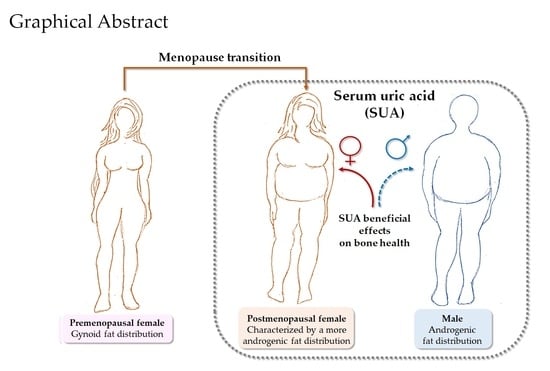
Changes in Adipose Tissue Distribution and Association between Uric Acid and Bone Health during Menopause Transition
Abstract
1. Introduction
2. Results
2.1. General Characteristics, Adiposity Indexes, BMD at Selected Skeletal Sites, Bone Markers and Uric Acid in the Study Sample
2.2. Correlations of SUA with BMD and Bone Markers
2.3. Multivariable Association between SUA and Total Hip BMD
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Measures of Anthropometric and DXA Indexes of Adiposity
4.3. Bone Densitometry Assessment
4.4. Biochemical Assays
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Romani, A.; Cremonini, E.; Bergamini, C.M.; Fila, E.; Squerzanti, M.; Greco, P.; Massari, L.; Bonaccorsi, G. Higher Urinary Levels of 8-Hydroxy-2′-deoxyguanosine Are Associated with a Worse RANKL/OPG Ratio in Postmenopausal Women with Osteopenia. Oxid. Med. Cell. Longev. 2016, 2016, 6038798. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Bonaccorsi, G.; Cremonini, E.; Bergamini, C.M.; Patella, A.; Castaldini, C.; Ferrazzini, S.; Capatti, A.; Picarelli, V.; Pansini, F.S.; et al. Bone mass density selectively correlates with serum markers of oxidative damage in post-menopausal women. Clin. Chem. Lab. Med. 2013, 51, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Sendur, O.F.; Turan, Y.; Tastaban, E.; Serter, M. Antioxidant status in patients with osteoporosis: A controlled study. Jt. Bone Spine 2009, 76, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Maggio, D.; Barabani, M.; Pierandrei, M.; Polidori, M.C.; Catani, M.; Mecocci, P.; Senin, U.; Pacifici, R.; Cherubini, A. Marked decrease in plasma antioxidants in aged osteoporotic women: Results of a cross-sectional study. J. Clin. Endocrinol. Metab. 2003, 88, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Lean, J.M.; Davies, J.T.; Fuller, K.; Jagger, C.J.; Kirstein, B.; Partington, G.A.; Urry, Z.L.; Chambers, T.J. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J. Clin. Investig. 2003, 112, 915–923. [Google Scholar] [CrossRef]
- Jun, J.H.; Lee, S.H.; Kwak, H.B.; Lee, Z.H.; Seo, S.B.; Woo, K.M.; Ryoo, H.M.; Kim, G.S.; Baek, J.H. N-acetylcysteine stimulates osteoblastic differentiation of mouse calvarial cells. J. Cell. Biochem. 2008, 103, 1246–1255. [Google Scholar] [CrossRef]
- Valacchi, G.; Virgili, F.; Cervellati, C.; Pecorelli, A. OxInflammation: From Subclinical Condition to Pathological Biomarker. Front. Physiol. 2018, 9, 858. [Google Scholar] [CrossRef]
- Cremonini, E.; Bonaccorsi, G.; Bergamini, C.M.; Castaldini, C.; Ferrazzini, S.; Capatti, A.; Massari, L.; Romani, A.; Marci, R.; Fila, E.; et al. Metabolic transitions at menopause: In post-menopausal women the increase in serum uric acid correlates with abdominal adiposity as assessed by DXA. Maturitas 2013, 75, 62–66. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Sautin, Y.Y.; Johnson, R.J. Uric acid: The oxidant-antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008, 27, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Lee, S.H.; Kim, B.J.; Lim, K.H.; Bae, S.J.; Kim, E.H.; Kim, H.K.; Choe, J.W.; Koh, J.M.; Kim, G.S. Higher serum uric acid is associated with higher bone mass, lower bone turnover, and lower prevalence of vertebral fracture in healthy postmenopausal women. Osteoporos. Int. 2013, 24, 2961–2970. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.; Miyao, M.; Mizuno, Y.; Tanaka-Ishikawa, M.; Akishita, M.; Ouchi, Y. Association between serum uric acid and lumbar spine bone mineral density in peri- and postmenopausal Japanese women. Osteoporos. Int. 2014, 25, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.D.; Wang, J.; Hou, X.H.; Bao, Y.Q.; Zhang, Z.L.; Hu, C.; Jia, W.P. Association of serum uric acid levels with osteoporosis and bone turnover markers in a Chinese population. Acta Pharmacol. Sin. 2018, 39, 626–632. [Google Scholar] [CrossRef]
- Zhang, D.; Bobulescu, I.A.; Maalouf, N.M.; Adams-Huet, B.; Poindexter, J.; Park, S.; Wei, F.; Chen, C.; Moe, O.W.; Sakhaee, K. Relationship between serum uric Acid and bone mineral density in the general population and in rats with experimental hyperuricemia. J. Bone Miner. Res. 2015, 30, 992–999. [Google Scholar] [CrossRef]
- Dalbeth, N.; Topless, R.; Flynn, T.; Cadzow, M.; Bolland, M.J.; Merriman, T.R. Mendelian randomization analysis to examine for a causal effect of urate on bone mineral density. J. Bone Miner. Res. 2015, 30, 985–991. [Google Scholar] [CrossRef]
- Dong, H.; Xu, Y.; Zhang, X.; Tian, S. Visceral adiposity index is strongly associated with hyperuricemia independently of metabolic health and obesity phenotypes. Sci. Rep. 2017, 7, 8822. [Google Scholar] [CrossRef]
- Hikita, M.; Ohno, I.; Mori, Y.; Ichida, K.; Yokose, T.; Hosoya, T. Relationship between hyperuricemia and body fat distribution. Intern. Med. 2007, 46, 1353–1358. [Google Scholar] [CrossRef]
- Cervellati, C.; Pansini, F.S.; Bonaccorsi, G.; Pascale, G.; Bagni, B.; Castaldini, C.; Ferrazini, S.; Ridolfi, F.; Pedriali, M.; Guariento, A.; et al. Body mass index is a major determinant of abdominal fat accumulation in pre-, peri- and post-menopausal women. Gynecol. Endocrinol. 2009, 25, 413–417. [Google Scholar] [CrossRef]
- Franklin, R.M.; Ploutz-Snyder, L.; Kanaley, J.A. Longitudinal changes in abdominal fat distribution with menopause. Metabolism 2009, 58, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Ha, S.K. Uric Acid Puzzle: Dual Role as Anti-oxidantand Pro-oxidant. Electrolytes Blood Press. 2014, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Passaro, A.; Bergamini, C.M.; Pilotto, A.; Zuliani, G. Oxidative balance, homocysteine, and uric acid levels in older patients with Late Onset Alzheimer’s Disease or Vascular Dementia. J. Neurol. Sci. 2014, 337, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Feig, D.I.; Kang, D.H.; Johnson, R.J. Uric acid and cardiovascular risk. N. Engl. J. Med. 2008, 359, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Sritara, C.; Ongphiphadhanakul, B.; Chailurkit, L.; Yamwong, S.; Ratanachaiwong, W.; Sritara, P. Serum uric acid levels in relation to bone-related phenotypes in men and women. J. Clin. Densitom. 2013, 16, 336–340. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, W.; Feng, X.; Liu, W.; Zhang, Z.; He, L.; Ye, Z. Serum uric acid is associated with lumbar spine bone mineral density in healthy Chinese males older than 50 years. Clin. Interv. Aging 2017, 12, 445–452. [Google Scholar] [CrossRef][Green Version]
- Lee, Y.J.; Hong, J.Y.; Kim, S.C.; Joo, J.K.; Na, Y.J.; Lee, K.S. The association between oxidative stress and bone mineral density according to menopausal status of Korean women. Obstet. Gynecol. Sci. 2015, 58, 46–52. [Google Scholar] [CrossRef]
- Cervellati, C.; Bonaccorsi, G.; Cremonini, E.; Romani, A.; Castaldini, C.; Ferrazzini, S.; Giganti, M.; Fila, E.; Massari, L.; Bergamini, C.M. Waist circumference and dual-energy X-ray absorptiometry measures of overall and central obesity are similarly associated with systemic oxidative stress in women. Scand. J. Clin. Lab. Investig. 2014, 74, 102–107. [Google Scholar] [CrossRef]
- Snijder, M.B.; Visser, M.; Dekker, J.M.; Seidell, J.C.; Fuerst, T.; Tylavsky, F.; Cauley, J.; Lang, T.; Nevitt, M.; Harris, T.B. The prediction of visceral fat by dual-energy X-ray absorptiometry in the elderly: A comparison with computed tomography and anthropometry. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 984–993. [Google Scholar] [CrossRef]
- Makovey, J.; Macara, M.; Chen, J.S.; Hayward, C.S.; March, L.; Seibel, M.J.; Sambrook, P.N. Serum uric acid plays a protective role for bone loss in peri- and postmenopausal women: A longitudinal study. Bone 2013, 52, 400–406. [Google Scholar] [CrossRef]
- Kim, S.; Jung, J.; Jung, J.H.; Kim, S.K.; Kim, R.B.; Hahm, J.R. Risk Factors of Bone Mass Loss at the Lumbar Spine: A Longitudinal Study in Healthy Korean Pre- and Perimenopausal Women Older than 40 Years. PLoS ONE 2015, 10, e0136283. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Occhionorelli, S.; Tisato, V.; Vigliano, M.; Longo, G.; Gonelli, A.; Sibilla, M.G.; Serino, M.L.; Zamboni, P. Inherited genetic predispositions in F13A1 and F13B genes predict abdominal adhesion formation: Identification of gender prognostic indicators. Sci. Rep. 2018, 8, 16916. [Google Scholar] [CrossRef] [PubMed]
- Ansani, L.; Marchesini, J.; Pestelli, G.; Luisi, G.A.; Scillitani, G.; Longo, G.; Milani, D.; Serino, M.L.; Tisato, V.; Gemmati, D. F13A1 Gene Variant (V34L) and Residual Circulating FXIIIA Levels Predict Short- and Long-Term Mortality in Acute Myocardial Infarction after Coronary Angioplasty. Int. J. Mol. Sci. 2018, 19, 2766. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.; Gorog, D.A.; Rihal, C.; Prasad, A.; Srinivasan, M. “Mind the gap” acute coronary syndrome in women: A contemporary review of current clinical evidence. Int. J. Cardiol. 2017, 227, 840–849. [Google Scholar] [CrossRef]
- Herrera, A.; Lobo-Escolar, A.; Mateo, J.; Gil, J.; Ibarz, E.; Gracia, L. Male osteoporosis: A review. World J. Orthop. 2012, 3, 223–234. [Google Scholar] [CrossRef]
- Cervellati, C.; Bonaccorsi, G.; Bergamini, C.M.; Fila, E.; Greco, P.; Valacchi, G.; Massari, L.; Gonelli, A.; Tisato, V. Association between circulatory levels of adipokines and bone mineral density in postmenopausal women. Menopause 2016, 23, 984–992. [Google Scholar] [CrossRef]
- Fassio, A.; Idolazzi, L.; Rossini, M.; Gatti, D.; Adami, G.; Giollo, A.; Viapiana, O. The obesity paradox and osteoporosis. Eat. Weight Disord. 2018, 23, 293–302. [Google Scholar] [CrossRef]
- Harlow, S.D.; Gass, M.; Hall, J.E.; Lobo, R.; Maki, P.; Rebar, R.W.; Sherman, S.; Sluss, P.M.; de Villiers, T.J.; Group, S.C. Executive summary of the Stages of Reproductive Aging Workshop + 10: Addressing the unfinished agenda of staging reproductive aging. J. Clin. Endocrinol. Metab. 2012, 97, 1159–1168. [Google Scholar] [CrossRef]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Bergamini, C.M.; Valacchi, G.; Pilotto, A.; Zuliani, G. Systemic oxidative stress and conversion to dementia of elderly patients with mild cognitive impairment. Biomed. Res. Int. 2014, 2014, 309507. [Google Scholar] [CrossRef]
- Pluskiewicz, W.; Drozdzowska, B. Comments on Sandhu et al.: Prognosis of fracture: Evaluation of predictive accuracy of the FRAX(TM) algorithm and Garvan nomogram. Osteoporos. Int. 2011, 22, 2561–2562. [Google Scholar] [CrossRef]
- Duplancic, D.; Kukoc-Modun, L.; Modun, D.; Radic, N. Simple and rapid method for the determination of uric acid-independent antioxidant capacity. Molecules 2011, 16, 7058–7068. [Google Scholar] [CrossRef] [PubMed]

| Demographic Parameters | Premenopausal Women (n = 124) | Postmenopausal Women (n = 234) |
|---|---|---|
| Age, years | 35 ± 10 | 55 ± 4 b |
| Years since menopause, years | - | 4.0 ± 0.1 |
| Hormone treatment, % * | 29 | 3 a |
| Smokers, % | 27 | 11 a |
| Osteoporosis, % | 0 | 27 a |
| Anthropometric parameters and DXA indexes of body fat distribution | ||
| BMI, kg/m2 | 23 ± 4 | 25 ± 3 b |
| Waist circumference, cm | 77 ± 10 | 85 ± 9 b |
| Trunk FM, kg | 7.8 ± 4 | 10.2 ± 3.8 a |
| Legs FM, kg | 7.7 ± 2.4 | 8.6 ± 2.3 a |
| Total FM, kg | 18.4 ± 6.9 | 22.3 ± 6.1 a |
| Markers of bone health | ||
| Lumbar spine BMD, g/cm2 | 0.99 ± 0.11 | 0.89 ± 0.09 a |
| Total hip, BMD, g/cm2 | 0.89 ± 0.10 | 0.82 ± 0.09 a |
| Femoral neck BMD, g/cm2 | 0.79 ± 0.11 | 0.70 ± 0.10 a |
| Trochanter BMD, g/cm2 | 0.67 ± 0.10 | 0.62 ± 0.09 a |
| CTX-1, ng/mL | - | 0.64 ± 0.40 |
| BAP, µg/L | - | 20.9 ± 6.8 |
| RANKL, pmol/L | - | 334.1 ± 251.9 |
| OPG, pmol/L | - | 226.8 ± 147.3 |
| RANKL/OPG | - | 2.7 ± 4.3 |
| Uric acid, µmol/L | 225 ± 76 | 239 ± 76 |
| Variables | Premenopausal Women (n = 124) | Postmenopausal Women (n = 234) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SUA | Lumbar Spine BMD | Total Hip BMD | Femoral Neck BMD | Trochanter BMD | SUA | Lumbar Spine BMD | Total Hip BMD | Femoral Neck BMD | Trochanter BMD | |
| BMI | −0.013 * | 0.227 a | 0.252 a | 0.190 | 0.261 a | 0.345 b | 0.264 b | 0.298 b | 0.240 b | 0.254 b |
| WC | 0.036 | 0.088 | 0.190 | 0.108 | 0.206 | 0.369 b | 0.286 b | 0.271 b | 0.270 b | 0.251 b |
| Trunk FM | 0.048 | 0.145 | 0.147 | 0.076 | 0.162 | 0.390 b | 0.285 b | 0.321 b | 0.291 b | 0.269 b |
| Arms FM | 0.007 | 0.128 | 0.134 | 0.078 | 0.157 | 0.292 b | 0.278 b | 0.327 b | 0.283 b | 0.266 b |
| Legs FM | 0.010 | 0.098 | 0.061 | 0.044 | 0.035 | 0.251 b | 0.162 a | 0.282 b | 0.293 b | 0.192 a |
| Total FM | 0.092 | −0.017 | −0.057 | −0.113 | −0.062 | 0.275 b | 0.241 b | 0.260 b | 0.219 b | 0.119 |
| Total FM % | 0.076 | 0.002 | −0.072 | −0.104 | −0.062 | 0.365 b | 0.159 a | 0.233 b | 0.223 b | 0.140 |
| Trunk FM % | 0.105 | 0.043 | −0.011 | −0.058 | −0.024 | 0.330 b | 0.211 b | 0.236 b | 0.202 b | 0.207 a |
| Legs FM % | 0.023 | −0.104 | −0.188 | −0.188 | −0.197 | −0.202 a | −0.055 | 0.084 | 0.123 | −0.040 |
| Multiple Regression Models | Covariate Added to Model 1 | β (R2 of Regression Model, %) | % Contribution of Total Hip BMD in Uric Acid Variance |
|---|---|---|---|
| 1 | - | 0.220 a (17.6) | 5.2 |
| 2 | BMI | 0.119 (24.0) | 1.6 ↓↓ * |
| 3 | Waist circumference | 0.119 (23.0) | 1.5 ↓↓ |
| 4 | Trunk FM | 0.100 (22.1) | 1.0 ↓↓ |
| 5 | Legs FM | 0.173 a (22.1) | 3.4 ↓ |
| 6 | Total FM | 0.101 (26.1) | 1.2 ↓↓ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaccorsi, G.; Trentini, A.; Greco, P.; Tisato, V.; Gemmati, D.; Bianchi, N.; Giganti, M.; Rossini, M.; Guglielmi, G.; Cervellati, C. Changes in Adipose Tissue Distribution and Association between Uric Acid and Bone Health during Menopause Transition. Int. J. Mol. Sci. 2019, 20, 6321. https://doi.org/10.3390/ijms20246321
Bonaccorsi G, Trentini A, Greco P, Tisato V, Gemmati D, Bianchi N, Giganti M, Rossini M, Guglielmi G, Cervellati C. Changes in Adipose Tissue Distribution and Association between Uric Acid and Bone Health during Menopause Transition. International Journal of Molecular Sciences. 2019; 20(24):6321. https://doi.org/10.3390/ijms20246321
Chicago/Turabian StyleBonaccorsi, Gloria, Alessandro Trentini, Pantaleo Greco, Veronica Tisato, Donato Gemmati, Nicoletta Bianchi, Melchiore Giganti, Maurizio Rossini, Giuseppe Guglielmi, and Carlo Cervellati. 2019. "Changes in Adipose Tissue Distribution and Association between Uric Acid and Bone Health during Menopause Transition" International Journal of Molecular Sciences 20, no. 24: 6321. https://doi.org/10.3390/ijms20246321
APA StyleBonaccorsi, G., Trentini, A., Greco, P., Tisato, V., Gemmati, D., Bianchi, N., Giganti, M., Rossini, M., Guglielmi, G., & Cervellati, C. (2019). Changes in Adipose Tissue Distribution and Association between Uric Acid and Bone Health during Menopause Transition. International Journal of Molecular Sciences, 20(24), 6321. https://doi.org/10.3390/ijms20246321