Glucocorticoids Equally Stimulate Epithelial Na+ Transport in Male and Female Fetal Alveolar Cells
Abstract
1. Introduction
2. Results
2.1. Glucocorticoid Receptor mRNA Expression in Sex-Specific FDLE Cells
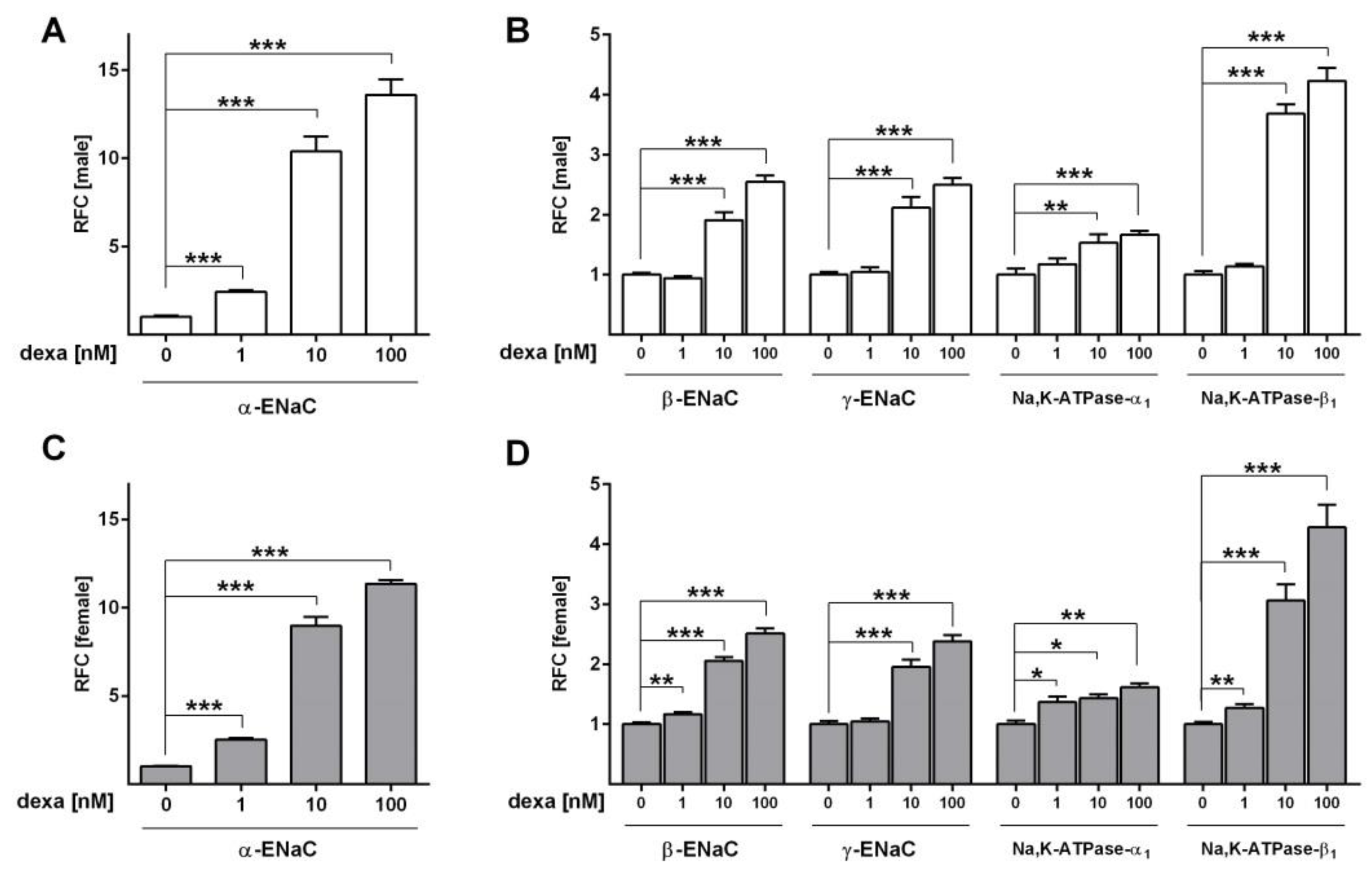
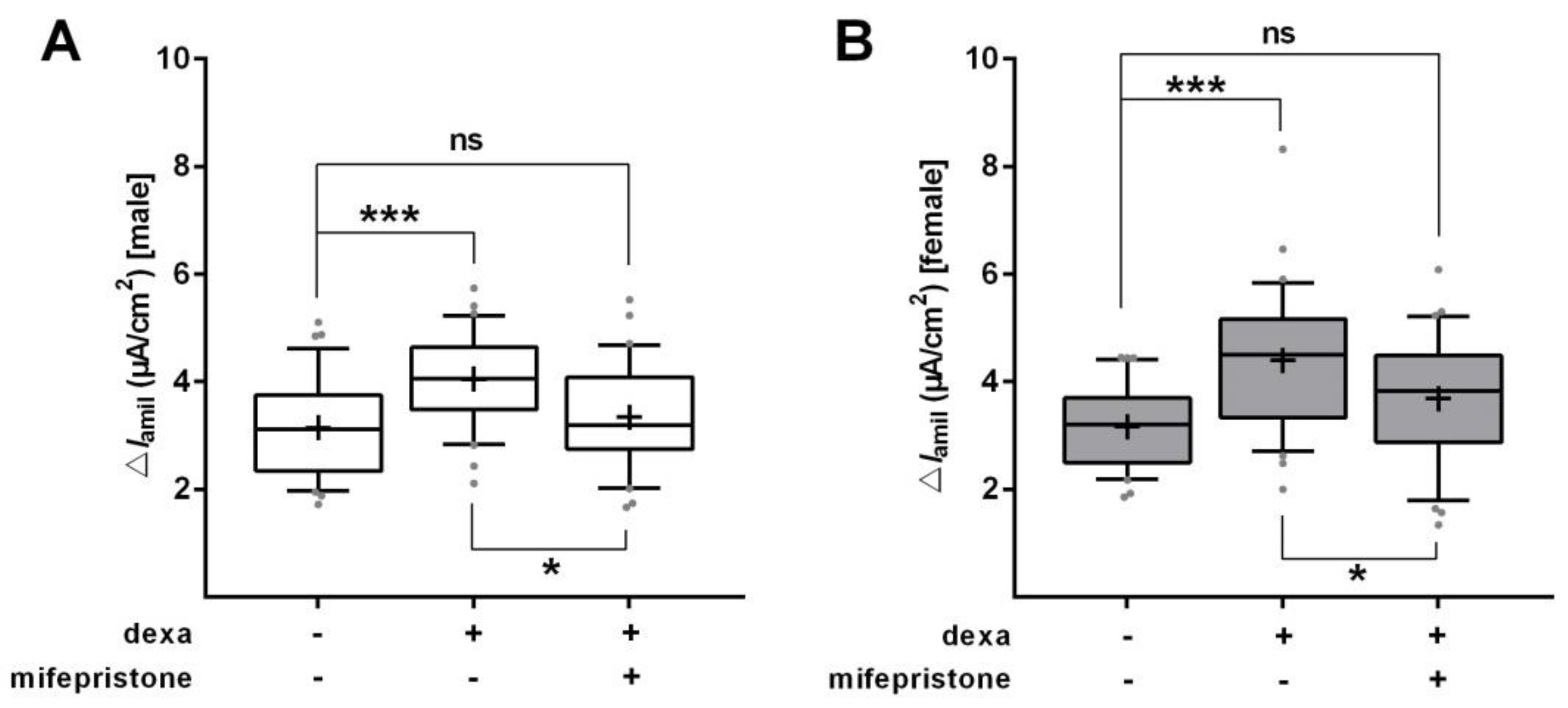
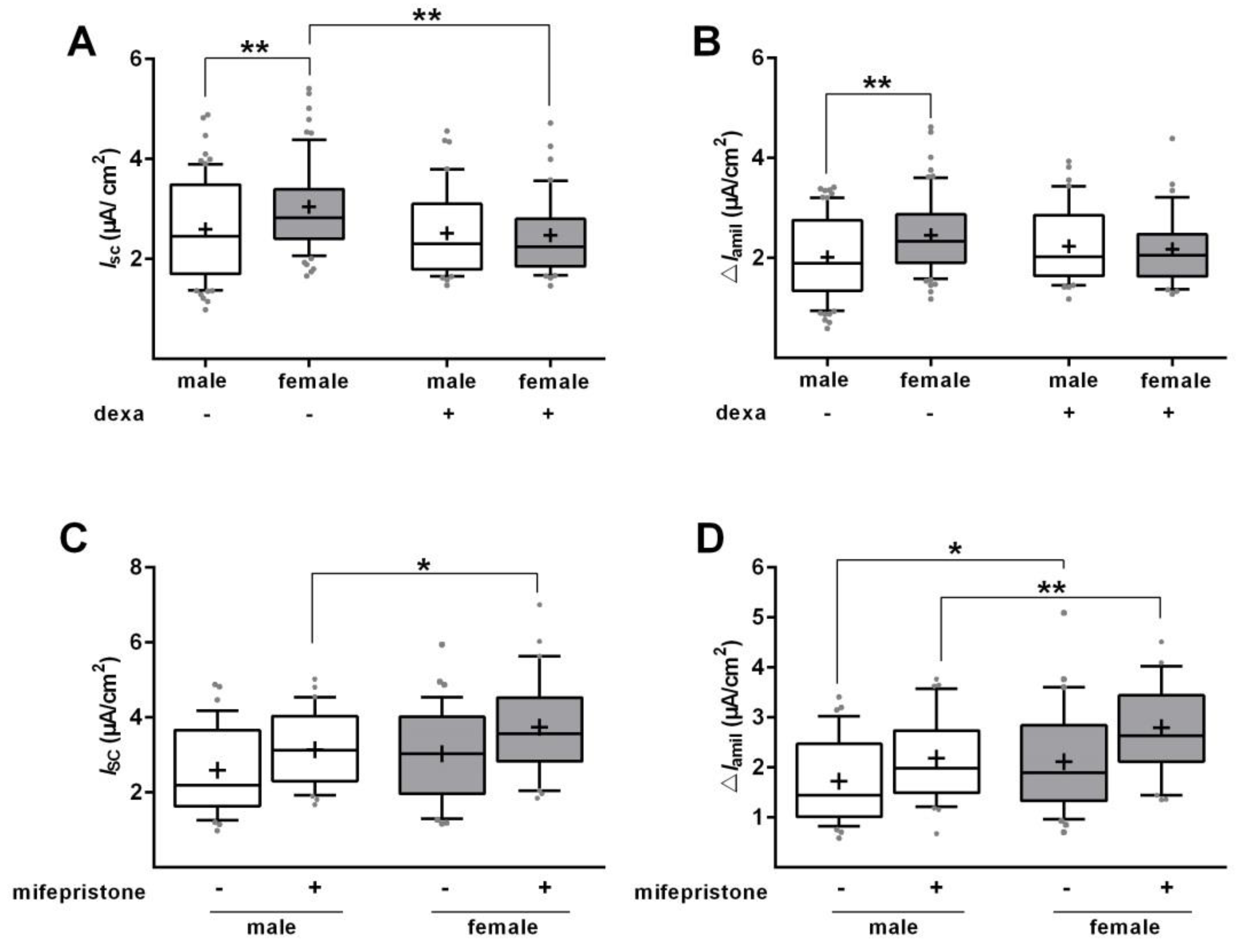
2.2. Glucocorticoids Increase Na+ Transport and Channel Expression in the Absence of Serum
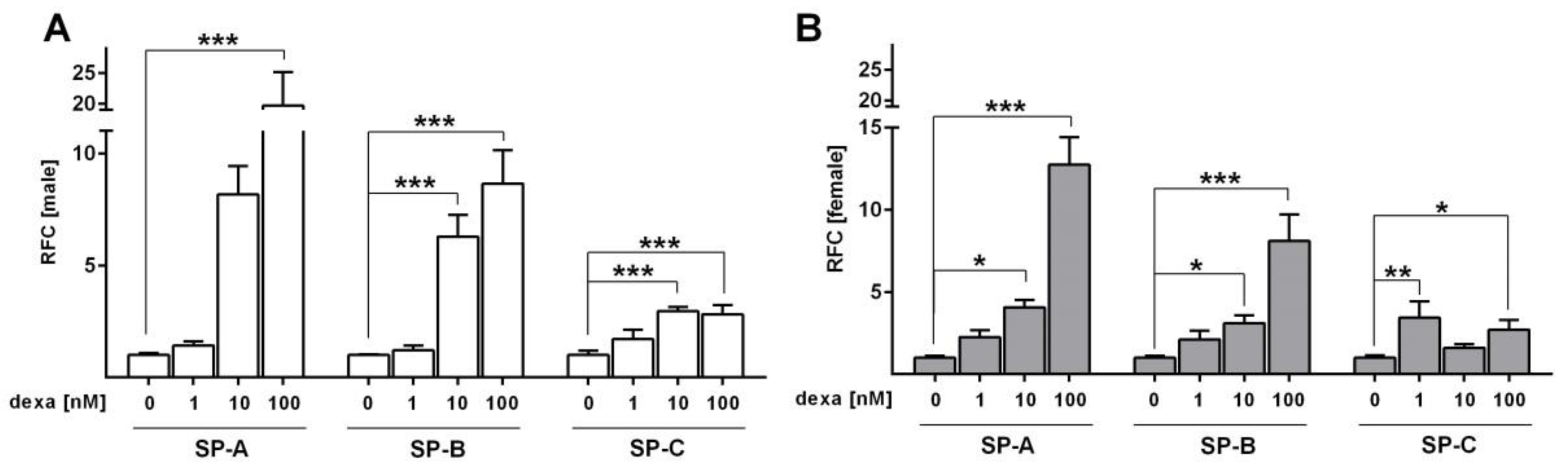
2.3. Effect of Glucocorticoids on Surfactant Protein Expression
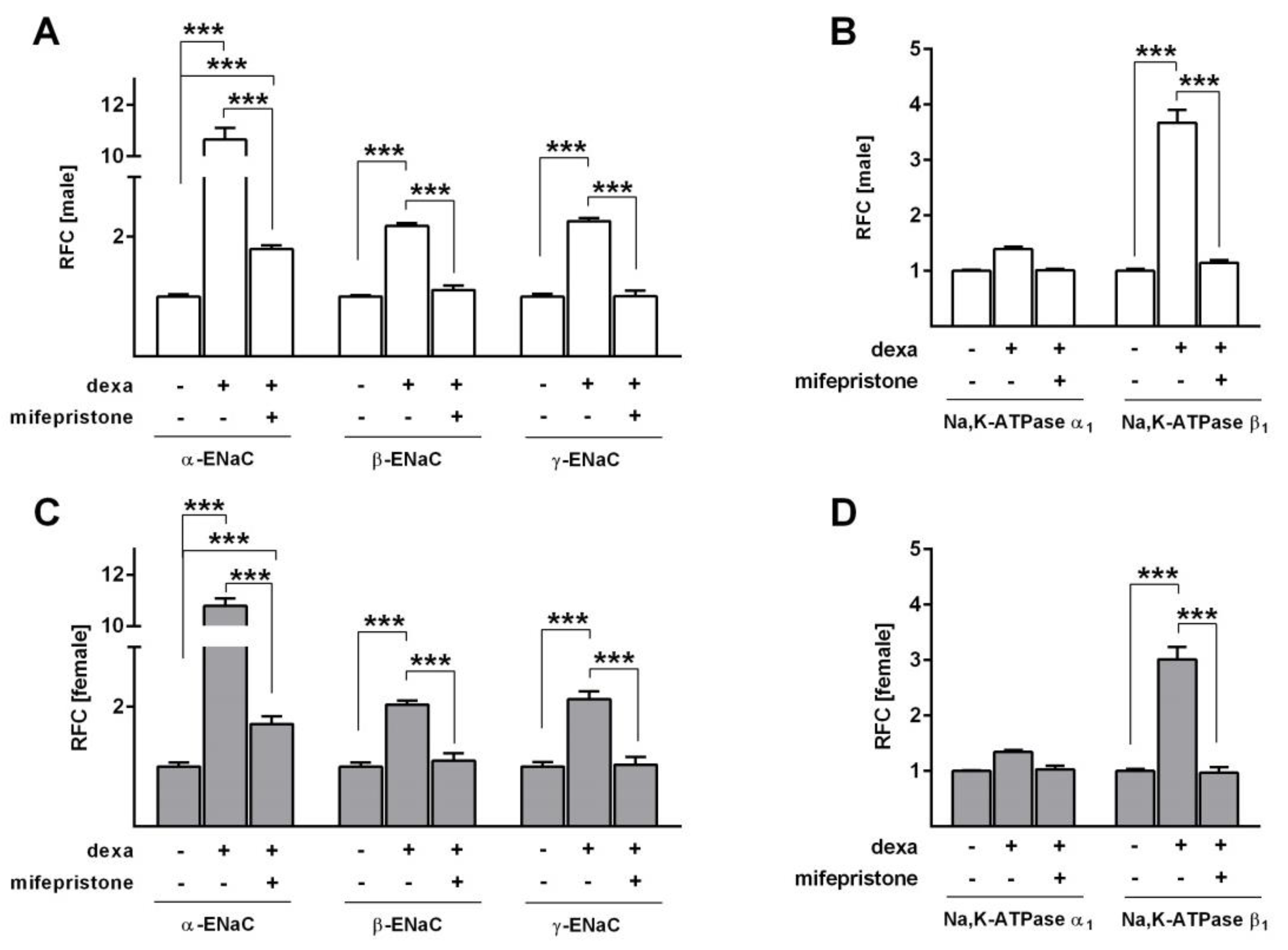
2.4. Effect of Glucocorticoids on Na+ Transport and Channel Expression in the Presence of Serum
3. Discussion
4. Materials and Methods
4.1. Cell Isolation and Culture
4.2. Ussing Chamber Measurements
4.3. mRNA Expression Analyses
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barker, P.M.; Gowen, C.W.; Lawson, E.E.; Knowles, M.R. Decreased sodium ion absorption across nasal epithelium of very premature infants with respiratory distress syndrome. J. Pediatr. 1997, 130, 373–377. [Google Scholar] [CrossRef]
- Helve, O.; Pitkänen, O.; Janér, C.; Andersson, S. Pulmonary Fluid Balance in the Human Newborn Infant. Neonatology 2009, 95, 347–352. [Google Scholar] [CrossRef]
- Altman, M.; Vanpee, M.; Cnattingius, S.; Norman, M. Risk factors for acute respiratory morbidity in moderately preterm infants. Paediatr. Perinat. Epidemiol. 2013, 27, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Anadkat, J.S.; Kuzniewicz, M.W.; Chaudhari, B.P.; Cole, F.S.; Hamvas, A. Increased risk for respiratory distress among white, male, late preterm and term infants. J. Perinatol. 2012, 32, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Farrell, P.M.; Avery, M.E. Hyaline membrane disease. Am. Rev. Respir. Dis. 1975, 111, 657–688. [Google Scholar]
- Ingemarsson, I. Gender aspects of preterm birth. BJOG 2003, 110 (Suppl. 20), 34–38. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.K.; Verter, J.; Fanaroff, A.A.; Oh, W.; Ehrenkranz, R.A.; Shankaran, S.; Donovan, E.F.; Wright, L.L.; Lemons, J.A.; Tyson, J.E.; et al. Sex differences in outcomes of very low birthweight infants: The newborn male disadvantage. Arch. Dis. Child. Fetal. Neonatal. Ed. 2000, 83, F182–F185. [Google Scholar] [CrossRef] [PubMed]
- Kaltofen, T.; Haase, M.; Thome, U.H.; Laube, M. Male Sex is Associated with a Reduced Alveolar Epithelial Sodium Transport. PLoS ONE 2015, 10, e0136178. [Google Scholar] [CrossRef] [PubMed]
- Canessa, C.M.; Schild, L.; Buell, G.; Thorens, B.; Gautschi, I.; Horisberger, J.D.; Rossier, B.C. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 1994, 367, 463–467. [Google Scholar] [CrossRef]
- Ridge, K.M.; Rutschman, D.H.; Factor, P.; Katz, A.I.; Bertorello, A.M.; Sznajder, J.L. Differential expression of Na-K-ATPase isoforms in rat alveolar epithelial cells. Am. J. Physiol. 1997, 273, L246–L255. [Google Scholar] [CrossRef]
- Haase, M.; Laube, M.; Thome, U.H. Sex-specific effects of sex steroids on alveolar epithelial Na+ transport. Am J Physiol Lung Cell Mol. Physiol. 2017, 312, L405–L414. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Dalziel, S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2006, CD004454. [Google Scholar] [CrossRef]
- Ballard, P.L.; Ning, Y.; Polk, D.; Ikegami, M.; Jobe, A.H. Glucocorticoid regulation of surfactant components in immature lambs. Am. J. Physiol. 1997, 273, L1048–L1057. [Google Scholar] [CrossRef] [PubMed]
- Folkesson, H.G.; Norlin, A.; Wang, Y.; Abedinpour, P.; Matthay, M.A. Dexamethasone and thyroid hormone pretreatment upregulate alveolar epithelial fluid clearance in adult rats. J. Appl. Physiol. 2000, 88, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.M.; Rodgers, H.F.; O’Brien, J.A.; Mahli, N.; Magliato, S.A.; Durham, P.L. Glucocorticoid effects on rabbit fetal lung maturation in vivo: An ultrastructural morphometric study. Anat. Rec. 1992, 232, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.J.; Blendy, J.A.; Monaghan, A.P.; Krieglstein, K.; Schmid, W.; Aguzzi, A.; Fantuzzi, G.; Hummler, E.; Unsicker, K.; Schütz, G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995, 9, 1608–1621. [Google Scholar] [CrossRef]
- Tchepichev, S.; Ueda, J.; Canessa, C.; Rossier, B.C.; O’Brodovich, H. Lung epithelial Na channel subunits are differentially regulated during development and by steroids. Am. J. Physiol. 1995, 269, C805–C812. [Google Scholar] [CrossRef]
- Thome, U.H.; Davis, I.C.; Nguyen, S.V.; Shelton, B.J.; Matalon, S. Modulation of sodium transport in fetal alveolar epithelial cells by oxygen and corticosterone. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 284, L376–L385. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Sutton, P.D.; Ventura, S.J.; Menacker, F.; Kirmeyer, S. Births: Final data for 2004. Natl. Vital. Stat. Rep. 2006, 55, 1–101. [Google Scholar]
- Willet, K.E.; Jobe, A.H.; Ikegami, M.; Polk, D.; Newnham, J.; Kohan, R.; Gurrin, L.; Sly, P.D. Postnatal Lung Function after Prenatal Steroid Treatment in Sheep: Effect of Gender. Pediatr. Res. 1997, 42, 885–892. [Google Scholar] [CrossRef]
- Townsend, E.A.; Miller, V.M.; Prakash, Y.S. Sex Differences and Sex Steroids in Lung Health and Disease. Endocr. Rev. 2012, 33, 1–47. [Google Scholar] [CrossRef] [PubMed]
- Roberge, S.; Lacasse, Y.; Tapp, S.; Tremblay, Y.; Kari, A.; Liu, J.; Fekih, M.; Qublan, H.S.; Amorim, M.M.; Bujold, E. Role of fetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: Systematic review and meta-analysis. J. Obstet. Gynaecol. Can. 2011, 33, 216–226. [Google Scholar] [CrossRef]
- Correia, C.; Rocha, G.; Flor-DE-Lima, F.; Guimaraes, H.; Correia, C.; Rocha, G.; Flor-DE-Lima, F.; Guimaraes, H. Respiratory morbidity in late preterm infants. Minerva. Pediatr. 2018, 70, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, N.; Liu, Y. High-risk Factors of Respiratory Distress Syndrome in Term Neonates: A Retrospective Case-control Study. Balkan. Med. J. 2014, 31, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.B. Postnatal glucocorticoids induce a-ENaC formation and regulate glucocorticoid receptors in the preterm rabbit lung. Am. J. Physiol. Lung Cell Mol. Physiol. 2003, 286, L73–L80. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Stokes, J.B.; JR McCray, P.B. Endogenous and exogenous glucocorticoid regulation of ENaC mRNA expression in developing kidney and lung. Am. J. Physiol. Renal. Physiol. 2002, 283, C762–C772. [Google Scholar] [CrossRef][Green Version]
- Peacock, J.L.; Marston, L.; Marlow, N.; Calvert, S.A.; Greenough, A. Neonatal and infant outcome in boys and girls born very prematurely. Pediatr. Res. 2012, 71, 305–310. [Google Scholar] [CrossRef]
- Ballard, P.L.; Ballard, R.A.; Granberg, J.P.; Sniderman, S.; Gluckman, P.D.; Kaplan, S.L.; Grumbach, M.M. Fetal sex and prenatal betamethasone therapy. J. Pediatr. 1980, 97, 451–454. [Google Scholar] [CrossRef]
- Liggins, G.C.; Howie, R.N. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972, 50, 515–525. [Google Scholar]
- Consensus Development Conference, N.I.H. Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA 1995, 273, 413–418. [Google Scholar]
- Collaborative group on antenatal steroid therapy. Effect of antenatal dexamethasone administration on the prevention of respiratory distress syndrome. Am. J. Obstet. Gynecol. 1981, 141, 276–287. [Google Scholar] [CrossRef]
- Sweezey, N.B.; Ghibu, F.; Gagnon, S.; Schotman, E.; Hamid, Q. Glucocorticoid receptor mRNA and protein in fetal rat lung in vivo: Modulation by glucocorticoid and androgen. Am. J. Physiol. 1998, 275, L103–L109. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Klammt, J.; Thome, U.H.; Laube, M. The interaction of glucocorticoids and progesterone distinctively affects epithelial sodium transport. Lung 2014, 192, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.; Waddell, B.J.; Sly, P.D.; Willet, K.E. Sex differences in response to steroids in preterm sheep lungs are not explained by glucocorticoid receptor number or binding affinity. Pediatr. Pulmonol. 2001, 32, 8–13. [Google Scholar] [CrossRef]
- Itani, O.A.; Auerbach, S.D.; Husted, R.F.; Volk, K.A.; Ageloff, S.; Knepper, M.A.; Stokes, J.B.; Thomas, C.P. Glucocorticoid-stimulated lung epithelial Na(+) transport is associated with regulated ENaC and sgk1 expression. Am. J. Physiol. Renal. Physiol. 2002, 282, L631–L641. [Google Scholar] [CrossRef]
- Lazrak, A.; Samanta, A.; Venetsanou, K.; Barbry, P.; Matalon, S. Modification of biophysical properties of lung epithelial Na(+) channels by dexamethasone. Am. J. Physiol. Cell Physiol. 2000, 279, C762–C770. [Google Scholar] [CrossRef]
- Otulakowski, G.; Rafii, B.; Harris, M.; O’Brodovich, H. Oxygen and glucocorticoids modulate alphaENaC mRNA translation in fetal distal lung epithelium. Am. J. Respir. Cell Mol. Biol. 2006, 34, 204–212. [Google Scholar] [CrossRef]
- Barquin, N.; Ciccolella, D.E.; Ridge, K.M.; Sznajder, J.I. Dexamethasone upregulates the Na-K-ATPase in rat alveolar epithelial cells. Am. J. Physiol. 1997, 273, L825–L830. [Google Scholar] [CrossRef]
- O’Brodovich, H.; Canessa, C.; Ueda, J.; Rafii, B.; Rossier, B.C.; Edelson, J. Expression of the epithelial Na+ channel in the developing rat lung. Am. J. Physiol. 1993, 265, C491–C496. [Google Scholar] [CrossRef]
- Voilley, N.; Lingueglia, E.; Champigny, G.; Mattei, M.G.; Waldmann, R.; Lazdunski, M.; Barbry, P. The lung amiloride-sensitive Na+ channel: Biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc. Natl. Acad. Sci. USA 1994, 91, 247–251. [Google Scholar] [CrossRef]
- McDonald, F.J.; Price, M.P.; Snyder, P.M.; Welsh, M.J. Cloning and expression of the beta- and gamma-subunits of the human epithelial sodium channel. Am. J. Physiol. 1995, 268, C1157–C1163. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, C.M.; Canessa, C.M. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J. Gen. Physiol. 1997, 109, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Pollak, A.; Birnbacher, R. Preterm male infants need more initial respiratory support than female infants. Acta. Paediatr. 2004, 93, 447–448. [Google Scholar] [CrossRef]
- Nielsen, H.C.; Torday, J.S. Sex differences in fetal rabbit pulmonary surfactant production. Pediatr. Res. 1981, 15, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Gandelman, R.; Vom Saal, F.S.; Reinisch, J.M. Contiguity to male foetuses affects morphology and behaviour of female mice. Nature 1977, 266, 722–724. [Google Scholar] [CrossRef]
- Manno, F.A.M., III. Measurement of the digit lengths and the anogenital distance in mice. Physiol. Behav. 2008, 93, 364–368. [Google Scholar] [CrossRef]
- Jassal, D.; Han, R.N.; Caniggia, I.; Post, M.; Tanswell, A.K. Growth of distal fetal rat lung epithelial cells in a defined serum-free medium. In Vitro. Cell Dev. Biol. 1991, 27, 625–632. [Google Scholar] [CrossRef]
- Renaud, H.J.; Cui, J.Y.; Khan, M.; Klaassen, C.D. Tissue distribution and gender-divergent expression of 78 cytochrome P450 mRNAs in mice. Toxicol. Sci. 2011, 124, 261–277. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laube, M.; Riedel, D.; Ackermann, B.; Haase, M.; H. Thome, U. Glucocorticoids Equally Stimulate Epithelial Na+ Transport in Male and Female Fetal Alveolar Cells. Int. J. Mol. Sci. 2020, 21, 57. https://doi.org/10.3390/ijms21010057
Laube M, Riedel D, Ackermann B, Haase M, H. Thome U. Glucocorticoids Equally Stimulate Epithelial Na+ Transport in Male and Female Fetal Alveolar Cells. International Journal of Molecular Sciences. 2020; 21(1):57. https://doi.org/10.3390/ijms21010057
Chicago/Turabian StyleLaube, Mandy, Diana Riedel, Benjamin Ackermann, Melanie Haase, and Ulrich H. Thome. 2020. "Glucocorticoids Equally Stimulate Epithelial Na+ Transport in Male and Female Fetal Alveolar Cells" International Journal of Molecular Sciences 21, no. 1: 57. https://doi.org/10.3390/ijms21010057
APA StyleLaube, M., Riedel, D., Ackermann, B., Haase, M., & H. Thome, U. (2020). Glucocorticoids Equally Stimulate Epithelial Na+ Transport in Male and Female Fetal Alveolar Cells. International Journal of Molecular Sciences, 21(1), 57. https://doi.org/10.3390/ijms21010057




