Impact of Aging on the 6-OHDA-Induced Rat Model of Parkinson’s Disease
Abstract
1. Introduction
2. Results
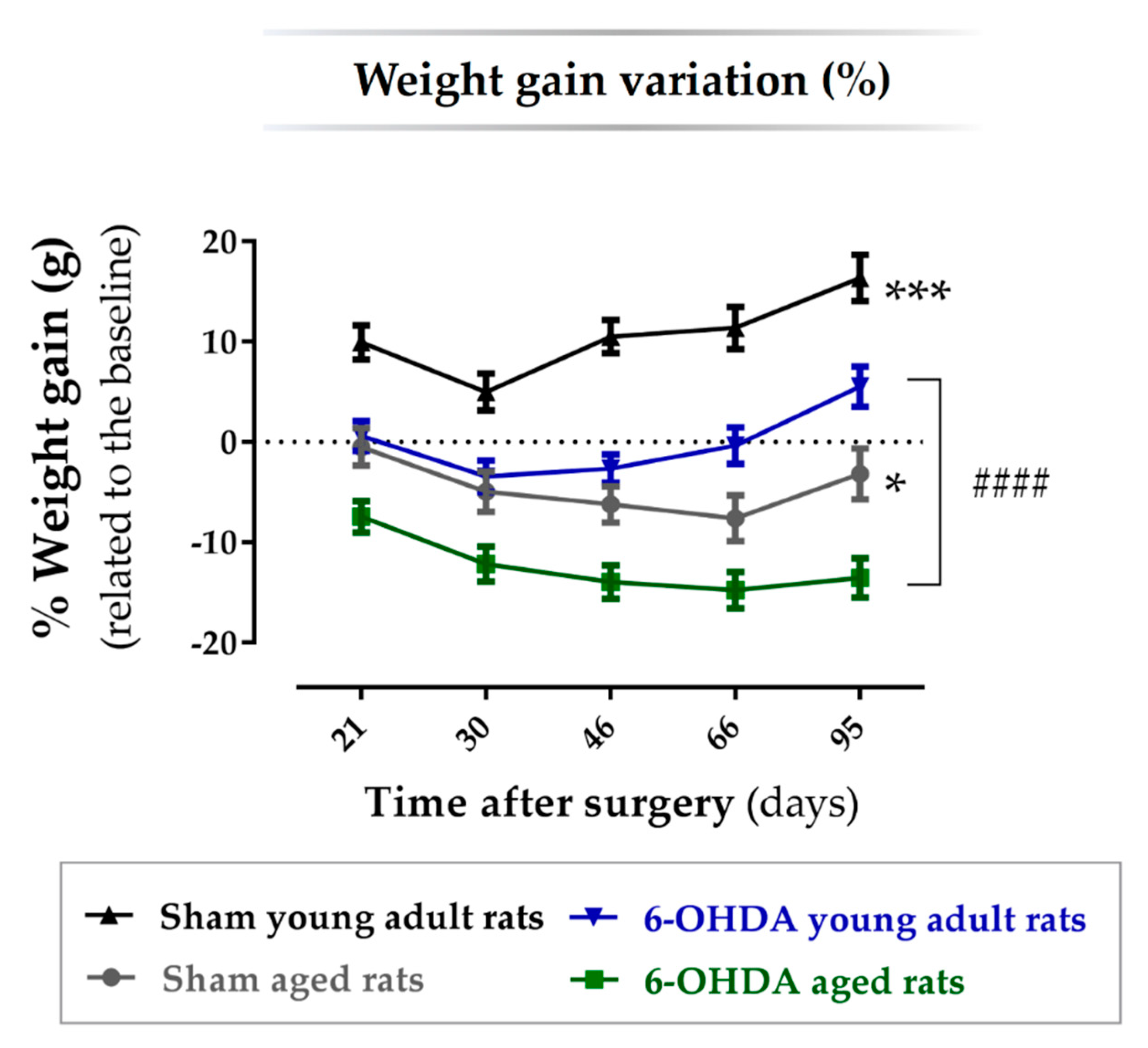
2.1. Animal Welfare Varied among Experimental Groups
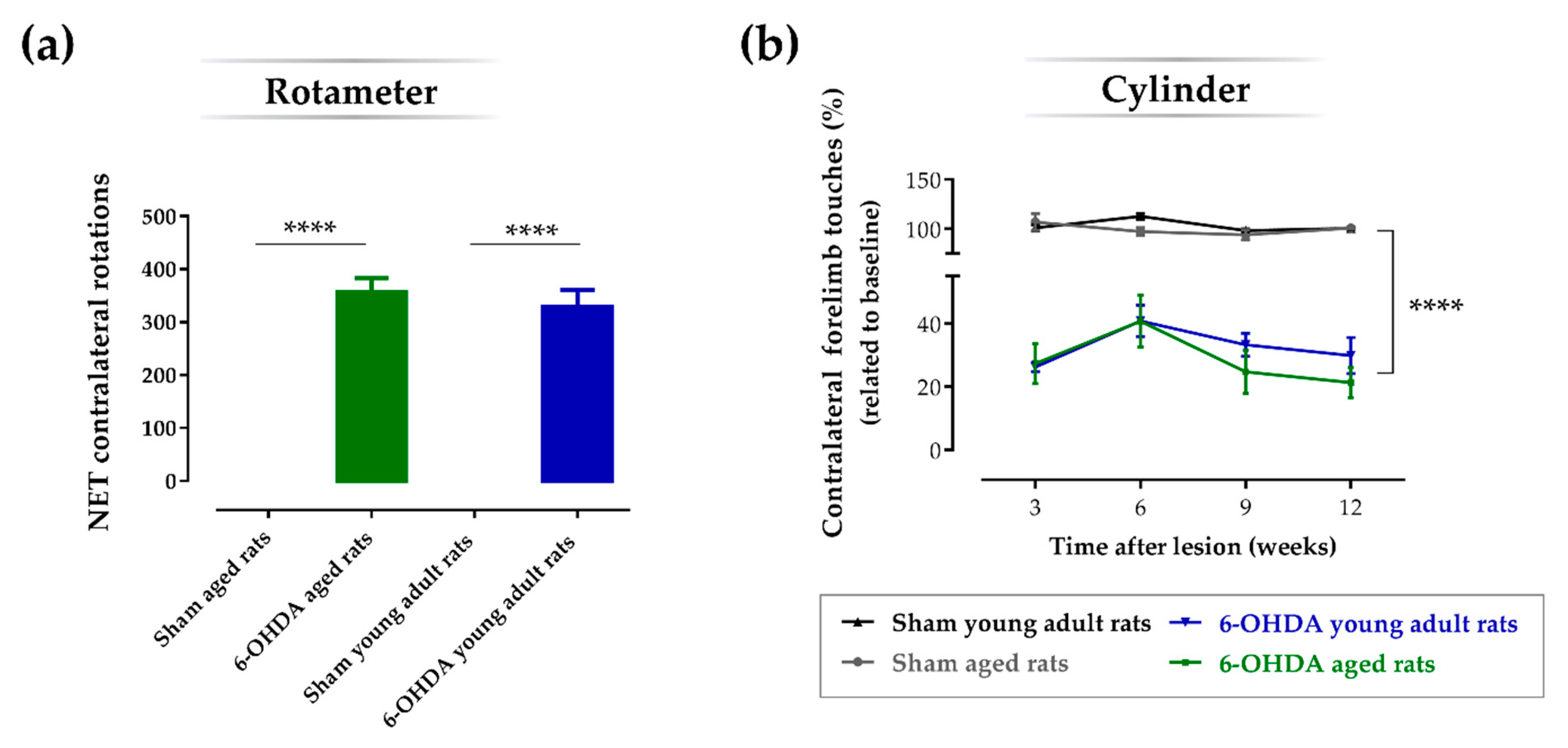
2.2. Aging Does Not Affect the Impact of the Lesion on the Turning Behavior and Degree of Forelimb Use Asymmetry
2.3. Aging Affects the Impact of Lesions on Skilled Motor Function
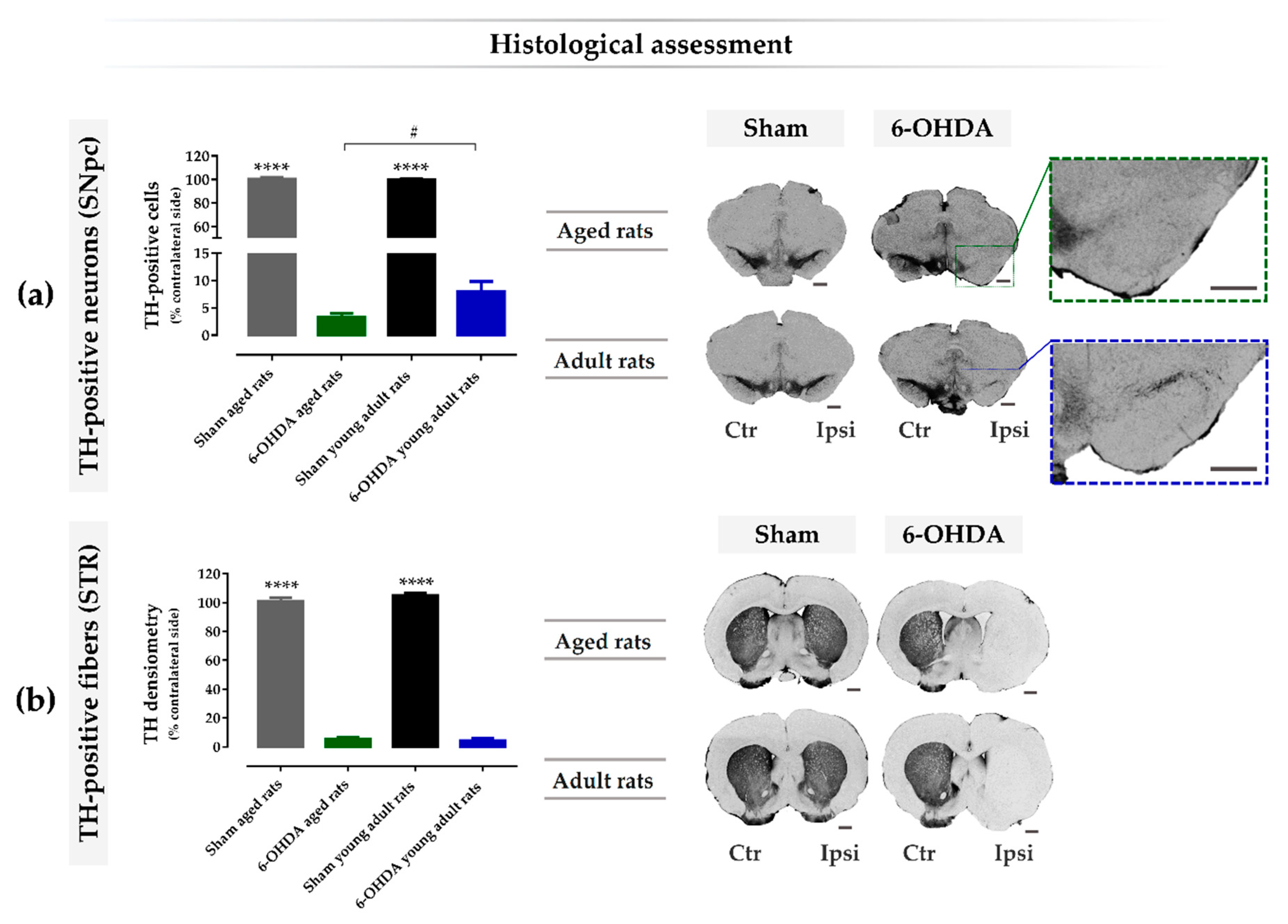
2.4. Aging Exacerbates Lesion-Induced Dopaminergic Degeneration within SNpc
3. Discussion
4. Materials and Methods
4.1. Animals
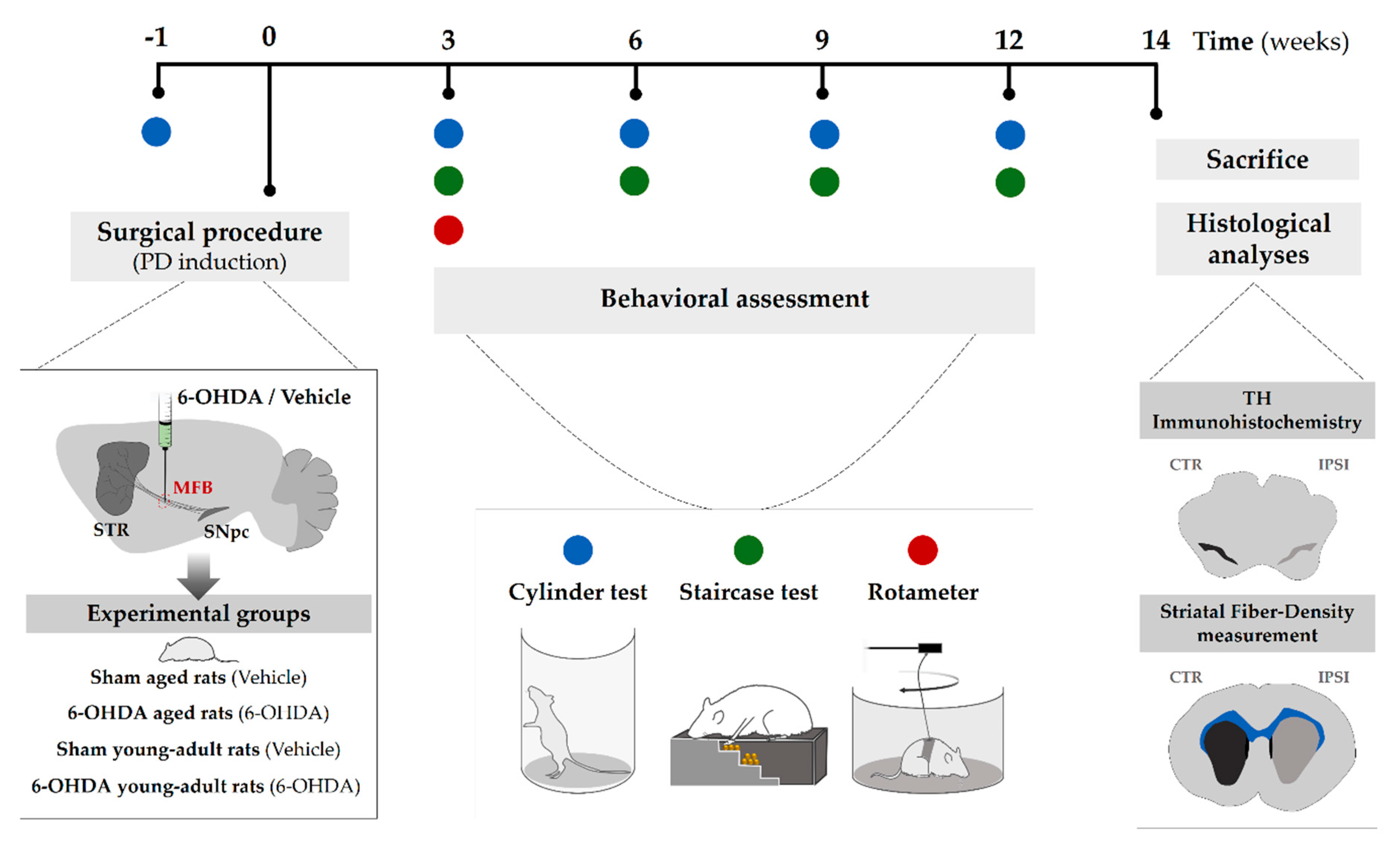
4.2. Experimental Design
4.3. Surgical Procedure (6-Hydroxidopamine Lesion)
4.4. Animal Welfare—Body Weight Measurement
4.5. Behavioral Assessment
4.5.1. Apomorphine-Induced Rotation Test
4.5.2. Cylinder Test
4.5.3. Staircase Test
4.6. Histological Assessment
4.6.1. TH Immunohistochemistry
4.6.2. Striatal Fiber-Density Measurement
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| 6-OHDA | 6-hydroxydopamine |
| SNpc | Substantia nigra pars compacta |
| DA neurons | Dopamine-containing neurons |
| MFB | Medial forebrain bundle |
| SEM | Standard error of the mean |
| TH | Tyrosine hydroxylase |
| STR | Striatum |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| SN | Substantia nigra |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| BDNF | Brain-derived neurotrophic factor |
| GDNF | Glial cell line-derived neurotrophic factor |
| PFA | Paraformaldehyde |
| PBS | Phosphate-buffered saline |
| FCS | Fetal calf serum |
| RT | Room temperature |
| PBS-T | Phosphate-buffered saline -Triton |
| DAB | 3,3’- diaminobenzidine tetrahydrochloride |
References
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J. Parkinson disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Klingelhoefer, L.; Reichmann, H. Pathogenesis of Parkinson disease—The gut–brain axis and environmental factors. Nat. Rev. Neurol. 2015, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Spatola, M.; Wider, C. Genetics of Parkinson’s disease: The yield. Parkinsonism Relat. Disord. 2014, 20, S35–S38. [Google Scholar] [CrossRef]
- Pang, S.Y.-Y.; Ho, P.W.-L.; Liu, H.-F.; Leung, C.-T.; Li, L.; Chang, E.E.S.; Ramsden, D.B.; Ho, S.-L. The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 2019, 8, 23. [Google Scholar] [CrossRef]
- Collier, T.J.; Kanaan, N.M.; Kordower, J.H. Ageing as a primary risk factor for Parkinson’s disease: Evidence from studies of non-human primates. Nat. Rev. Neurosci. 2011, 12, 359–366. [Google Scholar] [CrossRef]
- Reeve, A.; Simcox, E.; Turnbull, D. Ageing and Parkinson’s disease: Why is advancing age the biggest risk factor? Ageing Res. Rev. 2014, 14, 19–30. [Google Scholar] [CrossRef]
- Lotti, M. Age-related sensitivity of the nervous system to neurotoxic insults. Toxicol. Lett. 2002, 127, 183–187. [Google Scholar] [CrossRef]
- Phinney, A.L.; Andringa, G.; Bol, J.G.; Wolters, E.C.; van Muiswinkel, F.L.; van Dam, A.-M.W.; Drukarch, B. Enhanced sensitivity of dopaminergic neurons to rotenone-induced toxicity with aging. Parkinsonism Relat. Disord. 2006, 12, 228–238. [Google Scholar] [CrossRef]
- Boger, H.A.; Middaugh, L.D.; Zaman, V.; Hoffer, B.; Granholm, A.-C. Differential effects of the dopamine neurotoxin MPTP in animals with a partial deletion of the GDNF receptor, GFRα1, gene. Brain Res. 2008, 1241, 18–28. [Google Scholar] [CrossRef]
- Jankovic, J.; Aguilar, L.G. Current approaches to the treatment of Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2008, 4, 743. [Google Scholar] [CrossRef] [PubMed]
- Oertel, W.; Schulz, J.B. Current and experimental treatments of Parkinson disease: A guide for neuroscientists. J. Neurochem. 2016, 139, 325–337. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Carvalho, M.M.; Panchalingam, K.M.; Rodrigues, A.J.; Mendes-Pinheiro, B.; Anjo, S.; Manadas, B.; Behie, L.A.; Sousa, N.; Salgado, A.J. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson’s disease. Stem Cells Transl. Med. 2017, 6, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Manadas, B.; Behie, L.A.; Salgado, A.J. Secretome of undifferentiated neural progenitor cells induces histological and motor improvements in a rat model of Parkinson’s disease. Stem Cells Transl. Med. 2018, 7, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, B.M.; Anjo, S.I.; Manadas, B.; da Silva, J.D.; Marore, A.; Teixeira, F.G.; Salgado, A.J. Bone marrow mesenchymal stem cells’ secretome exerts neuroprotective effects in a Parkinson’s disease rat model. Front. Bioeng. Biotechnol. 2019, 7, 294. [Google Scholar] [CrossRef] [PubMed]
- De Jesús-Cortés, H.; Miller, A.D.; Britt, J.K.; DeMarco, A.J.; De Jesús-Cortés, M.; Stuebing, E.; Naidoo, J.; Vázquez-Rosa, E.; Morlock, L.; Williams, N.S. Protective efficacy of P7C3-S243 in the 6-hydroxydopamine model of Parkinson’s disease. NPJ Parkinson’s Dis. 2015, 1, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Espay, A.J. Disease modification in Parkinson’s disease: Current approaches, challenges, and future considerations. Mov. Disord. 2018, 33, 660–677. [Google Scholar] [CrossRef]
- Dawson, T.M.; Golde, T.E.; Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1370–1379. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [PubMed]
- Ricaurte, G.; DeLanney, L.; Finnegan, K.; Irwin, I.; Langston, J. The dopamine-depleting effect of 6-hydroxydopamine does not increase with aging. Brain Res. 1988, 438, 395–398. [Google Scholar] [CrossRef]
- Cass, W.A.; Harned, M.E.; Bailey, S.L. Enhanced effects of 6-hydroxydopamine on evoked overflow of striatal dopamine in aged rats. Brain Res. 2002, 938, 29–37. [Google Scholar] [CrossRef]
- Cass, W.A.; Peters, L.E.; Smith, M.P. Reductions in spontaneous locomotor activity in aged male, but not female, rats in a model of early Parkinson’s disease. Brain Res. 2005, 1034, 153–161. [Google Scholar] [CrossRef]
- Tamás, A.; Lubics, A.; Szalontay, L.; Lengvári, I.; Reglődi, D. Age and gender differences in behavioral and morphological outcome after 6-hydroxydopamine-induced lesion of the substantia nigra in rats. Behav. Brain Res. 2005, 158, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Tamás, A.; Lubics, A.; Lengvári, I.; Reglődi, D. Effects of age, gender, and gonadectomy on neurochemistry and behavior in animal models of Parkinson’s disease. Endocrine 2006, 29, 275–287. [Google Scholar] [CrossRef]
- Villar-Cheda, B.; Valenzuela, R.; Rodriguez-Perez, A.I.; Guerra, M.J.; Labandeira-Garcia, J.L. Aging-related changes in the nigral angiotensin system enhances proinflammatory and pro-oxidative markers and 6-OHDA-induced dopaminergic degeneration. Neurobiol. Aging 2012, 33, 204.e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.M.; Campos, F.L.; Coimbra, B.; Pêgo, J.M.; Rodrigues, C.; Lima, R.; Rodrigues, A.J.; Sousa, N.; Salgado, A.J. Behavioral characterization of the 6-hydroxidopamine model of Parkinson’s disease and pharmacological rescuing of non-motor deficits. Mol. Neurodegener. 2013, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhan, M.; OuYang, L.; Li, Y.; Chen, S.; Wu, J.; Chen, J.; Luo, C.; Lei, W. The effects of unilateral 6-OHDA lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav. Brain Res. 2014, 266, 37–45. [Google Scholar] [CrossRef]
- Teixeira, F.G.; Vilaça-Faria, H.; Domingues, A.V.; Campos, J.; Salgado, A.J. Preclinical Comparison of Stem Cells Secretome and Levodopa Application in a 6-Hydroxydopamine Rat Model of Parkinson’s Disease. Cells 2020, 9, 315. [Google Scholar] [CrossRef]
- Rentsch, P.; Stayte, S.; Morris, G.P.; Vissel, B. Time dependent degeneration of the nigrostriatal tract in mice with 6-OHDA lesioned medial forebrain bundle and the effect of activin A on l-Dopa induced dyskinesia. BMC Neurosci. 2019, 20, 5. [Google Scholar] [CrossRef] [PubMed]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, Y.Y.; van der Lijn, F.; Vernooij, M.W.; Hofman, A.; Niessen, W.J.; van der Lugt, A.; Ikram, M.A.; van der Geest, J.N. Older age relates to worsening of fine motor skills: A population-based study of middle-aged and elderly persons. Front. Aging Neurosci. 2014, 6, 259. [Google Scholar] [CrossRef] [PubMed]
- Voelcker-Rehage, C. Motor-skill learning in older adults—A review of studies on age-related differences. Eur. Rev. Aging Phys. Act. 2008, 5, 5–16. [Google Scholar] [CrossRef]
- Barnéoud, P.; Descombris, E.; Aubin, N.; Abrous, D.N. Evaluation of simple and complex sensorimotor behaviours in rats with a partial lesion of the dopaminergic nigrostriatal system. Eur. J. Neurosci. 2000, 12, 322–336. [Google Scholar] [CrossRef]
- Zucca, F.A.; Segura-Aguilar, J.; Ferrari, E.; Muñoz, P.; Paris, I.; Sulzer, D.; Sarna, T.; Casella, L.; Zecca, L. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog. Neurobiol. 2017, 155, 96–119. [Google Scholar] [CrossRef]
- Collier, T.J.; Kanaan, N.M.; Kordower, J.H. Aging and Parkinson’s disease: Different sides of the same coin? Mov. Disord. 2017, 32, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J. Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef]
- Sugama, S.; Yang, L.; Cho, B.P.; DeGiorgio, L.A.; Lorenzl, S.; Albers, D.S.; Beal, M.F.; Volpe, B.T.; Joh, T.H. Age-related microglial activation in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003, 964, 288–294. [Google Scholar] [CrossRef]
- Ali, S.; David, S.; Newport, G. Age-related susceptibility to MPTP-induced neurotoxicity in mice. Neurotoxicology 1993, 14, 29–34. [Google Scholar]
- Collier, T.J.; Lipton, J.; Daley, B.F.; Palfi, S.; Chu, Y.; Sortwell, C.; Bakay, R.A.; Sladek, J.R., Jr.; Kordower, J.H. Aging-related changes in the nigrostriatal dopamine system and the response to MPTP in nonhuman primates: Diminished compensatory mechanisms as a prelude to parkinsonism. Neurobiol. Dis. 2007, 26, 56–65. [Google Scholar] [CrossRef]
- Wang, X.; Guan, Q.; Wang, M.; Yang, L.; Bai, J.; Yan, Z.; Zhang, Y.; Liu, Z. Aging-related rotenone-induced neurochemical and behavioral deficits: Role of SIRT2 and redox imbalance, and neuroprotection by AK-7. Drug Des. Dev. Ther. 2015, 9, 2553. [Google Scholar] [CrossRef]
- Ureshino, R.P.; Costa, A.J.; Erustes, A.G.; Pereira, G.J.D.S.; Sinigaglia-Coimbra, R.; Smaili, S.S. Effects of aging in the striatum and substantia nigra of a Parkinson’s disease animal model. Toxicol. Pathol. 2018, 46, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Yurek, D.M.; Fletcher-Turner, A. Lesion-induced increase of BDNF is greater in the striatum of young versus old rat brain. Exp. Neurol. 2000, 161, 392–396. [Google Scholar] [CrossRef]
- Yurek, D.M.; Fletcher-Turner, A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001, 891, 228–235. [Google Scholar] [CrossRef]
- Collier, T.J.; Ling, Z.D.; Carvey, P.M.; Fletcher-Turner, A.; Yurek, D.M.; Sladek, J.R., Jr.; Kordower, J.H. Striatal trophic factor activity in aging monkeys with unilateral MPTP-induced parkinsonism. Exp. Neurol. 2005, 191, S60–S67. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.-Y.; Zhang, J.; Bing, G. Aging enhances the neuroinflammatory response and α-synuclein nitration in rats. Neurobiol. Aging 2010, 31, 1649–1653. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.N.; Schreier, W.A.; Ou, X.; Holcomb, L.A.; Morgan, D.G. Exaggerated astrocyte reactivity after nigrostriatal deafferentation in the aged rat. J. Comp. Neurol. 1997, 388, 106–119. [Google Scholar] [CrossRef]
- Koprich, J.B.; Reske-Nielsen, C.; Mithal, P.; Isacson, O. Neuroinflammation mediated by IL-1β increases susceptibility of dopamine neurons to degeneration in an animal model of Parkinson’s disease. J. Neuroinflamm. 2008, 5, 8. [Google Scholar] [CrossRef]
- Singh, S.; Ahmad, R.; Mathur, D.; Sagar, R.K.; Krishana, B.; Arora, R.; Sharma, R.K. Neuroprotective effect of BDNF in young and aged 6-OHDA treated rat model of Parkinson disease. Indian J. Exp. Biol. 2006, 44, 699–704. [Google Scholar]
- Grimmig, B.; Daly, L.; Subbarayan, M.; Hudson, C.; Williamson, R.; Nash, K.; Bickford, P.C. Astaxanthin is neuroprotective in an aged mouse model of Parkinson’s disease. Oncotarget 2018, 9, 10388. [Google Scholar] [CrossRef]
- Sortwell, C.E.; Camargo, M.D.; Pitzer, M.R.; Gyawali, S.; Collier, T.J. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp. Neurol. 2001, 169, 23–29. [Google Scholar] [CrossRef]
- Misal, U.S.; Joshi, S.A.; Shaikh, M.M. Delayed recovery from anesthesia: A postgraduate educational review. Anesth. Essays Res. 2016, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Chemali, J.; Kenny, J.; Olutola, O.; Taylor, N.; Kimchi, E.; Purdon, P.; Brown, E.; Solt, K. Ageing delays emergence from general anaesthesia in rats by increasing anaesthetic sensitivity in the brain. Br. J. Anaesth. 2015, 115 (Suppl. 1), i58–i65. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Compact, 6th ed.; Academic Press: New York, NY, USA, 2009. [Google Scholar]
- Schallert, T.; Tillerson, J.L. Intervention strategies for degeneration of dopamine neurons in parkinsonism. In Central Nervous System Diseases; Springer: Berlin, Germany, 2000; pp. 131–151. [Google Scholar]
- Heuer, A.; Smith, G.A.; Lelos, M.J.; Lane, E.L.; Dunnett, S.B. Unilateral nigrostriatal 6-hydroxydopamine lesions in mice I: Motor impairments identify extent of dopamine depletion at three different lesion sites. Behav. Brain Res. 2012, 228, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Montoya, C.; Campbell-Hope, L.; Pemberton, K.; Dunnett, S. The “staircase test”: A measure of independent forelimb reaching and grasping abilities in rats. J. Neurosci. Methods 1991, 36, 219–228. [Google Scholar] [CrossRef]
- Campos, F.L.; Carvalho, M.M.; Cristovão, A.C.; Je, G.; Baltazar, G.; Salgado, A.J.; Kim, Y.-S.; Sousa, N. Rodent models of Parkinson’s disease: Beyond the motor symptomatology. Front. Behav. Neurosci. 2013, 7, 175. [Google Scholar] [CrossRef]

| Comparison between Groups | Mean Difference | SEM | p-Value |
|---|---|---|---|
| Sham aged rats vs. 6-OHDA aged rats | 7.88 | 2.40 | p = 0.019 |
| Sham aged rats vs. sham young adult rats | −15.1 | 2.61 | p < 0.0001 |
| Sham aged rats vs. 6-OHDA young adult rats | −4.43 | 2.46 | p = 0.410 |
| 6-OHDA aged rats vs. 6-OHDA young adult rats | −12.3 | 2.09 | p < 0.0001 |
| Sham young adult rats vs. 6-OHDA young adult rats | 10.7 | 2.33 | p = 0.001 |
| Sham young adult rats vs. 6-OHDA aged rats | 23.0 | 2.27 | p < 0.0001 |
| Comparison between Groups | Mean Difference | SEM | p-Value |
|---|---|---|---|
| Sham aged rats vs. 6-OHDA aged rats | 71.3 | 4.78 | p < 0.0001 |
| Sham aged rats vs. Sham young adult rats | −3.39 | 4.99 | 0.986 |
| Sham aged rats vs. 6-OHDA young adult rats | 67.3 | 4.99 | p < 0.0001 |
| 6-OHDA aged rats vs. 6-OHDA young adult rats | −4.05 | 4.78 | 0.958 |
| Sham young adult rats vs. 6-OHDA young adult rats | 70.7 | 4.99 | p < 0.0001 |
| Sham young adult rats vs. 6-OHDA aged rats | 74.7 | 4.78 | p < 0.0001 |
| Staircase | |||
| Comparison between Groups | Mean Difference | SEM | p-Value |
| Sham aged rats vs. 6-OHDA aged rats | 57.9 | 6.15 | p < 0.0001 |
| Sham aged rats vs. sham young adult rats | 0.127 | 6.35 | p = 1.0 |
| Sham aged rats vs. 6-OHDA young adult rats | 39.7 | 6.15 | p < 0.0001 |
| 6-OHDA aged rats vs. 6-OHDA young adult rats | −18.3 | 5.02 | p = 0.013 |
| Sham young adult rats vs. 6-OHDA young adult rats | 36.6 | 5.26 | p < 0.0001 |
| Sham young adult rats vs. 6-OHDA aged rats | 57.8 | 5.26 | p < 0.0001 |
| Staircase–Forced-Choice | |||
| Comparison between Groups | Mean Difference | SEM | p-Value |
| Sham aged rats vs. 6-OHDA aged rats | 58.7 | 6.22 | p < 0.0001 |
| Sham aged rats vs. sham young adult rats | 0.061 | 6.43 | p = 1.0 |
| Sham aged rats vs. 6-OHDA young adult rats | 41.1 | 6.22 | p < 0.0001 |
| 6-OHDA aged rats vs. 6-OHDA young adult rats | −17.6 | 5.08 | p = 0.019 |
| Sham young adult rats vs. 6-OHDA young adult rats | 41.1 | 5.33 | p < 0.0001 |
| Sham young adult rats vs. 6-OHDA aged rats | 58.7 | 5.33 | p < 0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barata-Antunes, S.; Teixeira, F.G.; Mendes-Pinheiro, B.; Domingues, A.V.; Vilaça-Faria, H.; Marote, A.; Silva, D.; Sousa, R.A.; Salgado, A.J. Impact of Aging on the 6-OHDA-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3459. https://doi.org/10.3390/ijms21103459
Barata-Antunes S, Teixeira FG, Mendes-Pinheiro B, Domingues AV, Vilaça-Faria H, Marote A, Silva D, Sousa RA, Salgado AJ. Impact of Aging on the 6-OHDA-Induced Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2020; 21(10):3459. https://doi.org/10.3390/ijms21103459
Chicago/Turabian StyleBarata-Antunes, Sandra, Fábio G. Teixeira, Bárbara Mendes-Pinheiro, Ana V. Domingues, Helena Vilaça-Faria, Ana Marote, Deolinda Silva, Rui A. Sousa, and António J. Salgado. 2020. "Impact of Aging on the 6-OHDA-Induced Rat Model of Parkinson’s Disease" International Journal of Molecular Sciences 21, no. 10: 3459. https://doi.org/10.3390/ijms21103459
APA StyleBarata-Antunes, S., Teixeira, F. G., Mendes-Pinheiro, B., Domingues, A. V., Vilaça-Faria, H., Marote, A., Silva, D., Sousa, R. A., & Salgado, A. J. (2020). Impact of Aging on the 6-OHDA-Induced Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences, 21(10), 3459. https://doi.org/10.3390/ijms21103459






