buxboim, A.; Spinler, K.R.; Swift, J.; Christian, D.A.; Hunter, C.A.; Leon, C.; Gachet, C.; Dingal, P.C.; Ivanovska, I.L.; et al. Contractile forces sustain and polarize hematopoiesis from stem and progenitor cells. Cell Stem. Cell 2014, 14, 81–93. [Google Scholar] [CrossRef]
- Andzelm, M.M.; Chen, X.; Krzewski, K.; Orange, J.S.; Strominger, J.L. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J. Exp. Med. 2007, 204, 2285–2291. [Google Scholar] [CrossRef]
- Huse, M. Mechanical forces in the immune system. Nat. Rev. Immunol. 2017, 17, 679–690. [Google Scholar] [CrossRef]
- Shin, J.W.; Swift, J.; Spinler, K.R.; Discher, D.E. Myosin-II inhibition and soft 2D matrix maximize multinucleation and cellular projections typical of platelet-producing megakaryocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 11458–11463. [Google Scholar] [CrossRef]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell adhesion: Integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Rayment, I. The structural basis of the myosin ATPase activity. J. Biol. Chem. 1996, 271, 15850–15853. [Google Scholar] [CrossRef] [PubMed]
- Newell-Litwa, K.A.; Horwitz, R.; Lamers, M.L. Non-muscle myosin II in disease: Mechanisms and therapeutic opportunities. Dis. Model. Mech. 2015, 8, 1495–1515. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Cox, J.; Ostasiewicz, P.; Wisniewski, J.R.; Mann, M. Super-SILAC mix for quantitative proteomics of human tumor tissue. Nat. Methods 2010, 7, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, G.Y.; Soroceanu, L.; Manning, T.J., Jr.; Gladson, C.L.; Rosenfeld, S.S. Glioma migration can be blocked by nontoxic inhibitors of myosin II. Cancer Res. 1999, 59, 2076–2082. [Google Scholar]
- Jacobs, K.; Van Gele, M.; Forsyth, R.; Brochez, L.; Vanhoecke, B.; De Wever, O.; Bracke, M. P-cadherin counteracts myosin II-B function: Implications in melanoma progression. Mol. Cancer 2010, 9, 255. [Google Scholar] [CrossRef]
- Kaneko, K.; Satoh, K.; Masamune, A.; Satoh, A.; Shimosegawa, T. Myosin light chain kinase inhibitors can block invasion and adhesion of human pancreatic cancer cell lines. Pancreas 2002, 24, 34–41. [Google Scholar] [CrossRef]
- Wu, Q.; Sahasrabudhe, R.M.; Luo, L.Z.; Lewis, D.W.; Gollin, S.M.; Saunders, W.S. Deficiency in myosin light-chain phosphorylation causes cytokinesis failure and multipolarity in cancer cells. Oncogene 2010, 29, 4183–4193. [Google Scholar] [CrossRef]
- Khwaja, A.; Bjorkholm, M.; Gale, R.E.; Levine, R.L.; Jordan, C.T.; Ehninger, G.; Bloomfield, C.D.; Estey, E.; Burnett, A.; Cornelissen, J.J.; et al. Acute myeloid leukaemia. Nat. Rev. Dis. Primers 2016, 2, 16010. [Google Scholar] [CrossRef]
- Mali, R.S.; Ramdas, B.; Ma, P.; Shi, J.; Munugalavadla, V.; Sims, E.; Wei, L.; Vemula, S.; Nabinger, S.C.; Goodwin, C.B.; et al. Rho kinase regulates the survival and transformation of cells bearing oncogenic forms of KIT, FLT3, and BCR-ABL. Cancer Cell 2011, 20, 357–369. [Google Scholar] [CrossRef]
- Liu, L.; Li, G.; Li, Q.; Jin, Z.; Zhang, L.; Zhou, J.; Hu, X.; Zhou, T.; Chen, J.; Gao, N. Triptolide induces apoptosis in human leukemia cells through caspase-3-mediated ROCK1 activation and MLC phosphorylation. Cell Death Dis. 2013, 4, e941. [Google Scholar] [CrossRef] [PubMed]
- Makishima, M.; Honma, Y.; Hozumi, M.; Sampi, K.; Hattori, M.; Motoyoshi, K. Induction of differentiation of human leukemia cells by inhibitors of myosin light chain kinase. FEBS Lett. 1991, 287, 175–177. [Google Scholar] [CrossRef]
- Wigton, E.J.; Thompson, S.B.; Long, R.A.; Jacobelli, J. Myosin-IIA regulates leukemia engraftment and brain infiltration in a mouse model of acute lymphoblastic leukemia. J. Leukoc. Biol. 2016, 100, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.J. The HL-60 promyelocytic leukemia cell line: Proliferation, differentiation, and cellular oncogene expression. Blood 1987, 70, 1233–1244. [Google Scholar] [CrossRef] [PubMed]
- Bagger, F.O.; Kinalis, S.; Rapin, N. BloodSpot: A database of healthy and malignant haematopoiesis updated with purified and single cell mRNA sequencing profiles. Nucleic Acids Res. 2019, 47, D881–D885. [Google Scholar] [CrossRef] [PubMed]
- Allingham, J.S.; Smith, R.; Rayment, I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat. Struct. Mol. Biol. 2005, 12, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, J.S.; Sakakeeny, M.A.; Humphries, R.K.; Eaves, C.J.; Eckner, R.J. Demonstration of permanent factor-dependent multipotential (erythroid/neutrophil/basophil) hematopoietic progenitor cell lines. Proc. Natl. Acad. Sci. USA 1983, 80, 2931–2935. [Google Scholar] [CrossRef]
- Kagoya, Y.; Yoshimi, A.; Kataoka, K.; Nakagawa, M.; Kumano, K.; Arai, S.; Kobayashi, H.; Saito, T.; Iwakura, Y.; Kurokawa, M. Positive feedback between NF-kappaB and TNF-alpha promotes leukemia-initiating cell capacity. J. Clin. Investig. 2014, 124, 528–542. [Google Scholar] [CrossRef]
- Volk, A.; Li, J.; Xin, J.; You, D.; Zhang, J.; Liu, X.; Xiao, Y.; Breslin, P.; Li, Z.; Wei, W.; et al. Co-inhibition of NF-kappaB and JNK is synergistic in TNF-expressing human AML. J. Exp. Med. 2014, 211, 1093–1108. [Google Scholar] [CrossRef]
- Rulina, A.V.; Spirin, P.V.; Prassolov, V.S. Activated leukemic oncogenes AML1-ETO and c-kit: Role in development of acute myeloid leukemia and current approaches for their inhibition. Biochemistry (Moscow) 2010, 75, 1650–1666. [Google Scholar] [CrossRef]
- Song, J.H.; Kim, S.H.; Cho, D.; Lee, I.K.; Kim, H.J.; Kim, T.S. Enhanced invasiveness of drug-resistant acute myeloid leukemia cells through increased expression of matrix metalloproteinase-2. Int. J. Cancer 2009, 125, 1074–1081. [Google Scholar] [CrossRef]
- Frelin, C.; Imbert, V.; Griessinger, E.; Peyron, A.C.; Rochet, N.; Philip, P.; Dageville, C.; Sirvent, A.; Hummelsberger, M.; Berard, E.; et al. Targeting NF-kappaB activation via pharmacologic inhibition of IKK2-induced apoptosis of human acute myeloid leukemia cells. Blood 2005, 105, 804–811. [Google Scholar] [CrossRef]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar] [CrossRef]
- Clark, K.; Langeslag, M.; Figdor, C.G.; van Leeuwen, F.N. Myosin II and mechanotransduction: A balancing act. Trends Cell Biol. 2007, 17, 178–186. [Google Scholar] [CrossRef]
- Rath, N.; Olson, M.F. Rho-associated kinases in tumorigenesis: Re-considering ROCK inhibition for cancer therapy. EMBO Rep. 2012, 13, 900–908. [Google Scholar] [CrossRef]
- Colin-York, H.; Li, D.; Korobchevskaya, K.; Chang, V.T.; Betzig, E.; Eggeling, C.; Fritzsche, M. Cytoskeletal actin patterns shape mast cell activation. Commun. Biol. 2019, 2, 93. [Google Scholar] [CrossRef]
- Pecci, A.; Ma, X.; Savoia, A.; Adelstein, R.S. MYH9: Structure, functions and role of non-muscle myosin IIA in human disease. Gene 2018, 664, 152–167. [Google Scholar] [CrossRef]
- Ross, M.E.; Mahfouz, R.; Onciu, M.; Liu, H.C.; Zhou, X.; Song, G.; Shurtleff, S.A.; Pounds, S.; Cheng, C.; Ma, J.; et al. Gene expression profiling of pediatric acute myelogenous leukemia. Blood 2004, 104, 3679–3687. [Google Scholar] [CrossRef]
- Sedzinski, J.; Biro, M.; Oswald, A.; Tinevez, J.Y.; Salbreux, G.; Paluch, E. Polar actomyosin contractility destabilizes the position of the cytokinetic furrow. Nature 2011, 476, 462–466. [Google Scholar] [CrossRef]
- Ting, S.B.; Deneault, E.; Hope, K.; Cellot, S.; Chagraoui, J.; Mayotte, N.; Dorn, J.F.; Laverdure, J.P.; Harvey, M.; Hawkins, E.D.; et al. Asymmetric segregation and self-renewal of hematopoietic stem and progenitor cells with endocytic Ap2a2. Blood 2012, 119, 2510–2522. [Google Scholar] [CrossRef]
- Holst, J.; Watson, S.; Lord, M.S.; Eamegdool, S.S.; Bax, D.V.; Nivison-Smith, L.B.; Kondyurin, A.; Ma, L.; Oberhauser, A.F.; Weiss, A.S.; et al. Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat. Biotechnol. 2010, 28, 1123–1128. [Google Scholar] [CrossRef]
- Shin, J.W.; Swift, J.; Ivanovska, I.; Spinler, K.R.; Buxboim, A.; Discher, D.E. Mechanobiology of bone marrow stem cells: From myosin-II forces to compliance of matrix and nucleus in cell forms and fates. Differentiation 2013, 86, 77–86. [Google Scholar] [CrossRef]
- Straight, A.F.; Cheung, A.; Limouze, J.; Chen, I.; Westwood, N.J.; Sellers, J.R.; Mitchison, T.J. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science 2003, 299, 1743–1747. [Google Scholar] [CrossRef]
- Storchova, Z.; Pellman, D. From polyploidy to aneuploidy, genome instability and cancer. Nat. Rev. Mol. Cell Biol. 2004, 5, 45–54. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, Y.M.; Fan, X.; Shi, J.Y.; Wang, Q.R.; Yan, X.J.; Gu, Z.H.; Wang, Y.Y.; Chen, B.; Jiang, C.L.; et al. Gene mutation patterns and their prognostic impact in a cohort of 1185 patients with acute myeloid leukemia. Blood 2011, 118, 5593–5603. [Google Scholar] [CrossRef]
- Heo, S.K.; Noh, E.K.; Kim, J.Y.; Jeong, Y.K.; Jo, J.C.; Choi, Y.; Koh, S.; Baek, J.H.; Min, Y.J.; Kim, H. Targeting c-KIT (CD117) by dasatinib and radotinib promotes acute myeloid leukemia cell death. Sci. Rep. 2017, 7, 15278. [Google Scholar] [CrossRef]
- Guzman, M.L.; Neering, S.J.; Upchurch, D.; Grimes, B.; Howard, D.S.; Rizzieri, D.A.; Luger, S.M.; Jordan, C.T. Nuclear factor-kappaB is constitutively activated in primitive human acute myelogenous leukemia cells. Blood 2001, 98, 2301–2307. [Google Scholar] [CrossRef]
- Furukawa, K.T.; Yamashita, K.; Sakurai, N.; Ohno, S. The epithelial circumferential actin belt regulates YAP/TAZ through nucleocytoplasmic shuttling of Merlin. Cell Rep. 2017, 20, 1435–1447. [Google Scholar] [CrossRef]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Cottini, F.; Hideshima, T.; Xu, C.; Sattler, M.; Dori, M.; Agnelli, L.; ten Hacken, E.; Bertilaccio, M.T.; Antonini, E.; Neri, A.; et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat. Med. 2014, 20, 599–606. [Google Scholar] [CrossRef]
- Maruyama, J.; Inami, K.; Michishita, F.; Jiang, X.; Iwasa, H.; Nakagawa, K.; Ishigami-Yuasa, M.; Kagechika, H.; Miyamura, N.; Hirayama, J.; et al. Novel YAP1 Activator, Identified by Transcription-Based Functional Screen, Limits Multiple Myeloma Growth. Mol. Cancer Res. 2018, 16, 197–211. [Google Scholar] [CrossRef]
- Rauscher, A.A.; Gyimesi, M.; Kovacs, M.; Malnasi-Csizmadia, A. Targeting myosin by blebbistatin derivatives: Optimization and pharmacological potential. Trends Biochem. Sci. 2018, 43, 700–713. [Google Scholar] [CrossRef]
- Varkuti, B.H.; Kepiro, M.; Horvath, I.A.; Vegner, L.; Rati, S.; Zsigmond, A.; Hegyi, G.; Lenkei, Z.; Varga, M.; Malnasi-Csizmadia, A. A highly soluble, non-phototoxic, non-fluorescent blebbistatin derivative. Sci. Rep. 2016, 6, 26141. [Google Scholar] [CrossRef]
- Chon, H.J.; Lee, W.S.; Yang, H.; Kong, S.J.; Lee, N.K.; Moon, E.S.; Choi, J.; Han, E.C.; Kim, J.H.; Ahn, J.B.; et al. Tumor microenvironment remodeling by intratumoral oncolytic vaccinia virus enhances the efficacy of immune-checkpoint blockade. Clin. Cancer Res. 2019, 25, 1612–1623. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, F.; Kong, S.J.; Wang, L.; Choi, B.K.; Lee, H.; Kim, C.; Kim, J.M.; Park, K. Targeting Actomyosin Contractility Suppresses Malignant Phenotypes of Acute Myeloid Leukemia Cells. Int. J. Mol. Sci. 2020, 21, 3460. https://doi.org/10.3390/ijms21103460
Chang F, Kong SJ, Wang L, Choi BK, Lee H, Kim C, Kim JM, Park K. Targeting Actomyosin Contractility Suppresses Malignant Phenotypes of Acute Myeloid Leukemia Cells. International Journal of Molecular Sciences. 2020; 21(10):3460. https://doi.org/10.3390/ijms21103460
Chicago/Turabian StyleChang, Fengjiao, So Jung Kong, Lele Wang, Beom K. Choi, Hyewon Lee, Chan Kim, Jin Man Kim, and Kyungpyo Park. 2020. "Targeting Actomyosin Contractility Suppresses Malignant Phenotypes of Acute Myeloid Leukemia Cells" International Journal of Molecular Sciences 21, no. 10: 3460. https://doi.org/10.3390/ijms21103460
APA StyleChang, F., Kong, S. J., Wang, L., Choi, B. K., Lee, H., Kim, C., Kim, J. M., & Park, K. (2020). Targeting Actomyosin Contractility Suppresses Malignant Phenotypes of Acute Myeloid Leukemia Cells. International Journal of Molecular Sciences, 21(10), 3460. https://doi.org/10.3390/ijms21103460




