Abstract
Pterostilbene (PTER), a natural dimethylated analog of resveratrol, has been demonstrated to produce anti-neoplastic or neuroprotective actions. However, how and whether this compound can entail any perturbations on ionic currents in electrically excitable cells remains unknown. In whole-cell current recordings, addition of PTER decreased the amplitude of macroscopic Ih during long-lasting hyperpolarization in GH3 cells in a concentration-dependent manner, with an effective IC50 value of 0.84 μM. Its presence also shifted the activation curve of Ih along the voltage axis to a more hyperpolarized potential, by 11 mV. PTER at a concentration greater than 10 μM could also suppress l-type Ca2+ and transient outward K+ currents in GH3 cells. With the addition of PTER, IK(Ca) amplitude was increased, with an EC50 value of 2.23 μM. This increase in IK(Ca) amplitude was attenuated by further addition of verruculogen, but not by tolbutamide or TRAM-39. Neither atropine nor nicotine, in the continued presence of PTER, modified the PTER-stimulated IK(Ca). PTER (10 μM) slightly suppressed the amplitude of l-type Ca2+ current and transient outward K+ current. The presence of PTER (3 μM) was also effective at increasing the open-state probability of large-conductance Ca2+-activated K+ (BKCa) channels identified in hippocampal mHippoE-14 neurons; however, its inability to alter single-channel conductance was detected. Our study highlights evidence to show that PTER has the propensity to perturb ionic currents (e.g., Ih and IK(Ca)), thereby influencing the functional activities of neurons, and neuroendocrine or endocrine cells.
1. Introduction
Pterostilbene (PTER, trans-3,5-dimethoxy-4′-hydroxystilbene) is a natural dimethylated analog of resveratrol and was named after a natural phenolic compound found in Pterocarpus marsupium Roxb. (Fabaceae), which is native to India, Nepal, and Sri Lanka, and is also one of the active compounds in the extracts of P. marsupium that were used in Ayurvedic medicine for the treatment of various disorders [1]. This compound has been reported to have benefits for the prevention or treatment of different kinds of cancers, as mounting evidence has demonstrated its inhibitory effects on almost every cellular event that promotes tumor progression toward metastasis in apoptosis-dependent or apoptosis-independent manners [2,3,4,5,6,7,8,9,10,11,12,13].
Recent evidence has revealed that PTER could exert protective actions against the oxidative stress or inflammatory reaction in various types of neurons, such as neuronal SH-SY5Y cells and spinal cord neurons, as well as in ischemia- or hypoxia-induced brain injuries [14,15,16,17,18,19,20,21]. PTER and other resveratrol analogs were previously reported to exert antiepileptic actions [22,23,24] to decrease dopamine release in striatal slices, and to substantially ameliorate depressive or cognitive behavioral deficits in rats [25,26,27]. It is also noted that the presence of PTER could disrupt pituitary function [3,28]. However, whether this agent is capable of perturbing different types of membrane ionic currents in central neurons is not thoroughly investigated, although resveratrol, a phytoalexin, has previously been demonstrated to modify large-conductance Ca2+-activated K+ (BKCa) channels and voltage-gated Na+ currents in various types of cells including vascular endothelial cells, cardiac fibroblasts and cortical neurons [29,30,31].
Hyperpolarization-activated cation current (Ih) has increasingly been considered to be an important determinant of repetitive electrical activity inherent in heart cells and in a variety of neurons, and neuroendocrine or endocrine cells [32,33,34,35,36,37,38,39]. This current is a mixed inward Na+/K+ current, which is sensitive to blocking by different compounds such as CsCl, ivabradine or zatebradine [40,41], and increased amplitude of its own accord can act to depolarize membrane potential to the threshold required for elicitation of action potential (AP) [33,35,36,37,42]. The current is regarded to be carried by channels of the hyperpolarization-activated cyclic nucleotide-gated (HCN) gene family, which belongs to the superfamily of voltage-gated K+ channels and cyclic nucleotide-gated channels [36,43]. Notably, the magnitude of Ih existing in central neurons has been burgeoningly reported to be intimately linked either to spatial working memory in behaving mice or in patients with schizophrenia, or to cognitive dysfunction [25,44,45,46,47,48]. However, how PTER or other stilbenoids can directly and functionally interact with the activity of HCN channels to modify the amplitude and gating of Ih remains largely unanswered.
In the current study, we intended to explore the perturbating effects of PTER on different types of ionic currents (e.g., hyperpolarization-activated cation current [Ih], l-type Ca2+ current [ICa,l], transient outward K+ current [IK(TO)], and Ca2+-activated K+ current [IK(Ca)]) in pituitary GH3 cells. Its effect on large-conductance Ca2+-activated K+ [BKCa] channels in hippocampal mHippoE-14 neurons [4] was also examined. Findings from the present results highlight evidence to reveal that the addition of PTER is capable of suppressing Ih but stimulating IK(Ca) in a concentration-dependent manner. Those actions on the activity of ionic currents could concertedly and substantially influence the functional activities of different electrically excitable cells (e.g., GH3 cells and mHippoE-14 neurons).
2. Results
2.1. Depressant Effect of PTER on Hyperpolarization-Activated Cation Current (Ih) Identified in Pituitary GH3 Cells
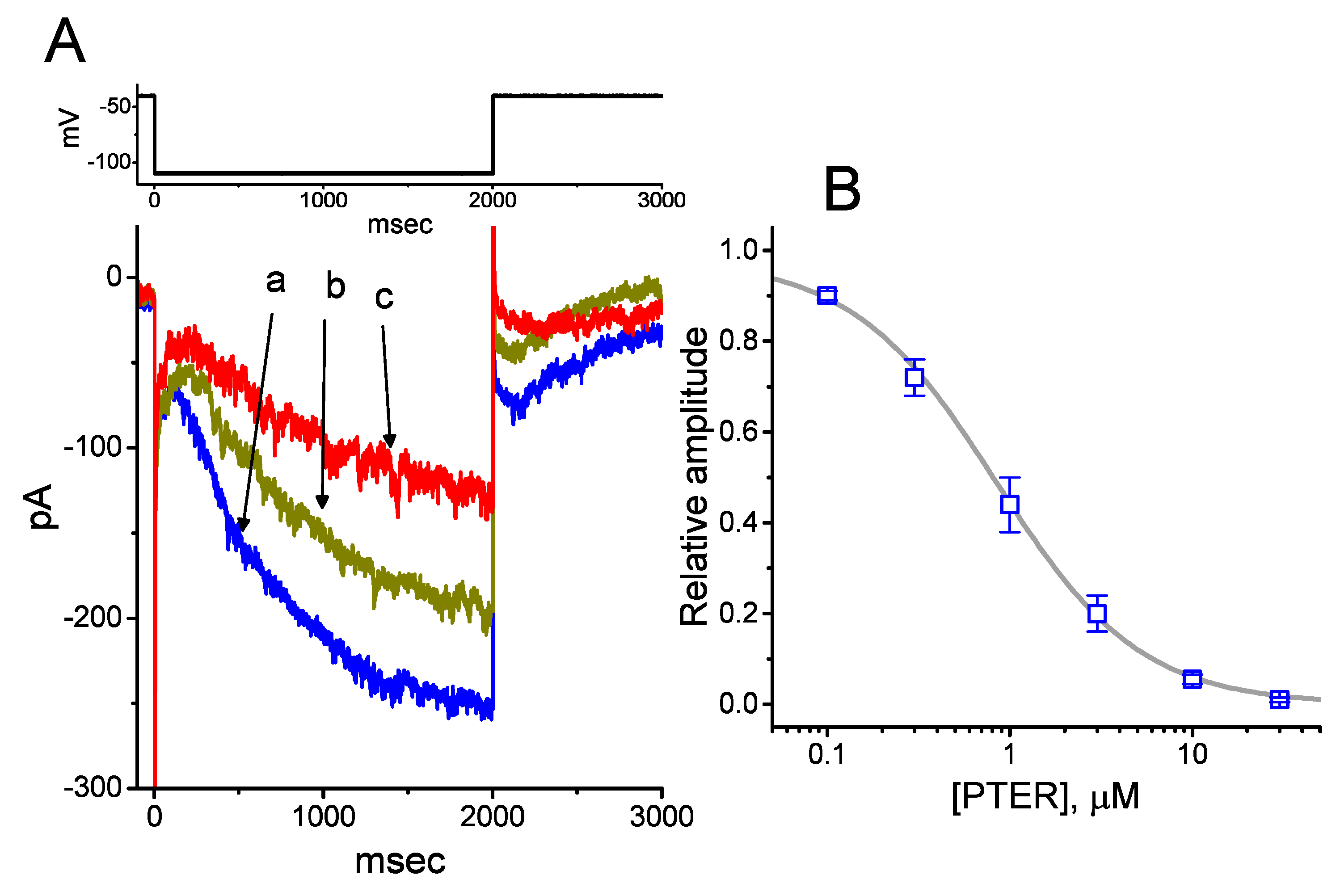
We initially evaluated whether the Ih observed in these cells [34,38,39] was subject to modification by PTER. Whole-cell experiments were conducted in cells that were suspended in Ca2+-free Tyrode’s solution, and the recording pipette used was filled with a K+-containing solution. Within 1 min of exposing cells to different concentrations of PTER, the amplitude of Ih activated during 2-s membrane hyperpolarization to −110 mV from a holding potential of −40 mV was progressively reduced (Figure 1A). For example, the addition of PTER (1 μM) substantially decreased Ih amplitude from 255 ± 18 to 118 ± 12 pA (n = 9, p < 0.05). After washout of the agent, current amplitude returned to 249 ± 17 pA (n = 8, p < 0.05). At the same time, the addition of PTER at a concentration of 0.3 or 1 μM noticeably increased the activation time constant (τ) of Ih during 2-s step hyperpolarization to 811 ± 19 ms (n = 8, p < 0.05) or 931 ± 23 ms (n = 8, p < 0.05), respectively, from a control value of 628 ± 15 ms (n = 8).
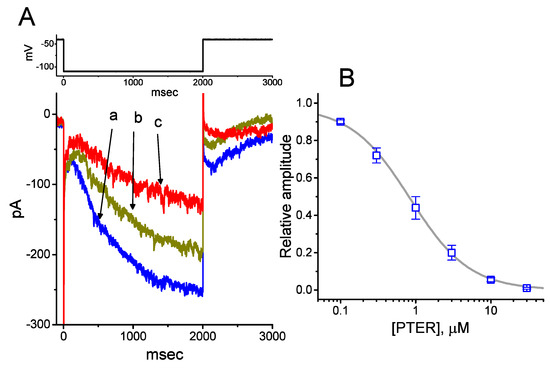
Figure 1.
Effect of pterostilbene (PTER) on hyperpolarization-activated cation current (Ih) taken from pituitary GH3 cells. In these experiments, we immersed cells in Ca2+-free Tyrode’s solution containing 1 μM tetrodotoxin (TTX), and the recording pipette used was filled with a K+-containing solution. The composition of these solutions is described under Materials and Methods. (A) Representative records of macroscopic Ih achieved under control conditions (i.e., PTER was not present, a) and during cell exposure to 0.3 μM PTER (b) and 1 μM PTER (c). The upper part is the voltage profile delivered. (B) Concentration–response relationship for PTER-induced inhibition of Ih amplitude activated during step hyperpolarization (mean ± SEM; n = 8 for each data point). Current amplitude was obtained at the end of 2-s hyperpolarizing step −110 mV from a holding potential of −40 mV. The bold sigmoidal curve indicates the best fit to the Hill equation, as detailed under Materials and Methods. The estimated values for IC50 and the Hill coefficient were 0.84 μM and 1.1, respectively.
Figure 1B illustrates that PTER can depress the amplitude of Ih activated during step hyperpolarization in a concentration-dependent fashion. The IC50 value for PTER-mediated suppression of Ih identified in GH3 cells was 0.84 μM. However, in the continued presence of PTER 1 μM, subsequent addition of atropine (10 μM) or nicotine (10 μM) to the bath medium was unable to attenuate the PTER-mediated inhibition of Ih. For example, the Ih amplitude did not differ between PTER (1 μM) alone and PTER (1 μM) plus atropine (10 μM) (133 ± 15 pA [PTER alone] versus 132 ± 16 pA [PTER plus atropine], n = 7, p > 0.05). The current–voltage (I–V) relationships of Ih established at various levels of hyperpolarizing steps were also constructed and are depicted in Figure 2. It was noted that the presence of PTER (1 μM) substantially reduced the slope of the linear fit of Ih amplitudes to the voltages between −130 and −100 mV from 27.2 ± 1.3 to 13.1 ± 1.1 nS (n = 8, p < 0.05). The results thus demonstrate that PTER has a conceivable depressant action on Ih functionally expressed in GH3 cells.
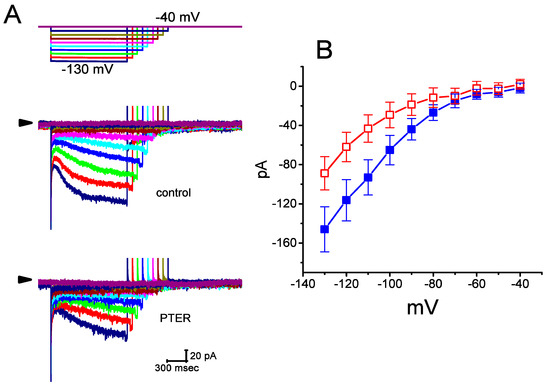
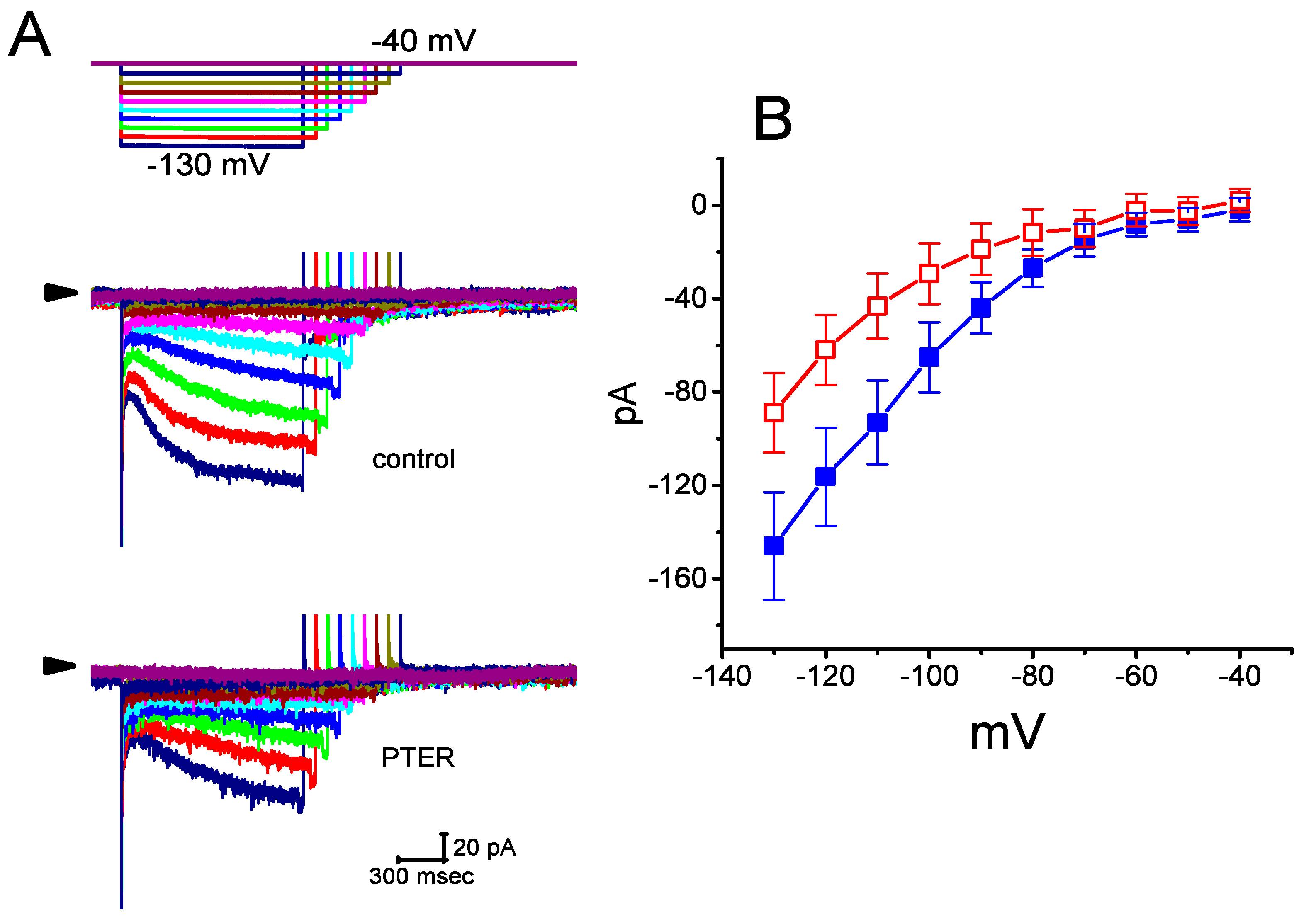
Figure 2.
Effect of PTER on the current–voltage (I–V) relationships of Ih in GH3 cells. Current traces were recorded during 2-s voltage steps to a family of membrane potentials ranging between −130 and −40 mV in 10-mV steps from a holding potential of −40 mV, as indicated in the uppermost part of (A). (A) Representative Ih traces achieved under the control conditions (i.e., PTER was not present) (upper) and during the exposure to 1 μM PTER (lower). The arrowhead in each panel depicts the zero-current level, and the calibration mark shown in the right lower side applies to all current traces. Of note, there are varying durations in the voltage-clamp profile for better illustration. (B) Averaged I–V relations of Ih achieved in the absence (■) and presence (□) of 1 μM PTER (mean ± SEM; n = 9 for each data point). Current amplitude was measured at the end of each voltage step.
2.2. Effect of PTER on the Activation Curve of Ih Recorded from GH3 Cells
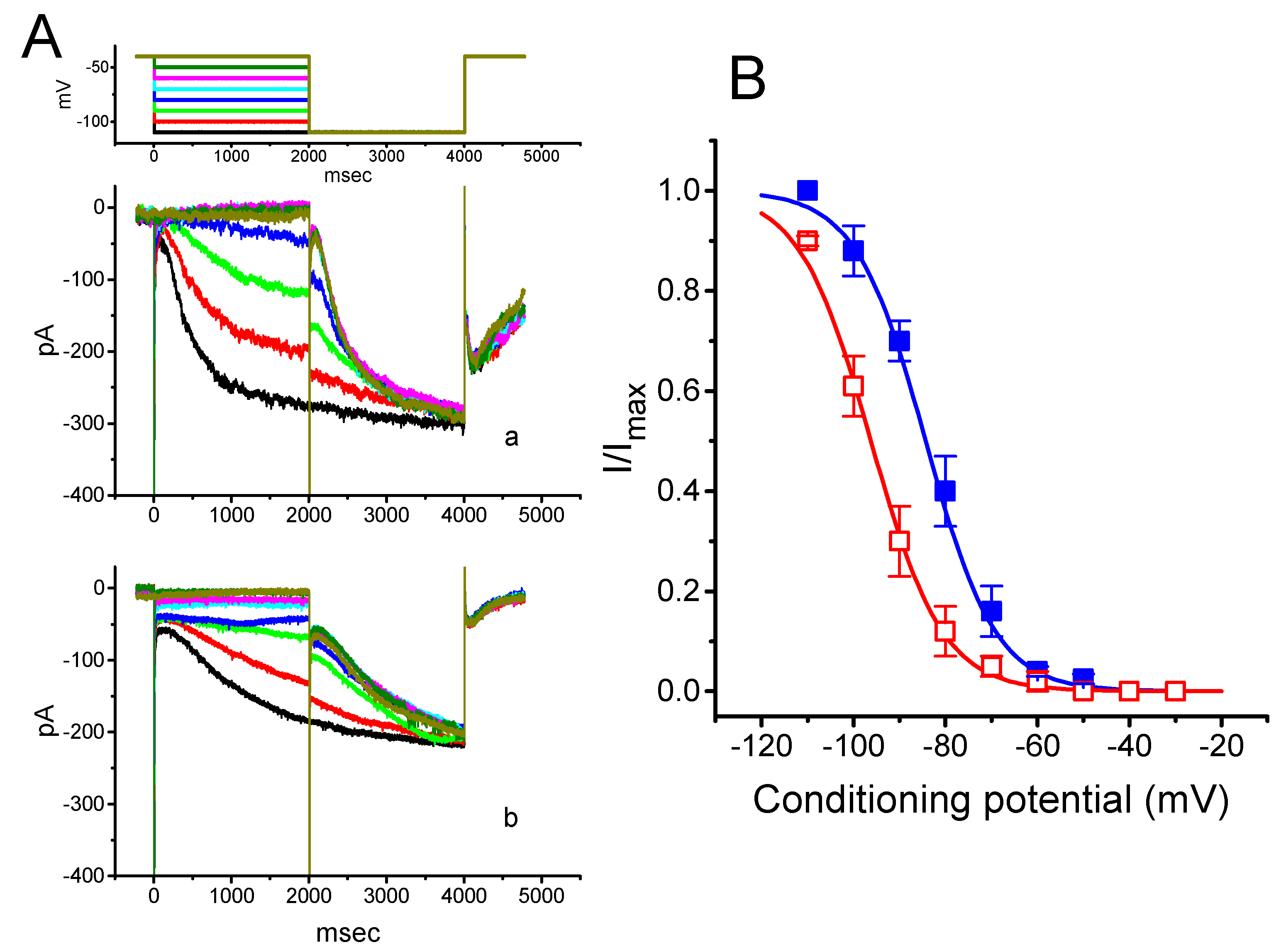
To further characterize the inhibitory effect of PTER on Ih, we investigated the voltage dependence of its effect on Ih in these cells. Figure 3 shows the quasi-steady state activation curve of Ih with or without addition of 1 μM PTER. These experiments were conducted with a two-step voltage pulse profile, that is, a 2-s conditioning pulse to various membrane potentials (i.e., the voltage between −110 and −30 mV in 10-mV steps) preceded the test pulse (2 s in duration) to −110 mV from a holding potential of −40 mV. To ensure complete recovery of Ih, the duration between two sets of voltage pulses applied was set at 60 s.
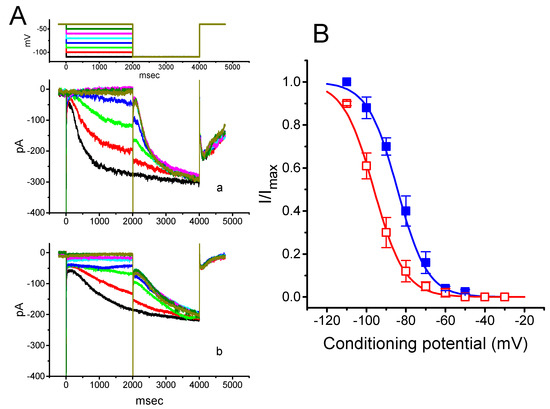
Figure 3.
Effect of PTER on the quasi-steady state activation curve of Ih recorded from GH3 cells. The experiments were conducted with a two-step voltage pulse. The conditioning potentials had a duration of 2 s to the voltage ranging from −110 to −30 mV in 10-mV steps. Following each conditioning potential, a 2-s hyperpolarizing pulse to −110 mV was applied to activate Ih. (A) Representative Ih traces evoked during this two-step voltage protocol (indicated in the uppermost part of (A)). Current records labeled “a” are controls, and those labeled “b” were taken during cell exposure to 1 μM PTER. (B) Steady-state activation curves of Ih obtained in the absence (■) and presence (□) of 1 μM PTER (mean ± SEM; n = 9) (9 for each data point). Notably, as the cells were exposed to PTER, there was a shift in the curve along the voltage axis toward more hyperpolarized potentials by 11 mV; however, no conceivable change in the gating charge of the curve was demonstrated.
The relationships between the normalized amplitudes of Ih and the conditioning potentials obtained with or without PTER (1 μM) addition were plotted and the data sets were thereafter approximately fitted to a Boltzmann function, as described in Materials and Methods. In the control, V1/2 = −83.5 ± 1.9 mV, and q = 3.07 ± 1.8 e (n = 9), whereas during exposure to 1 μM PTER, V1/2 = −94.2.5 ± 2.1 mV, and q = 3.07 ± 1.7 e (n = 9). Therefore, it is clear from these data that the presence of PTER did not merely suppress the maximal conductance of Ih, but also was able to significantly shift the activation curve of the current to a hyperpolarized potential by approximately 11 mV. However, we failed to find any change in the gating charge of the curve achieved during cell exposure to PTER. The results indicate that, besides the reduction of Ih amplitude during long-lasting step hyperpolarization, PTER can alter the voltage dependence of Ih in these cells.
2.3. Effect of PTER on l-Type Ca2+ Current (ICa,l) in GH3 Cells
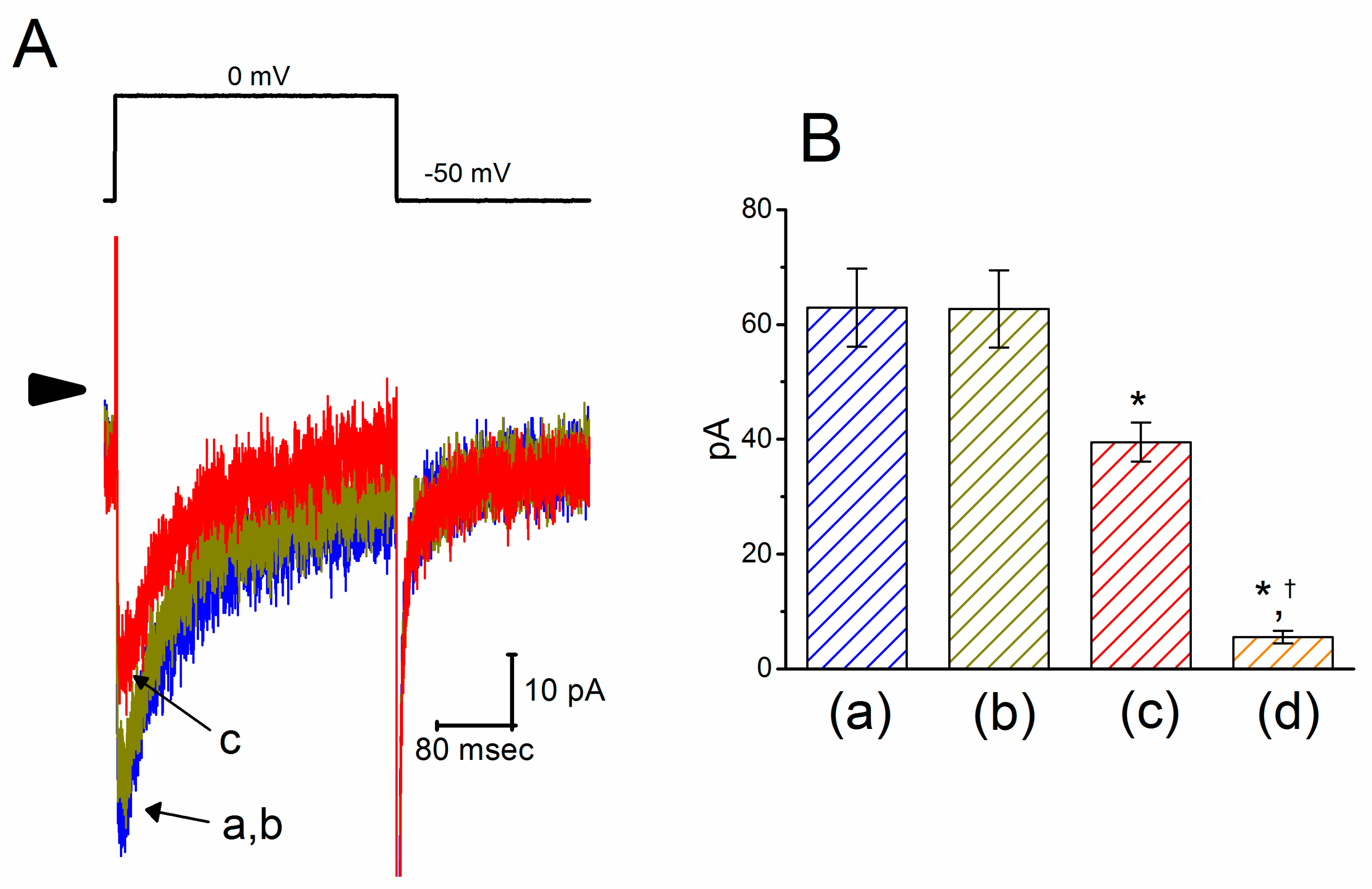
We next evaluated any possible perturbations of this agent on ICa,l previously described in these cells [49]. In these experiments, cells were bathed in normal Tyrode’s solution containing 1.8 mM CaCl2, 1 μM tetrodotoxin (TTX) and 10 mM tetraethylammonium chloride (TEA), and the pipette used was filled with a Cs+-containing solution. As shown in Figure 4, the peak amplitude of ICa,l activated during step depolarization to 0 mV from a holding potential of −50 mV was decreased in the presence of PTER (10 μM) to 39.5 ± 3.4 pA (n = 9, p < 0.05) from a control value of 62.9 ± 6.8 pA (n = 9). Moreover, subsequent addition of SDZ-202791 (3 μM), an inhibitor of ICa,l [50], but still in the continued presence of 10 μM PTER, further decreased the peak ICa,l to 5.5 ± 1.1 pA (n = 8, p < 0.05).
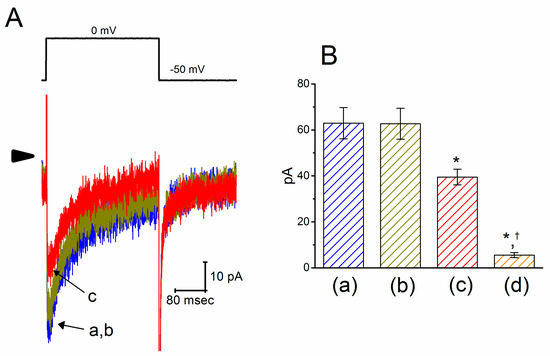
Figure 4.
Inhibitory effect of PTER on l-type Ca2+ current (ICa,l) identified in GH3 cells. This set of experiments was conducted in normal Tyrode’s solution, which contained 1.8 mM CaCl2, 10 mM tetraethylammonium chloride (TEA), and 1 μM TTX. (A) Original ICa,l traces evoked during 300-ms depolarizing pulse to 0 mV from a holding potential of −50 mV (indicated in the upper part). The arrowhead depicts the zero-current level, and the calibration mark in the right lower side applies to all current traces. The current trace labeled “a” is control, and that labeled “b” or “c” was obtained during exposure to 3 or 10 μM PTER, respectively. (B) Summary bar graph showing the effects of PTER and PTER plus SDZ-202791 on the peak amplitude of ICa,l in GH3 cells (mean ± SEM; n = 8–9 for each bar). Current amplitude was measured at the beginning of each depolarizing pulse from −50 to 0 mV. a: control; b: 3 μM PTER; c: 10 μM PTER; d: 10 μM PTER plus 3 μM SDZ-202791. *Significantly different from control (p < 0.05); †significantly different from the PTER (10 μM) alone group (p < 0.05).
2.4. Mild Inhibition by PTER of Transient Outward K+ Current (IK(TO)) Recorded from GH3 Cells
We continued to determine whether PTER could alter another type of K+ current, i.e., IK(TO). This set of whole-cell current recordings was conducted in cells bathed in Ca2+-free Tyrode’s solution, and the pipette was filled with K+-containing solution. However, unlike its modulatory effects on Ih or IK(Ca) mentioned above, IK(TO) was relatively resistant to being altered by the presence of PTER (3 μM). However, PTER at a concentration higher than 10 μM significantly suppressed the amplitude of IK(TO), accompanied by a slowing in the inactivation time course of the current (Figure 5). For example, the exposure to 10 μM or 30 μM PTER decreased the peak amplitude of IK(TO) to 387 ± 12 pA (n = 8, p < 0.05) or 256 ± 11 pA (n = 8, p < 0.05), respectively, from the control value of 517 ± 16 pA (n = 8). Concomitant with these data, the values for the inactivation time constant (τ) of IK(TO) during membrane depolarization in the presence of 10 or 30 μM PTER were significantly reduced to 90.9 ± 8.2 ms (n = 8, p < 0.05) or 83.1 ± 6.2 ms (n = 8, p < 0.05), respectively, from the control value of 94.8 ± 9.8 ms (n = 8).
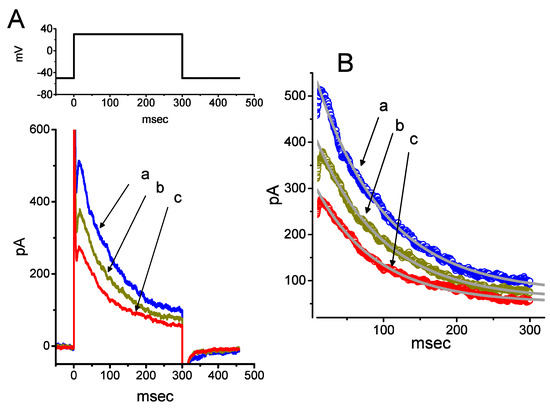
Figure 5.
Inhibitory effect of PTER on transient outward K+ current (IK(TO)) in GH3 cells. Cells were bathed in Ca2+-free Tyrode’s solution, and the recording pipette was filled with K+-containing solution containing 1 μM TTX. (A) Superimposed IK(TO) traces acquired in the control (a) and after the addition of 10 μM PTER (b) or 30 μM PTER (c). The upper part indicates the voltage protocol applied, i.e., the holding potential was −50 mV and clamp pulses to +50 mV with a duration of 300 ms were applied. (B) Effect of PTER on the inactivation time course of IK(TO) elicited during 300-ms membrane depolarization. The bold approximate curves are nonlinear least squares fits of a single exponential to the records. a: control; b: 10 μM PTER; c: 30 μM PTER. The values of the inactivation time constant (τ) for IK(TO) labeled “a”, “b” and “c” were estimated to be 94.8, 91.2 and 83.1 ms, respectively.
2.5. Effect of PTER on Ca2+-Activated K+ Current (IK(Ca)) in GH3 Cells
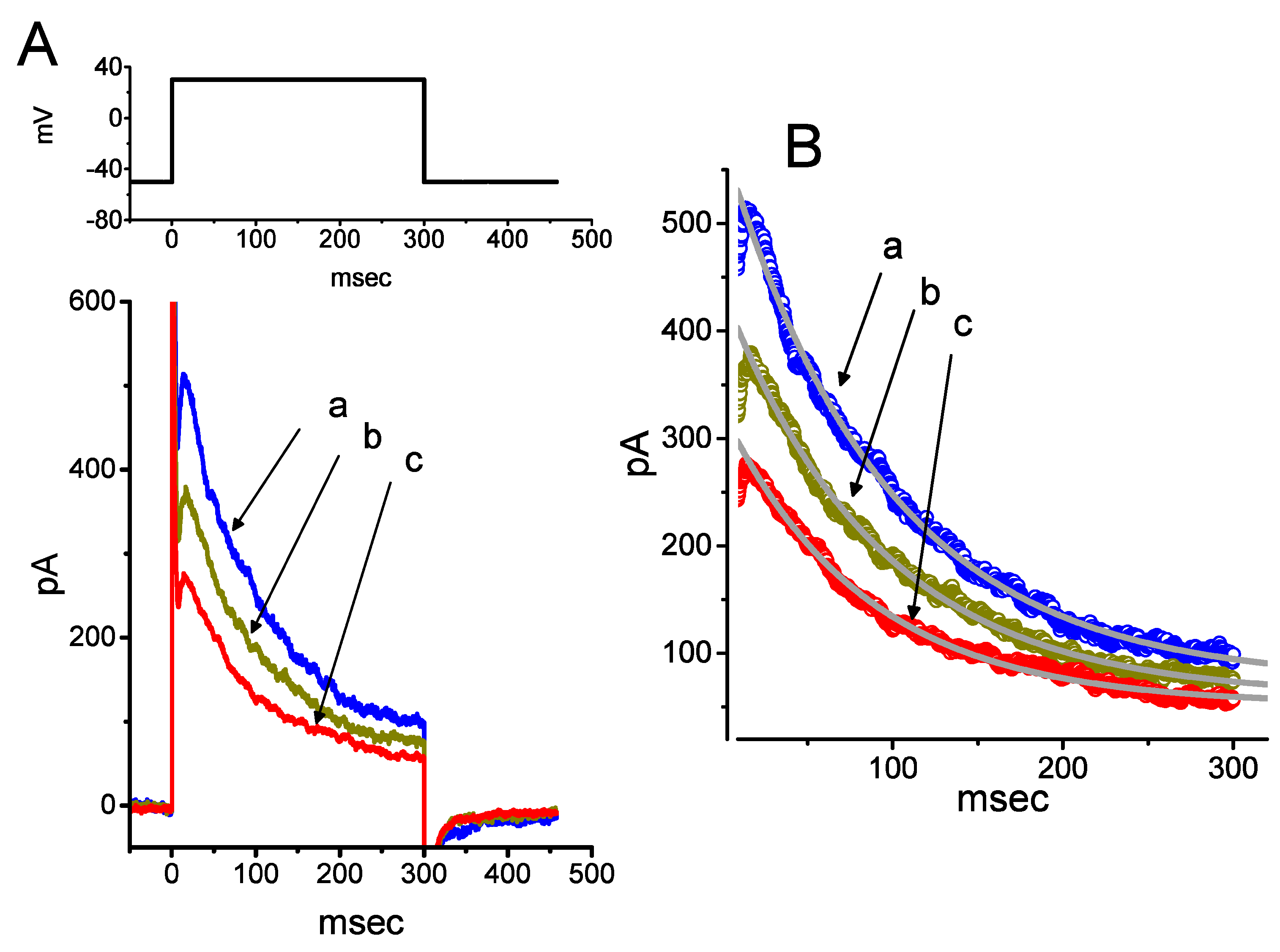
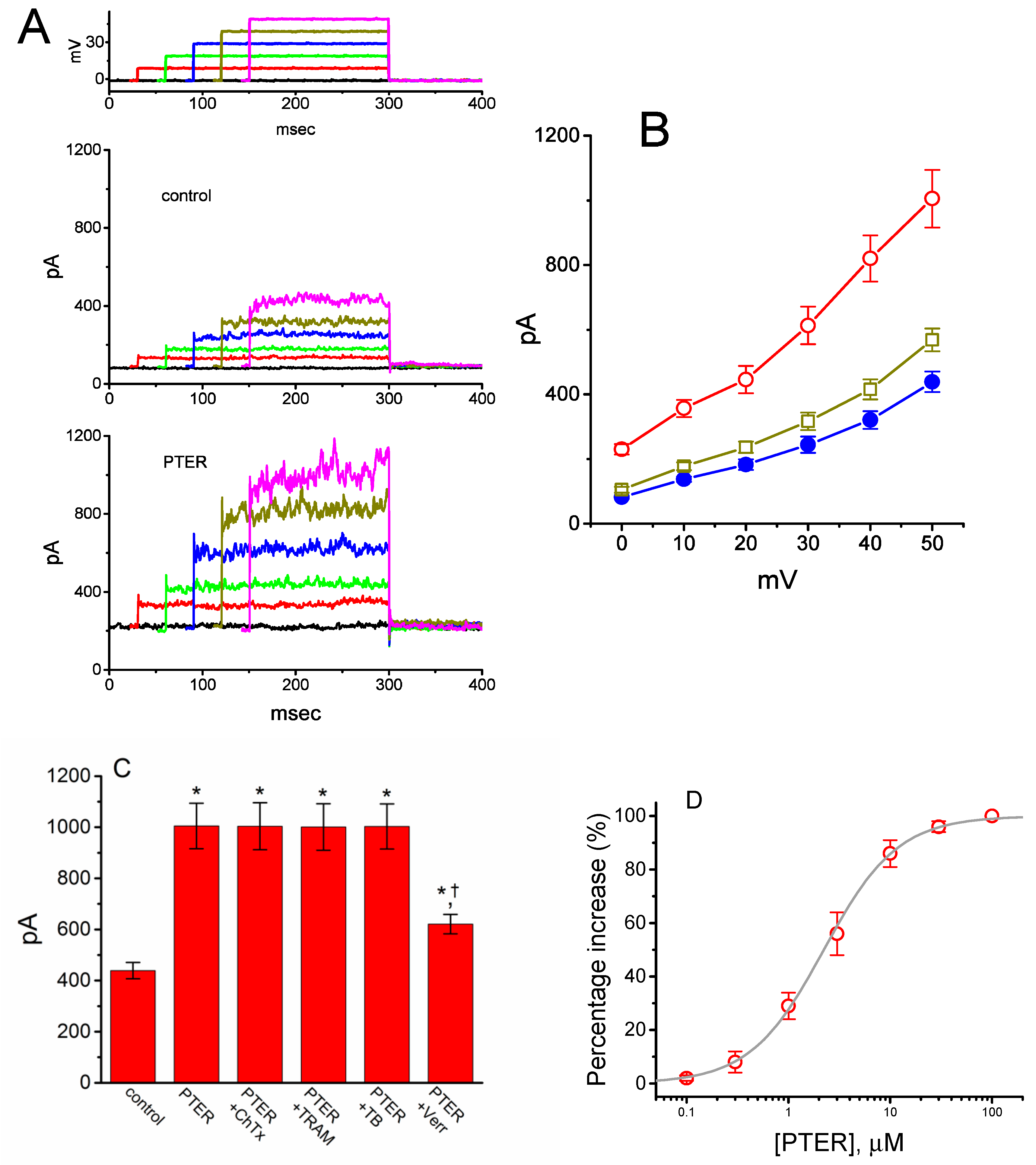
Earlier observations have reported the ability of resveratrol to activate BKCa channels [29,30,31]. In the next set of experiments, we determined whether PTER could alter the amplitude of IK(Ca) activated in response to membrane depolarization present in GH3 cells. In this set of whole-cell current recordings, we suspended GH3 cells in normal Tyrode’s solution containing 1.8 mM CaCl2, and the pipette used was filled with K+-containing solution, the composition of which is mentioned above. As cells settled down the bottom of the chamber, whole-cell current recordings were made. In an attempt to inactivate most voltage-gated K+ currents [51,52], we maintained the examined cells at the level of 0 mV, and then applied a series of voltage pulses between 0 and +50 mV with 10-mV steps. Within 1 min of exposing GH3 cells to PTER (3 μM), the amplitude of IK(Ca) elicited by this voltage profile evidently rose (Figure 6A). For example, the addition of 3 μM PTER substantially increased the IK(Ca) amplitude elicited by depolarizing pulse from 0 to +50 mV, from 568 ± 35 to 1005 ± 89 pA (n = 8, p < 0.05). As the agent was washed out, the current amplitude returned to 621 ± 38 pA (n = 8). The averaged I–V relationships of IK(Ca) amplitude in the control, during the exposure to 3 μM PTER and after washout of the compound are depicted in Figure 6B. The addition of 3μM PTER substantially increased the whole-cell conductance of IK(Ca) measured at the voltages between +30 and +50 mV to 19.6 ± 0.8 nS (n = 8, p < 0.05) from the control value of 12.6 ± 0.5 nS (n = 8). Moreover, as summarized in Figure 6C, IK(Ca) amplitude increased by 3 μM PTER was suppressed by subsequent addition of verruculogen (1 μM), yet not by chlorotoxin (1 μM), TRAM-39 (3 μM) or tolbutamide (10 μM). Chlorotoxin or verruculogen is a blocker of Cl− or BKCa channels, respectively, while TRAM-39 or tolbutamide was reported to inhibit intermediate-conductance Ca2+-activated K+ (IKCa) channels or ATP-sensitive K+ (KATP) channels, respectively [4]. For example, subsequent addition of verruculogen (1 μM), but still in the presence of 3 μM PTER, attenuated the IK(Ca) amplitude from 1012 ± 92 to 621 ± 38 pA (n = 8, p < 0.05). Therefore, unlike its inhibitory action on IK(TO), PTER could increase the amplitude of IK(Ca) observed in these cells, and its stimulation of IK(Ca) was largely attributable to the activation of large-conductance Ca2+-activated K+ (BKCa) channels.
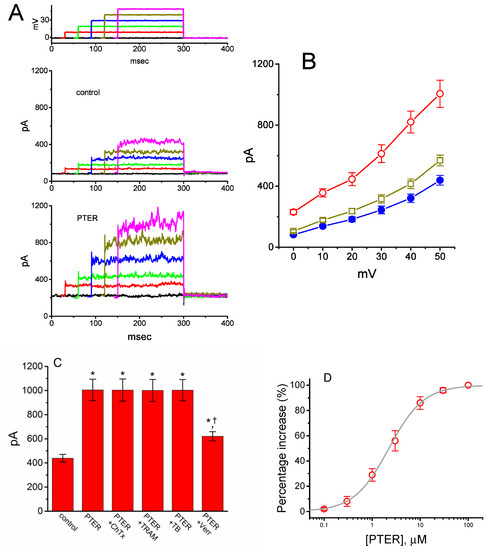
Figure 6.
Stimulatory effect of PTER on Ca2+-activated K+ current (IK(Ca)) identified in GH3 cells. This set of whole-cell current recordings was conducted in cells suspended in normal Tyrode’s solution, which contained 1.8 mM CaCl2. (A) Representative IK(Ca) traces obtained during the voltage steps to various membrane potentials between 0 and +50 mV in 10-mV steps from a holding potential of 0 mV (as indicated in the uppermost part of (A)). Records shown in the upper and lower panels were obtained under control conditions and during exposure to 3 μM PTER, respectively. The uppermost part depicts the voltage pulses applied. (B) Averaged I–V relations of IK(Ca) obtained in the control (●), during exposure to 3 μM PTER (○), and after washout of the agent (□) (mean ± SEM; n = 8 for each data point). (C) Summary bar graph showing the effects of PTER, PTER plus chlorotoxin, PTER plus TRAM-39, PTER plus tolbutamide, and PTER plus verruculogen on IK(Ca) amplitude (mean ± SEM; n = 8 for each bar). Current amplitude was measured at the end of the voltage step from 0 to +50 mV. PTER: 3 μM PTER; ChTx: 1 μM chlorotoxin; TRAM: 1 μM TRAM-39; TB: 10 μM tolbutamide; Verr: 1 μM verruculogen. *Significantly different from control (p < 0.05); †significantly different from the PTER (3 μM) alone group (p < 0.05). (D) Concentration-dependent effect of PTER on IK(Ca) amplitude activated by membrane depolarization (mean ± SEM; n = 7–8 for each data point). Current amplitude was measured at the end of the depolarizing pulse from 0 to +50 mV. As cells were exposed to PTER (100 μM), current amplitude was taken to be 100%, and current amplitude achieved at different concentrations was then compared with the control value. The bold sigmoidal curve indicates a best fit to a modified Hill function, as detailed in Materials and Methods. The estimated values for EC50 and the Hill coefficient were 2.23 μM and 1.2, respectively.
Figure 6D shows the relationship between the PTER concentration and the percentage increase of IK(Ca). PTER could increase the amplitude of IK(Ca) activated during membrane depolarization in a concentration-dependent manner. The half maximal concentration (EC50) required for the stimulatory effect of PTER on IK(Ca) was 2.23 μM, and at a concentration of 100 μM, it fully increased the current amplitude. These results thus demonstrate the effectiveness of PTER in producing a stimulatory action on IK(Ca) in GH3 cells.
2.6. Activity of Large-Conductance Ca2+-Activated K+ (BKCa) Channels Stimulated by PTER in mHippoE-14 Neurons
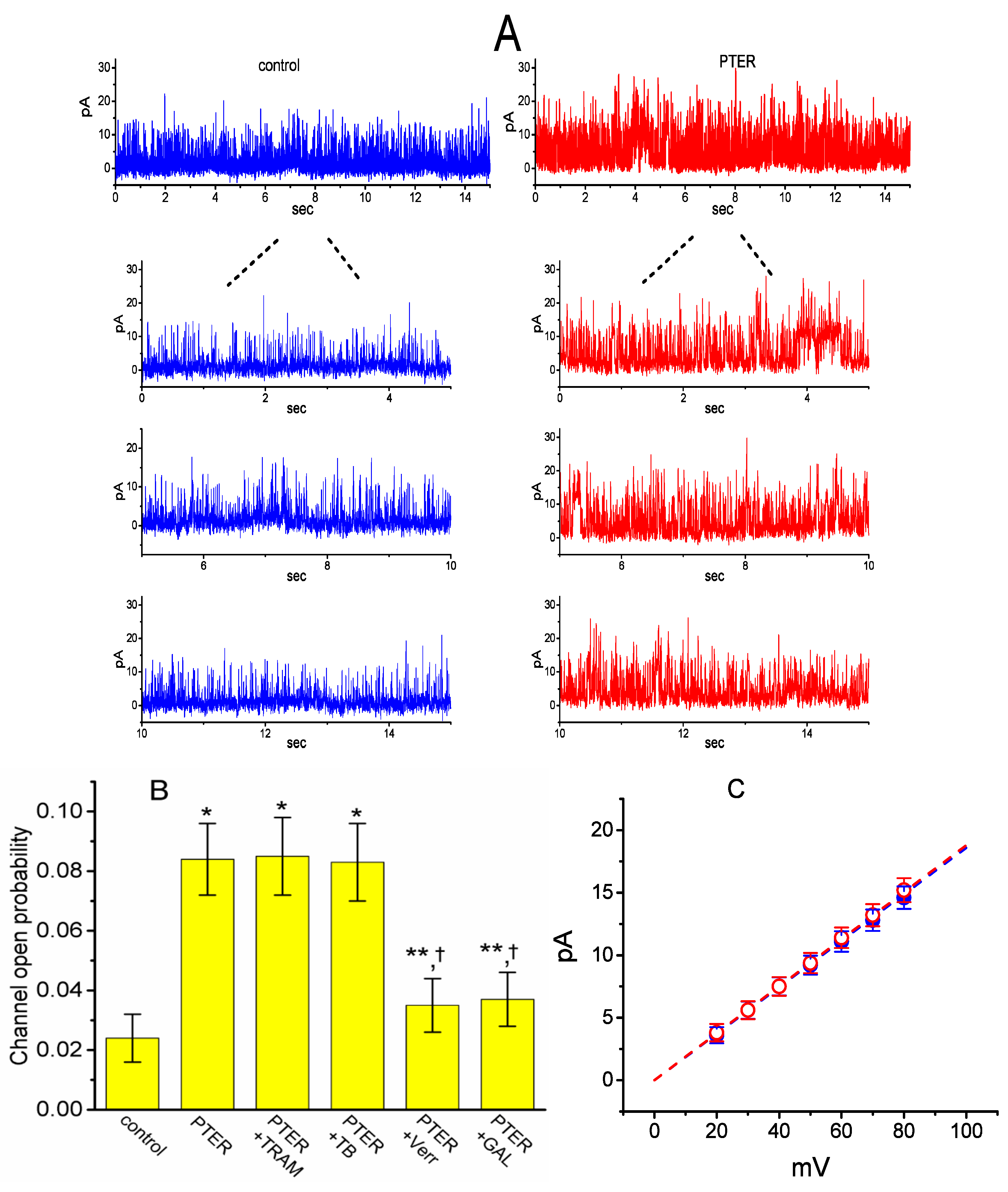
We next tested the question of whether the presence of PTER could perturb the single-channel currents flowing through BKCa channels identified in these cells [53]. As depicted in Figure 7A, under inside-out current recordings, when PTER at a concentration of 3 μM was applied to the intracellular leaflet of the detached patch, the probability of BKCa channels that would be open substantially rose, as evidenced by a significant increase in channel activity to 0.084 ± 0.012 (n = 10, p < 0.01) from a control value of 0.024 ± 0.008 (n = 10), although minimal change in single-channel current was noticeable in the presence of PTER. After washout of the agent, BKCa-channel activity returned to 0.029 ± 0.009 (n = 7, p < 0.01).
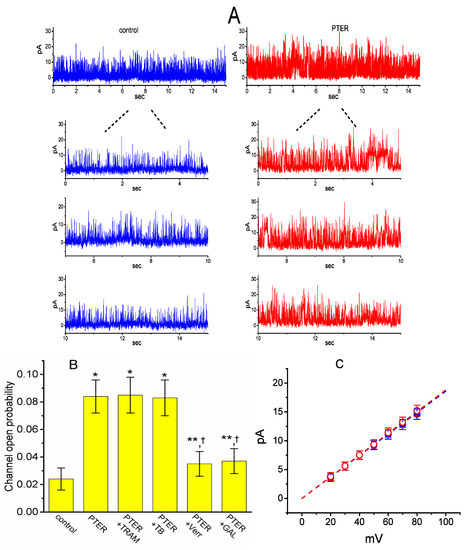
Figure 7.
Stimulatory effect of PTER on the activity of large-conductance Ca2+-activated K+ (BKCa) channels recorded from the detached patch of hippocampal mHippoE-14 neurons. In these experiments, cells were bathed in a high-K+ solution containing 0.1 μM Ca2+, and inside-out configuration with a holding potential of +60 mV was made. (A) Single-channel currents flowing through BKCa channels obtained under control conditions (i.e., PTER was not present, left) and after bath application of 3 μM PTER (i.e., at the cytosolic face of the detached patch, right). The lower portions in each panel indicate the expanded records of the uppermost part in the absence (left) and presence (right) of 3 μM PTER. Upward deflections represent channel openings. (B) Summary bar graph depicting the effects of PTER, PTER plus TRAM-39, PTER plus tolbutamide, PTER plus verruculogen, and PTER plus GAL-021 (mean ± SEM; n = 8–10 for each bar). Inside-out current recordings with a holding potential of +60 mV were performed to measure the channel opening probability. PTER: 3 μM pterostilbene; TRAM: 3 μM TRAM-39; TB: 10 μM tolbutamide; Verr: 1 μM verruculogen; GAL-021: 10 μM GAL-021. * or **: Significantly different from control (p < 0.01 or p < 0.05, respectively); †significantly different from the PTER (3 μM) alone group (p < 0.05). (C) Averaged I–V relations of BKCa channels in the absence (●) and presence (○) of 3 μM PTER (mean ± SEM; n = 8 for each data point). In inside-out current recordings, single-channel amplitude was measured at the level of each holding potential. Of note, the single-channel conductances (indicated by a dashed line) in the absence and presence of PTER are virtually superimposable with each other.
2.7. Comparisons of the Effects of PTER, PTER Plus TRAM-39, PTER Plus Tolbutamide, PTER Verruculogen, and PTER Plus GAL-021 on the Probability of BKCa-Channel Openings in mHippoE-14 Neurons
We further evaluated whether the stimulatory effect of PTER on BKCa-channel activity can be altered by subsequent application of TRAM-39, tolbutamide, verruculogen or GAL-021. In these experiments, in which cells were bathed in a high-K+ solution containing 0.1 μM Ca2+, inside-out patch configuration was made and the potential was constantly held at +60 mV. As summarized in Figure 7B, in the continued presence of 3 μM PTER, subsequent addition of neither TRAM-39 (3 μM) nor tolbutamide (10 μM) substantially modified PTER-mediated elevation in the channel opening probability, while that of verrculogen (1 μM) or GAL-021 (10 μM) was capable of reversing the PTER-induced raise in channel activity. GAL-021 is recognized as an intravenous inhibitor of BKCa channels [54]. For example, further application of GAL-021 (10 μM), but still in the presence of PTER (3 μM), attenuated channel activity from 0.084 ± 0.012 to 0.037 ± 0.009 (n = 8, p < 0.05).
2.8. Lack of PTER Effect on Single-Channel Conductance of BKCa Channels in mHippoE-14 Neurons
The activity of BKCa channels at different levels of membrane potentials obtained with or without PTER addition was further assessed and compared in these cells. Figure 6B depicts I–V relations of BKCa channels established under control conditions and during exposure to PTER (3 μM). The single-channel conductance of BKCa channels derived from the linear I–V relation in the control (i.e., when PTER was not present) was 186 ± 9 pS (n = 8), a value that did not differ significantly from that achieved after the application of PTER (188 ± 7 pS; n = 8, p > 0.05). It is clear, therefore, from these data that the presence of PTER resulted in no considerable change in the single-channel conductance of the channel; however, it did increase the open-state probability of BKCa channels in mHippoE-14 neurons.
3. Discussion
The principal findings from the present study are as follows. First, in pituitary GH3 cells, the addition of PTER inhibited Ih effectively in a concentration- and time-dependent manner. Second, the steady state activation curve of Ih was distinctly shifted to more hyperpolarizing potentials by 11 mV, producing channel opening at more negative voltages. Third, PTER at a concentration higher than 10 μM was able to suppress the amplitude of ICa,l and IK(TO). Fourth, its presence substantially raised the amplitude of macroscopic IK(Ca). Fifth, in hippocampal mHippoE-14 neurons, under inside-out patch recordings, the addition of PTER failed to change the single-channel conductance of BKCa channels; however, it did increase the probability of the channels that would be opened. The present observations, therefore, reflect that the effects on the modifications of ion-channel activity could conceivably be one of the ionic mechanisms underlying PTER-induced actions, if similar in vitro or in vivo results can emerge in central neurons (e.g., mHippoE-14 neurons), and in neuroendocrine or endocrine cells (e.g., GH3 cells).
Previous studies have shown that stilbenes might increase the sensitivity of striatal muscarinic receptors and suppress the activity of acetylcholinesterase [20,25]. PTER analogs were also recently reported to be antagonists of nicotinic acetylcholine receptors [55]. However, in the continued presence of PTER, further application of atropine, a blocker of muscarinic receptors, or nicotine, an activator of nicotinic receptors, was unable to modify PTER-mediated inhibition of Ih in GH3 cells. Thus, PTER-induced blocking of Ih or stimulation of IK(Ca) seen in GH3 cells could not be predominantly attributed to either an increase in acetylcholine level or its binding to cholinergic receptors.
Four mammalian subtypes (HCN1, HCN2, HCN3, and HCN4) have been cloned to date [36,37,43,56]. Previous work has mentioned that HCN2, HCN3, or mixed HCN2 + HCN3 channels could be functionally expressed in GH3 cells or various types of central neurons and endocrine or neuroendocrine cells [35,37,38,39,57]. Because of the crucial roles of Ih (i.e., HCNx-encoded currents) in contributing to the excitability and automaticity existing in various types of electrically excitable cells [33,35,37,38,39,42,49,57], findings from this study could provide substantial insights into the electrophysiological and pharmacological properties of PTER and other structurally related compounds.
It has previously been demonstrated that long-term treatment with PTER might be able to suppress cholesterol biosynthesis, thus causing the intracellular accumulation of oxysterols [58]. Depletion of membrane cholesterol with methyl-β-cyclodextrin was previously reported to increase the activity of large-conductance Ca2+-activated K+ (BKCa) channels [59]. Membrane cholesterol content was also shown to modify the amplitude of HCN-encoded currents [56]. However, the PTER-induced blocking of Ih observed in GH3 cells is rapid in onset and hence appears to be unlinked to its suppression in the conversion of 7-dehydrocholesterol to cholesterol occurring inside the cell.
The addition of PTER was observed to enhance the amplitude of IK(Ca) in GH3 cells. One would expect that IK(Ca) amplitude stimulated by PTER could result from the elevation of ICa,l flowing through l-type Ca2+ channels. However, in this study, the presence of PTER at a concentration higher than 10 μM decreased the peak amplitude of ICa,l in response to membrane depolarization. It therefore seems unlikely that the PTER stimulation of macroscopic IK(Ca) presented herein ascribes indirectly from an increase in intracellular Ca2+ concentration through elevation of membranous Ca2+-channel activity. In keeping with these observations, BKCa-channel activity observed in the detached patches of mHippoE-14 neurons was substantially raised after bath application of PTER.
Resveratrol was previously reported to stimulate BKCa-channel activity [29,31]. In the continued presence of PTER (3 μM), further addition of 3 μM resveratrol did not produce any stimulatory effect on the probability of BKCa-channel openings in mHippoE-14 neurons. The present results demonstrate that the stimulatory effect of PTER and resveratrol on the activity of BKCa channels was not additive, suggesting that these two compounds, which are structurally related, may functionally interact with similar binding sites existing in the BKCa channel.
Stilbenoids, including PTER, have the ability to modulate the magnitude of GABA-induced Cl− currents [60]. However, in the current study, the PTER-mediated increase in IK(Ca) amplitude was not substantially affected by further addition of either chlorotoxin (1 μM), a blocker of Cl− channels, or tolbutamide (10 μM), an inhibitor of KATP channels, or TRAM-39 (3 μM), an inhibitor of IKCa channels. Therefore, the possibility proposing that PTER-stimulated IK(Ca) observed in GH3 cells or hippocampal mHippoE-14 neurons is largely mediated through its modulation of Cl−-, KATP- or IKCa-channel activity would be virtually excluded.
Besides the suppression of Ih amplitude activated in response to membrane hyperpolarization, the activation time course of the current progressively became slowed during the exposure to PTER. In other words, the blocking of Ih produced by PTER is apparently not instantaneous, but it does develop over time after HCN channel(s) become opened (i.e., Ih was activated) upon long-lasting membrane hyperpolarization. It is also noted that the τ value of IK(TO) inactivation in response to membrane depolarization was increased in the presence of PTER higher than 10 μM. It is reasonable to assume that the PTER molecules exhibit a low affinity for the resting state and a high affinity for the activated state of the channel(s) in GH3 cells. The blocking site of PTER thus appears to be located within the channel pore only when the channel is open (i.e., when macroscopic Ih is activated). However, to what extent the PTER-induced block of Ih is linked to its ability to form hydrogen bonds and to generate stable resonance structures in the presence of oxidative electrophilic molecules, possibly in or around the HCN channels, will deserve vigorous further study.
It needs to be emphasized that the PTER addition to GH3 cells produced significant depressant action on the amplitude of Ih in a concentration-dependent manner, with an IC50 value of 0.84 μM, a value that is slightly lower than the EC50 value (i.e., 2.23 μM) required for its stimulation of IK(Ca). No discernible modification in the single-channel conductance of BKCa channels in mHippoE-14 neurons was demonstrated; however, it did enhance the probability of BKCa-channel openings. By use of a two-step voltage protocol, exposure to PTER (1 μM) could also distinctly shift the steady state activation curve of Ih along the voltage axis in a leftward direction by 11 mV. Therefore, the sensitivity of Ih to PTER could be dependent on the PTER concentration achieved, the level of resting potential, the firing rate of APs, or a combination. The serum PTER level measured in rats was reported to reach 0.12 μM [25]. The actions of PTER or other stilbenoids on ionic currents presented herein could have characteristics that make it significant from a therapeutic or toxicological standpoint. To this end, PTER-mediated perturbations of Ih, IK(Ca) or BKCa-channel activity are of particular importance, and they tend to be upstream of its actions on aberrant signaling pathways including regulation in DNA methylation, inhibition of telomerase activity, or other anti-neoplastic or anti-oxidative activities [7,10,16,18,19,58,61,62,63,64].
4. Materials and Methods
4.1. Chemicals, Drugs and Solutions
Pterostilbene (PTER, trans-3,5-dimethoxy-4-hydroxystilbene), atropine, nicotine, resveratrol (3,4′,5-trihydroxy-trans-stilbene), tetraethylammonium chloride (TEA), tetrodotoxin (TTX) and tolbutamide were acquired from Sigma-Aldrich (Merck, Taipei, Taiwan), while TRAM-39 (2-chloro-α,α-diphenylbenzeneacetonitrile) was from Tocris (Union Biomed Inc., Taipei, Taiwan). Verruculogen was obtained from Alomone (Asia Bioscience, Taipei, Taiwan), and GAL-021 (N2-methoxy-N2-methyl-N4,N6-dipropyl-1,3,5-triazine-2,4,6-triamine) was from MedChemExpress (Everything Biotech Ltd., New Taipei City, Taiwan), while SDZ-202791 was from Santa Cruz (Hong Jing, New Taipei City, Taiwan). Chlorotoxin was a kind gift provided by Professor Dr. Woei-Jer Chuang (Department of Biochemistry, National Cheng Kung University Medical College, Tainan City, Taiwan). Cell culture media, l-glutamine, horse serum, and fetal bovine or calf serum were generally obtained from HyClone™ (Thermo Fisher Scientific, Logan, UT, USA), whereas other chemicals, including CsCl, CsOH, aspartic acid, EGTA, and HEPES, were commercially available and of analytical reagent grade.
The composition of the standard extracellular solution (i.e., normal Tyrode’s solution), in which cells were suspended, was as follows (in mM): NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5, and HEPES-NaOH buffer 5.5 (pH 7.4). To measure Ih or IK(Ca) and preclude contamination of Cl− currents, the patch pipette was backfilled with a solution (in mM) containing: K-aspartate 130, KCl 20, KH2PO4 1, MgCl2 1, Na2ATP 3, Na2GTP 0.1, EGTA 0.1, and HEPES-KOH buffer 5 (pH 7.2). To record ICa,l, the pipette solution contained (in mM): CsCl 130, EGTA 0.1, MgCl2 1, Na2ATP 3, Na2GTP 0.1 and HEPES-CsOH buffer 5 (pH 7.2). For the recordings of BKCa-channel activity, the bath solution was replaced with a high K+ solution (in mM) containing: KCl 145, MgCl2 0.53, and HEPES-KOH buffer 5 (pH 7.2), and the pipettes were filled with a solution (in mM) consisting of KCl 145, MgCl2 2, and HEPES-KOH buffer 5 (pH 7.4). All solutions were prepared using deionized water from a Milli-Q water purification system (APS Water Services, Inc., Van Nuys, CA, USA), and both the pipette solution and culture medium used were commonly filtered on the day of use with a sterile Acrodisc® filter with a 0.2-μm Supor® membrane (Pall Corp., Port Washington, NY, USA).
4.2. Cell Preparations
GH3 pituitary tumor cells, acquired from the Bioresources Collection and Research Center ([BCRC-60015]; Hsinchu, Taiwan; originally derived from ATCC [CCL-82.1]), were maintained in Ham’s F-12 medium supplemented with 15% horse serum (v/v), 2.5% fetal calf serum (v/v), and 2 mM l-glutamine. An established embryonic mouse hippocampal cell line (mHippoE-14; CLU198) was acquired from Cedarlane (Burlington, ON, Canada) [65,66,67,68]. mHippoE-14 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and 2 mL l-glutamine. GH3 or mHippoE-14 cells were plated as a monolayer culture on 50-mL plastic culture flasks in a humidifier environment of 5% CO2/95% air at 37 °C. The medium was refreshed every 2 days to maintain a healthy cell population. Under our experimental conditions, the presence of neurites and varicosities during the preparations of mHippoE-14 neurons could often be observed. The recordings were performed 5 or 6 days after cells were subcultured (60%–80% confluence).
4.3. Electrophysiological Measurements
Prior to each experiment, cells (i.e., GH3 cells and mHippoE-14 neurons) were gently dissociated, and we transferred an aliquot of cell suspension to a home-made chamber mounted on the fixed stage of an inverted TMS-F microscope (Nikon, Tokyo, Japan). The microscope was coupled to a video camer system with magnification up to 1500×. Cells were immersed at room temperature (20–25 °C) in normal Tyrode’s solution. The patch electrodes used were drawn from Kimax-51 glass capillaries (#34500; Kimble, Vineland, NJ, USA) on a PP-830 vertical puller (Narishige, Tokyo, Japan), and their tips fire-polished with MF-83 microforge (Narishige). When the electrodes were filled with different internal solutions, their resistances generally ranged from 3 to 5 MΩ. A three-dimensional oil-driven micromanipulator (MO-103; Narishige, Tokyo, Japan) was used to precisely position the electrode near the cell examined. Ionic currents were measured in the whole-cell, cell-attached or inside-out configuration of the patch-clamp technique by using an RK-400 patch-clamp amplifier (Bio-Logic, Claix, France) [51,52]. The liquid junction potentials were generally nulled shortly before seal formation was made, and the whole-cell results were corrected by the potentials measured under our experimental conditions.
4.4. Data Recordings
The signals, comprising both potential and current traces, were acquired through analog-to-digital conversion and monitored on the organic light-emitting diode (OLED) display of an ASUS VivoBook Flip-14 touch-screen laptop computer (TP412U; Taipei City, Taiwan). The records were stored online at 10 kHz on the computer, connected with a Digidata 1440A interface (Molecular Devices, Sunnyvale, CA, USA). During the recordings, the latter device was controlled by the pCLAMP 10.7 software package (Molecular Devices). Current signals obtained were low-pass filtered at 2 kHz with an FL-4 four-pole Bessel filter (Dagan, Minneapolis, MN, USA). Through digital-to-analog conversion, pCLAMP-generated profiles comprising different rectangular or linear ramp waveforms were designed to acquire data for constructing either the current versus voltage (I–V) relationship or the activation curve of Ih. After the digital data were collected, we later analyzed them using various analytical tools including the LabChart 7.0 program (AD Instruments; Gerin, Tainan, Taiwan), 64-bit OriginPro 2016 (Microcal, Northampton, MA, USA), or custom-built macros run under Microsoft Excel™ 2016 (Redmond, WA, USA).
4.5. Data Analyses
To evaluate the concentration-dependent inhibition of PTER on the amplitude of Ih, the cells were immersed in Ca2+-free Tyrode’s solution, the cell examined was held at −40 mV, and a 2-s hyperpolarizing pulse to −110 mV was driven to activate Ih. Current amplitudes at the end of each hyperpolarizing pulse were measured under control conditions and during cell exposure to different PTER concentrations (0.1–30 μM). The concentration required to suppress 50% of Ih amplitude was determined with the goodness of fit by using a modified Hill function (Equation (1)):
where [PTER] is the PTER concentration applied; IC50 and nH are the concentration required for a 50% inhibition and the Hill coefficient, respectively; and maximal inhibition (i.e., 1 − a) was also estimated in this equation.
To determine the inhibitory action of PTER on the voltage dependence of Ih, the quasi-steady state activation curve of the current was achieved by using a two-step protocol. The relationships between the conditioning voltage pulses and the normalized amplitudes of Ih with and without PTER (1 μM) addition are described and were hence fitted by a Boltzmann function (Equation (2)) in the following form:
where Imax is the maximal activated Ih, V the conditioning potential, V1/2 the membrane for half-maximal activation, q the apparent gating charge, and F, R, or T is Faraday’s constant, the universal gas constant or the absolute temperature, respectively.
To assess the concentration-dependent effect of PTER on IK(Ca) amplitude, each cell was depolarized to +50 mV from a holding potential of 0 mV. Current amplitude measured at the end of depolarizing voltage during cell exposure to 100 μM PTER was considered to be 100%. Mean values in then concentration-dependent relation of PTER to the stimulation of IK(Ca) were least-squares fitted to the Hill equation (Equation (3)). That is,
where [PTER] represents the concentration of PTER, EC50 is the concentration that produces 50% of maximal stimulation, nH is the Hill coefficient, and Emax is the PTER-induced maximal stimulation of IK(Ca).
4.6. Single-Channel Analyses
Single-channel currents flowing through BKCa channels measured from surface membranes of GH3 cells were recorded and analyzed using pClamp 10.7 (Molecular Devices). Single-channel amplitudes were commonly evaluated by fitting Gaussian distributions to the amplitude histograms of the closed and open states. Channel activity was defined as N·PO, namely the product of the channel number (N) and open probability (PO). Single-channel conductance was calculated by linear regression using mean values of current amplitudes measured at the different levels of the holding potentials.
4.7. Statistical Analyses
The data regarding macroscopic and single-channel currents were collected and are provided as the mean values ± standard error of the mean (SEM), with sample sizes (n) indicating the number of cells used to analyze the experimental results, and error bars were plotted as SEM. We made assertions about the variability of means that could be collected from a random cohort derived from the population concerned; hence, the SEM could be more appropriate than the standard deviation. The paired or unpaired Student’s t-test or a one-way analysis of variance (ANOVA) followed by post-hoc Fisher’s least-significance difference test for multiple comparisons were commonly implemented for statistical evaluation. However, as the assumption of normality underlying ANOVA was possibly violated, we used the non-parametric Kruskal–Wallis test. Values of p < 0.05 were regarded as statistically significant, unless stated otherwise.
Author Contributions
Conceptualization, E.C.S.; Data curation, Z.-H.G.; Formal analysis, S.-N.W.; Project administration, S.Y.K. and S.-N.W.; Supervision, S.Y.K. and S.-N.W.; Visualization, Z.-H.G.; Writing—original draft, E.C.S.; Writing—review and editing, S.Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
Original research reported in this paper is partly supported by grants from National Cheng Kung University (D107-F2519 and NCKUH-10709001), from the Ministry of Education (D108-F2507), from the Ministry of Science and Technology (MOST-108-2314-B-006-094), An Nan Hospital, China Medical University (ANHRF108-03), and Chang Jung Christian University (K108041).
Acknowledgments
The authors thank Kaisen Lee and Sih-Wei Li for their helpful assistance.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AP | Action potential |
| BKCa | Channel large-conductance Ca2+-activated K+ channel |
| EC50 | The concentration required for 50% stimulation |
| HCN channel | Hyperpolarization-activated cyclic nucleotide-gated ion channel |
| IC50 | The concentration required for 50% inhibition |
| ICa,l | l-type Ca2+ current |
| Ih | Hyperpolarization-activated cation current |
| IK(Ca) | Ca2+-activated K+ current |
| IK(M) | M-type K+ current |
| IK(TO) | Transient outward K+ current |
| I–V | Current versus voltage |
| PTER | Pterostilbene (trans-3,5-dimethoxy-4′-hydroxystilbene) |
| SEM | Standard error of the mean |
| τ | Time constant |
| TEA | Tetraethylammonium chloride |
| TTX | Tetrodotoxin |
References
- Ahmad, H.; Rajagopal, K. Pharmacology of Pterocarpus marsupium Roxib. Med. Plant Res. 2015, 5, 1–6. [Google Scholar]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Benlloch, M.; Obrador, E.; Valles, S.L.; Rodriguez, M.L.; Sirerol, J.A.; Alcacer, J.; Pellicer, J.A.; Salvador, R.; Cerda, C.; Saez, G.T.; et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid. Redox Signal. 2016, 24, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Ruan, J.S.; Wu, S.N. Evidence of Decreased Activity in Intermediate-Conductance Calcium-Activated Potassium Channels During Retinoic Acid-Induced Differentiation in Motor Neuron-Like NSC-34 Cells. Cell Physiol. Biochem. 2018, 48, 2374–2388. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Mi, Y.; Li, T.; Gong, Y.; Ouyang, H.; Hou, Y. Long Non-coding RNAs Contribute to the Inhibition of Proliferation and EMT by Pterostilbene in Human Breast Cancer. Front. Oncol. 2018, 8, 629. [Google Scholar] [CrossRef]
- Pan, M.H.; Wu, J.C.; Ho, C.T.; Lai, C.S. Antiobesity molecular mechanisms of action: Resveratrol and pterostilbene. Biofactors 2018, 44, 50–60. [Google Scholar] [CrossRef]
- Chatterjee, K.; Mukherjee, S.; Vanmanen, J.; Banerjee, P.; Fata, J.E. Dietary Polyphenols, Resveratrol and Pterostilbene Exhibit Antitumor Activity on an HPV E6-Positive Cervical Cancer Model: An in vitro and in vivo Analysis. Front. Oncol. 2019, 9, 352. [Google Scholar] [CrossRef]
- Czop, M.; Bogucka-Kocka, A.; Kubrak, T.; Knap-Czop, K.; Makuch-Kocka, A.; Galkowski, D.; Wawer, J.; Kocki, T.; Kocki, J. Imaging Flow Cytometric Analysis of Stilbene-Dependent Apoptosis in Drug Resistant Human Leukemic Cell Lines. Molecules 2019, 24, 1896. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Chen, N.C.; Koh, Y.C.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Pterostilbene Inhibits Adipocyte Conditioned-Medium-Induced Colorectal Cancer Cell Migration through Targeting FABP5-Related Signaling Pathway. J. Agric. Food Chem. 2019, 67, 10321–10329. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, X.; Xu, L.; Liu, D.; Di, S.; Li, W.; Zhang, J.; Zhang, H.; Li, X.; Han, J.; et al. Pterostilbene: Mechanisms of its action as oncostatic agent in cell models and in vivo studies. Pharmacol. Res. 2019, 145, 104265. [Google Scholar] [CrossRef]
- Tan, K.T.; Chen, P.W.; Li, S.; Ke, T.M.; Lin, S.H.; Yang, C.C. Pterostilbene inhibits lung squamous cell carcinoma growth in vitro and in vivo by inducing S phase arrest and apoptosis. Oncol. Lett. 2019, 18, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Yang, S.F.; Hung, T.W.; Lin, C.L.; Hsieh, Y.H.; Chiou, H.L. Inhibition of eIF2alpha dephosphorylation accelerates pterostilbene-induced cell death in human hepatocellular carcinoma cells in an ER stress and autophagy-dependent manner. Cell Death Dis. 2019, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, B.; Feng, Q.; Xu, Z.; Huang, C.; Wu, H.; Chen, Z.; Hu, L.; Gao, L.; Liu, P.; et al. DCZ0801, a novel compound, induces cell apoptosis and cell cycle arrest via MAPK pathway in multiple myeloma. Acta Biochim. Biophys. Sin. 2019, 51, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Han, S.; Pan, X.; Gong, Y.; Wang, M. Pterostilbene mediates neuroprotection against oxidative toxicity via oestrogen receptor alpha signalling pathways. J. Pharm. Pharmacol. 2015, 67, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.M.; Ma, A.; Zhang, Y.L.; Chen, Y.Y.; Zhou, H.; Li, W.J.; Jin, X. Orally administrated pterostilbene attenuates acute cerebral ischemia-reperfusion injury in a dose- and time-dependent manner in mice. Pharmacol. Biochem. Behav. 2015, 135, 199–209. [Google Scholar] [CrossRef]
- Li, D.; Song, T.; Yang, L.; Wang, X.; Yang, C.; Jiang, Y. Neuroprotective actions of pterostilbene on hypoxic-ischemic brain damage in neonatal rats through upregulation of heme oxygenase-1. Int. J. Dev. Neurosci. 2016, 54, 22–31. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Li, Y.; Fan, C.; Jiang, S.; Zhao, L.; Di, S.; Xin, Z.; Wang, B.; Wu, G.; et al. HO-1 Signaling Activation by Pterostilbene Treatment Attenuates Mitochondrial Oxidative Damage Induced by Cerebral Ischemia Reperfusion Injury. Mol. Neurobiol. 2016, 53, 2339–2353. [Google Scholar] [CrossRef]
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene attenuates high glucose-induced oxidative injury in hippocampal neuronal cells by activating nuclear factor erythroid 2-related factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 827–837. [Google Scholar] [CrossRef]
- He, J.L.; Dong, X.H.; Li, Z.H.; Wang, X.Y.; Fu, Z.A.; Shen, N. Pterostilbene inhibits reactive oxygen species production and apoptosis in primary spinal cord neurons by activating autophagy via the mechanistic target of rapamycin signaling pathway. Mol. Med. Rep. 2018, 17, 4406–4414. [Google Scholar] [CrossRef]
- Nagumo, M.; Ninomiya, M.; Oshima, N.; Itoh, T.; Tanaka, K.; Nishina, A.; Koketsu, M. Comparative analysis of stilbene and benzofuran neolignan derivatives as acetylcholinesterase inhibitors with neuroprotective and anti-inflammatory activities. Bioorg. Med. Chem. Lett. 2019, 29, 2475–2479. [Google Scholar] [CrossRef]
- van den Brand, A.D.; Villevoye, J.; Nijmeijer, S.M.; van den Berg, M.; van Duursen, M.B.M. Anti-tumor properties of methoxylated analogues of resveratrol in malignant MCF-7 but not in non-tumorigenic MCF-10A mammary epithelial cell lines. Toxicology 2019, 422, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nieoczym, D.; Socala, K.; Gawel, K.; Esguerra, C.V.; Wyska, E.; Wlaz, P. Anticonvulsant Activity of Pterostilbene in Zebrafish and Mouse Acute Seizure Tests. Neurochem. Res. 2019, 44, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Nieoczym, D.; Socala, K.; Jedziniak, P.; Wyska, E.; Wlaz, P. Effect of Pterostilbene, a Natural Analog of Resveratrol, on the Activity of some Antiepileptic Drugs in the Acute Seizure Tests in Mice. Neurotox. Res. 2019, 36, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Hsieh, C.P.; Chan, M.H.; Chan, T.Y.; Chen, L.; Chen, H.H. Distinct effects of resveratrol on seizures and hyperexcitability induced by NMDA and 4-aminopyridine. Nutr. Neurosci. 2019, 22, 867–876. [Google Scholar] [CrossRef]
- Joseph, J.A.; Fisher, D.R.; Cheng, V.; Rimando, A.M.; Shukitt-Hale, B. Cellular and behavioral effects of stilbene resveratrol analogues: Implications for reducing the deleterious effects of aging. J. Agric. Food Chem. 2008, 56, 10544–10551. [Google Scholar] [CrossRef]
- La Spina, M.; Sansevero, G.; Biasutto, L.; Zoratti, M.; Peruzzo, R.; Berardi, N.; Sale, A.; Azzolini, M. Pterostilbene Improves Cognitive Performance in Aged Rats: An in Vivo Study. Cell Physiol. Biochem. 2019, 52, 232–239. [Google Scholar]
- Yang, L.; Ran, Y.; Quan, Z.; Wang, R.; Yang, Q.; Jia, Q.; Zhang, H.; Li, Y.; Peng, Y.; Liang, J.; et al. Pterostilbene, an active component of the dragon’s blood extract, acts as an antidepressant in adult rats. Psychopharmacology 2019, 236, 1323–1333. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Li, X.; Zhang, Y. Effects of pterostilbene on treating hyperprolactinemia and related mechanisms. Am. J. Trans. Res. 2016, 8, 3049–3055. [Google Scholar]
- Li, H.F.; Chen, S.A.; Wu, S.N. Evidence for the stimulatory effect of resveratrol on Ca2+-activated K+ current in vascular endothelial cells. Cardiovasc. Res. 2000, 45, 1035–1045. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lin, M.W.; Wu, S.N.; Sung, R.J. The activation by estrogen receptor agonists of the BKCa-channel in human cardiac fibroblasts. Biochem. Pharmacol. 2007, 73, 1347–1357. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chan, M.H.; Chen, L.; Wu, S.N.; Chen, H.H. Resveratrol attenuates cortical neuron activity: Roles of large conductance calcium-activated potassium channels and voltage-gated sodium channels. J. Biomed. Sci. 2016, 23, 47. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.A.; Pape, H.C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J. Physiol. 1990, 431, 291–318. [Google Scholar] [CrossRef] [PubMed]
- Irisawa, H.; Brown, H.F.; Giles, W. Cardiac pacemaking in the sinoatrial node. Physiol. Rev. 1993, 73, 197–227. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Wang, Y.J.; Wu, P.Y.; Wu, S.N. Tramadol-induced block of hyperpolarization-activated cation current in rat pituitary lactotrophs. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 127–135. [Google Scholar] [CrossRef]
- Stojilkovic, S.S.; Tabak, J.; Bertram, R. Ion channels and signaling in the pituitary gland. Endocr. Rev. 2010, 31, 845–915. [Google Scholar] [CrossRef]
- He, C.; Chen, F.; Li, B.; Hu, Z. Neurophysiology of HCN channels: From cellular functions to multiple regulations. Prog. Neurobiol. 2014, 112, 1–23. [Google Scholar] [CrossRef]
- Spinelli, V.; Sartiani, L.; Mugelli, A.; Romanelli, M.N.; Cerbai, E. Hyperpolarization-activated cyclic-nucleotide-gated channels: Pathophysiological, developmental, and pharmacological insights into their function in cellular excitability. Can. J. Physiol. Pharmacol. 2018, 96, 977–984. [Google Scholar] [CrossRef]
- Chang, W.T.; Gao, Z.H.; Lo, Y.C.; Wu, S.N. Evidence for Effective Inhibitory Actions on Hyperpolarization-Activated Cation Current Caused by Ganoderma Triterpenoids, the Main Active Constitutents of Ganoderma Spores. Molecules 2019, 24, 4256. [Google Scholar] [CrossRef]
- Hsiao, H.T.; Liu, Y.C.; Liu, P.Y.; Wu, S.N. Concerted suppression of Ih and activation of IK(M) by ivabradine, an HCN-channel inhibitor, in pituitary cells and hippocampal neurons. Brain Res. Bull. 2019, 149, 11–20. [Google Scholar] [CrossRef]
- Romanelli, M.N.; Cerbai, E.; Dei, S.; Guandalini, L.; Martelli, C.; Martini, E.; Scapecchi, S.; Teodori, E.; Mugelli, A. Design, synthesis and preliminary biological evaluation of zatebradine analogues as potential blockers of the hyperpolarization-activated current. Bioorg. Med. Chem. 2005, 13, 1211–1220. [Google Scholar] [CrossRef]
- Novella Romanelli, M.; Sartiani, L.; Masi, A.; Mannaioni, G.; Manetti, D.; Mugelli, A.; Cerbai, E. HCN Channels Modulators: The Need for Selectivity. Curr. Top. Med. Chem. 2016, 16, 1764–1791. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. Serious workings of the funny current. Prog. Biophys. Mol. Biol. 2006, 90, 13–25. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, J.C.; DiFrancesco, D. Dysfunctional HCN ion channels in neurological diseases. Front. Cell Neurosci. 2015, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ramos, B.P.; Paspalas, C.D.; Shu, Y.; Simen, A.; Duque, A.; Vijayraghavan, S.; Brennan, A.; Dudley, A.; Nou, E.; et al. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell 2007, 129, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gamo, N.J.; Yang, Y.; Jin, L.E.; Wang, X.J.; Laubach, M.; Mazer, J.A.; Lee, D.; Arnsten, A.F. Neuronal basis of age-related working memory decline. Nature 2011, 476, 210–213. [Google Scholar] [CrossRef]
- Paspalas, C.D.; Wang, M.; Arnsten, A.F. Constellation of HCN channels and cAMP regulating proteins in dendritic spines of the primate prefrontal cortex: Potential substrate for working memory deficits in schizophrenia. Cereb. Cortex 2013, 23, 1643–1654. [Google Scholar] [CrossRef] [PubMed]
- Omrani, A.; van der Vaart, T.; Mientjes, E.; van Woerden, G.M.; Hojjati, M.R.; Li, K.W.; Gutmann, D.H.; Levelt, C.N.; Smit, A.B.; Silva, A.J.; et al. HCN channels are a novel therapeutic target for cognitive dysfunction in Neurofibromatosis type 1. Mol. Psychiatry 2015, 20, 1311–1321. [Google Scholar] [CrossRef]
- Vargish, G.A.; McBain, C.J. The Hyperpolarization-Activated Cation Current Ih: The Missing Link Connecting Cannabinoids to Cognition. Neuron 2016, 89, 889–891. [Google Scholar] [CrossRef][Green Version]
- Wu, S.N.; Chiang, H.T.; Shen, A.Y.; Lo, Y.K. Differential effects of quercetin, a natural polyphenolic flavonoid, on L-type calcium current in pituitary tumor (GH3) cells and neuronal NG108-15 cells. J. Cell Physiol. 2003, 195, 298–308. [Google Scholar] [CrossRef]
- Triggle, D.J.; Rampe, D. 1,4-Dihydropyridine activators and antagonists: Structural and functional distinctions. Trends Pharmacol. Sci. 1989, 10, 507–511. [Google Scholar] [CrossRef]
- Wu, S.N.; Li, H.F.; Jan, C.R.; Shen, A.Y. Inhibition of Ca2+-activated K+ current by clotrimazole in rat anterior pituitary GH3 cells. Neuropharmacology 1999, 38, 979–989. [Google Scholar] [CrossRef]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Kuo, H.C.; Cheng, L.H.; Lee, Y.H.; Chang, W.T.; Wang, B.J.; Wang, Y.J.; Cheng, H.C. Apoptotic and Nonapoptotic Activities of Pterostilbene against Cancer. Int. J. Mol. Sci. 2018, 19, 287. [Google Scholar] [CrossRef] [PubMed]
- Golder, F.J.; Dax, S.; Baby, S.M.; Gruber, R.; Hoshi, T.; Ideo, C.; Kennedy, A.; Peng, S.; Puskovic, V.; Ritchie, D.; et al. Identification and Characterization of GAL-021 as a Novel Breathing Control Modulator. Anesthesiology 2015, 123, 1093–1104. [Google Scholar] [CrossRef]
- Bavo, F.; Pucci, S.; Fasoli, F.; Lammi, C.; Moretti, M.; Mucchietto, V.; Lattuada, D.; Viani, P.; De Palma, C.; Budriesi, R.; et al. Potent Antiglioblastoma Agents by Hybridizing the Onium-Alkyloxy-Stilbene Based Structures of an alpha7-nAChR, alpha9-nAChR Antagonist and of a Pro-Oxidant Mitocan. J. Med. Chem. 2018, 61, 10531–10544. [Google Scholar] [CrossRef]
- Furst, O.; D’Avanzo, N. Isoform dependent regulation of human HCN channels by cholesterol. Sci. Rep. 2015, 5, 14270. [Google Scholar] [CrossRef]
- Hao, X.M.; Xu, R.; Chen, A.Q.; Sun, F.J.; Wang, Y.; Liu, H.X.; Chen, H.; Xue, Y.; Chen, L. Endogenous HCN Channels Modulate the Firing Activity of Globus Pallidus Neurons in Parkinsonian Animals. Front. Aging Neurosci. 2019, 11, 190. [Google Scholar] [CrossRef]
- Chakraborty, A.; Bodipati, N.; Demonacos, M.K.; Peddinti, R.; Ghosh, K.; Roy, P. Long term induction by pterostilbene results in autophagy and cellular differentiation in MCF-7 cells via ROS dependent pathway. Mol. Cell Endocrinol. 2012, 355, 25–40. [Google Scholar] [CrossRef]
- Lin, M.W.; Wu, A.Z.; Ting, W.H.; Li, C.L.; Cheng, K.S.; Wu, S.N. Changes in membrane cholesterol of pituitary tumor (GH3) cells regulate the activity of large-conductance Ca2+-activated K+ channels. Chin. J. Physiol. 2006, 49, 1–13. [Google Scholar]
- Rueda, D.C.; Schoffmann, A.; De Mieri, M.; Raith, M.; Jahne, E.A.; Hering, S.; Hamburger, M. Identification of dihydrostilbenes in Pholidota chinensis as a new scaffold for GABAA receptor modulators. Bioorg. Med. Chem. 2014, 22, 1276–1284. [Google Scholar] [CrossRef]
- Beetch, M.; Lubecka, K.; Shen, K.; Flower, K.; Harandi-Zadeh, S.; Suderman, M.; Flanagan, J.M.; Stefanska, B. Stilbenoid-Mediated Epigenetic Activation of Semaphorin 3A in Breast Cancer Cells Involves Changes in Dynamic Interactions of DNA with DNMT3A and NF1C Transcription Factor. Mol. Nutr. Food Res. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Chen, Y.Y.; Yeh, Y.L.; Wang, Y.J.; Chen, R.J. Stilbene Compounds Inhibit Tumor Growth by the Induction of Cellular Senescence and the Inhibition of Telomerase Activity. Int. J. Mol. Sci. 2019, 20, 2716. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, G.; Pourgheysari, B.; Shirzad, H.; Sourani, Z. Pterostilbene increases Fas expression in T-lymphoblastic leukemia cell lines. Res. Pharm. Sci. 2019, 14, 55–63. [Google Scholar] [PubMed]
- Zhou, J.; Ci, X.; Ma, X.; Yu, Q.; Cui, Y.; Zhen, Y.; Li, S. Pterostilbene Activates the Nrf2-Dependent Antioxidant Response to Ameliorate Arsenic-Induced Intracellular Damage and Apoptosis in Human Keratinocytes. Front. Pharmacol. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, S.; Kim, G.L.; Chalmers, J.A.; Koletar, M.M.; Wang, X.; Wang, Y.; Belsham, D.D. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience 2010, 170, 54–66. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, M.C.; Hung, T.Y.; Lin, K.M.; Huang, C.W.; Wu, S.N. Pioglitazone, a PPAR-gamma Activator, Stimulates BKCa but Suppresses IK M in Hippocampal Neurons. Front. Pharmacol. 2018, 9, 977. [Google Scholar] [CrossRef]
- Huang, C.W.; Lin, K.M.; Hung, T.Y.; Chuang, Y.C.; Wu, S.N. Multiple Actions of Rotenone, an Inhibitor of Mitochondrial Respiratory Chain, on Ionic Currents and Miniature End-Plate Potential in Mouse Hippocampal (mHippoE-14) Neurons. Cell Physiol. Biochem. 2018, 47, 330–343. [Google Scholar] [CrossRef]
- So, E.C.; Foo, N.P.; Ko, S.Y.; Wu, S.N. Bisoprolol, Known to Be a Selective beta(1)-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons. Int. J. Mol. Sci. 2019, 20, 657. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).