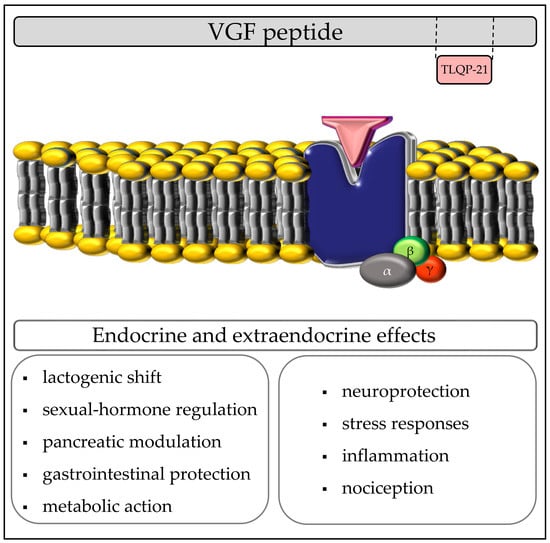
TLQP-21, A VGF-Derived Peptide Endowed of Endocrine and Extraendocrine Properties: Focus on In Vitro Calcium Signaling
Abstract
1. Introduction
2. VGF and VGF-Derived Peptides
2.1. TLQP-21: A VGF-Derived Peptide
2.2. TLQP-21: Endocrine Activities
2.2.1. Lactogenic Effect
2.2.2. Effect on the Reproductive Tract
2.2.3. Effect on Endocrine Pancreas
2.2.4. Effect on the Gastrointestinal Tract
2.3. TLQP-21 Metabolic Actions
2.4. TLQP-21: Extraendocrine Activities
2.4.1. Neuroprotection
2.4.2. Stress Responses, Inflammation and Nociception
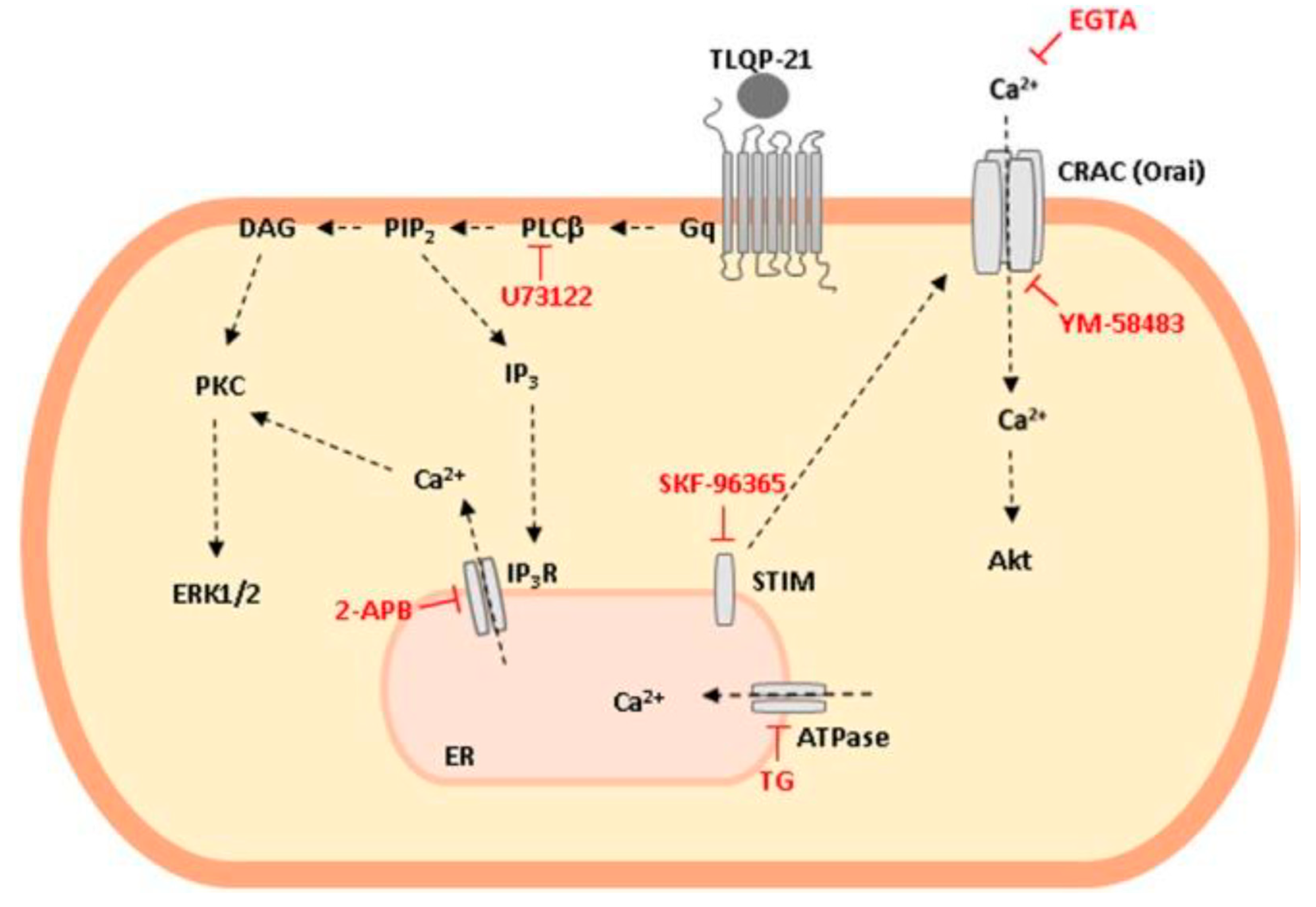
2.5. TLQP-21: Mechanism of Action
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| AGRP | Agouti-Related Peptide |
| ALS | Amyotrophic Lateral Sclerosis |
| BDNF | Brain-Derived Neurotrophic Factor |
| β3-AR | β3-adrenergic receptor |
| C3AR-1 | complement C3a receptor-1 |
| Ca2+ | calcium |
| CHO | Chinese Hamster Ovary |
| COX-2 | cyclooxygenase-2 |
| CRAC-Orai1 | Calcium Release-Activated Calcium Channel-Orai1 |
| CRH | Corticotropin-Releasing Hormone |
| CSF | cerebrospinal fluid |
| CSS | chronic subordination stress |
| DAG | Diacylglycerol |
| DRG | dorsal root ganglion |
| E | epinephrine |
| EE | energy expenditure |
| EGF | Epidermal Growth Factor |
| ER/SR | endoplasmic/sarcoplasmic reticulum |
| ERK | Extracellular Signal-regulated Kinase |
| FGF | Fibroblast Growth Factor |
| FSH | Follicle-stimulating Hormone |
| GH | Growth Hormone |
| GPCR | G protein-coupled receptor |
| hGC | human Chorionic Gonadotropin |
| HSL | Hormone-Sensitive Lipase |
| i.c.v. | Intra-cerebro-ventricular |
| IL-6 | interleukin-6 |
| IP | inositol phosphate |
| IP3 | Inositol Triphosphate |
| IP3R | inositol-3-phosphate receptor |
| i.v. | intravenous |
| KO | knock-out |
| LH | Luteinizing Hormone |
| LOX | lipoxygenase |
| MCH | Melanin-Concentrating Hormone |
| MAPK | Mitogen-Activated Protein Kinase |
| NCX | Sodium-Calcium Exchanger |
| NE | norepinephrine |
| NGF | Nerve Growth Factor |
| NT-3 | Neurotrophin-3 |
| NPY | Neuropeptide Y |
| Orai1 | Calcium Release-Activated Calcium Channel Protein 1 |
| PC | prohormone convertase |
| PLC | Phospholipase C |
| PG | prostaglandin |
| PKC | Protein kinase C |
| PNS and CNS | Peripheral and Central Nervous Systems |
| PM | plasma membrane |
| PMCA | Plasma Membrane Calcium ATPase |
| POMC | Proopiomelanocortin |
| PPAR-δ | Peroxisome Proliferative Activated Receptor δ |
| PRL | prolactin |
| PTX | pertussis toxin |
| RyR | ryanodine receptor |
| RS | restraint stress |
| s.c. | subcutaneous |
| SERCA | Sarco-Endoplasmic Reticulum Calcium ATPase |
| SOC | Store-Operated Calcium |
| SOCE | Store-Operated Ca2+ Entry |
| SOD1 | Superoxide Dismutase-1 |
| STIM | Stromal Interaction Molecule |
| TG | triglycerides |
| TP | thapsigargine |
| TRPC | Transient Receptor Potential Channels |
| UCP-1 | Uncoupling Protein 1 |
| VGCs | Voltage-gated ion channels |
| WAT | White Adipose Tissue |
References
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Intracellular calcium homeostasis and signaling. Met. Ions Life Sci. 2013, 12, 119–168. [Google Scholar] [CrossRef] [PubMed]
- Ziman, A.P.; Ward, C.W.; Rodney, G.G.; Lederer, W.J.; Bloch, R.J. Quantitative measurement of Ca(2+) in the sarcoplasmic reticulum lumen of mammalian skeletal muscle. Biophys. J. 2010, 99, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, A.; Makhro, A.; Wang, J.; Lipp, P.; Kaestner, L. Calcium in red blood cells-a perilous balance. Int. J. Mol. Sci. 2013, 14, 9848–9872. [Google Scholar] [CrossRef] [PubMed]
- Conrard, L.; Tyteca, D. Regulation of Membrane Calcium Transport Proteins by the Surrounding Lipid Environment. Biomolecules 2019, 9, 513. [Google Scholar] [CrossRef]
- Ferri, G.L.; Noli, B.; Brancia, C.; D’Amato, F.; Cocco, C. VGF: An inducible gene product, precursor of a diverse array of neuro-endocrine peptides and tissue-specific disease biomarkers. J. Chem. Neuroanat. 2011, 42, 249–261. [Google Scholar] [CrossRef]
- Salton, S.R.; Fischberg, D.-J.; Dong, K.W. Structure of the gene encoding VGF, a nervous system-specific mRNA that is rapidly and selectively induced by nerve growth factor in PC12 cells. Mol. Cell. Biol. 1991, 11, 2335–2349. [Google Scholar] [CrossRef][Green Version]
- Possenti, R.; Di Rocco, G.; Nasi, S.; Levi, A. Regulatory elements in the promoter region of VGF, a nerve growth factor-inducible gene. Proc. Natl. Acad. Sci. USA 1992, 89, 3815–3819. [Google Scholar] [CrossRef]
- Hawley, R.J.; Scheibe, R.J.; Wagner, J.A. NGF induces the expression of the VGF gene through a cAMP response element. J. Neurosci. 1992, 12, 2573–2581. [Google Scholar] [CrossRef]
- Levi, A.; Ferri, G.L.; Watson, E.; Possenti, R.; Salton, S.R. Processing, distribution, and function of VGF, a neuronal and endocrine peptide precursor. Cell. Mol. Neurobiol. 2004, 24, 517–533. [Google Scholar] [CrossRef]
- van den Pol, A.N.; Decavel, C.; Levi, A.; Paterson, B. Hypothalamic expression of a novel gene product, VGF: Immunocytochemical analysis. J. Neurosci. 1989, 9, 4122–4137. [Google Scholar] [CrossRef]
- Ferri, G.L.; Levi, A.; Possenti, R. A novel neuroendocrine gene product: Selective VGF8a gene expression and immuno-localisation of the VGF protein in endocrine and neuronal populations. Brain Res. Mol. Brain Res. 1992, 13, 139–143. [Google Scholar] [CrossRef]
- Salton, S.R.; Ferri, G.L.; Hahm, S.; Snyder, S.E.; Wilson, A.J.; Possenti, R.; Levi, A. VGF: A novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front. Neuroendocrinol. 2000, 21, 199–219. [Google Scholar] [CrossRef] [PubMed]
- Trani, E.; Giorgi, A.; Canu, N.; Amadoro, G.; Rinaldi, A.M.; Halban, P.A.; Ferri, G.L.; Possenti, R.; Schininà, M.E.; Levi, A. Isolation and characterization of VGF peptides in rat brain. Role of PC1/3 and PC2 in the maturation of VGF precursor. J. Neurochem. 2002, 81, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Canu, N.; Possenti, R.; Ricco, A.S.; Rocchi, M.; Levi, A. Cloning, structural organization analysis, and chromosomal assignment of the human gene for the neurosecretory protein VGF. Genomics 1997, 45, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Possenti, R.; Eldridge, J.D.; Paterson, B.M.; Grasso, A.; Levi, A. A protein-induced by NGF in PC12 cells is stored in secretory vescicles and released through the regulated pathway. EMBO J. 1989, 8, 2217–2223. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Possenti, R.; Levi, A.; Pavone, F.; Moles, A. The vgf gene and VGF derived peptides in nutrition and metabolism. Genes Nutr. 2007, 2, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Andrews, P.C.; Mershon, J.L.; Yan, C.; Allen, D.L.; Ben-Jonathan, N. Peptide V: A VGF-derived neuropeptide purified from bovine posterior pituitary. J. Endocrinol. 1994, 135, 2742–2748. [Google Scholar] [CrossRef]
- Noli, B.; Sanna, F.; Brancia, C.; D’Amato, F.; Manconi, B.; Vincenzoni, F.; Messana, I.; Melis, M.R.; Argiolas, A.; Ferri, G.L.; et al. Profiles of VGF Peptides in the Rat Brain and Their Modulations after Phencyclidine Treatment. Front. Cell. Neurosci. 2017, 2, 11–158. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, F.; Noli, B.; Brancia, C.; Cocco, C.; Flore, G.; Collu, M.; Nicolussi, P.; Ferri, G.L. Differential distribution of VGF-derived peptides in the adrenal medulla and evidence for their selective modulation. J. Endocrinol. 2008, 197, 359–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brancia, C.; Cocco, C.; D’Amato, F.; Noli, B.; Sanna, F.; Possenti, R.; Argiolas, A.; Ferri, G.L. Selective expression of TLQP-21 and other VGF peptides in gastric neuroendocrine cells and modulation by feeding. J. Endocrinol. 2010, 207, 329–341. [Google Scholar] [CrossRef][Green Version]
- Turolla, E.A.; Valtorta, S.; Bresciani, E.; Fehrentz, J.; Giuliano, L.; Stucchi, S.; Belloli, S.; Rainone, P.; Sudati, F.; Rizzi, L.; et al. Study of the Tissue Distribution of TLQP-21 in Mice Using [18F]JMV5763, a Radiolabeled Analog Prepared via [18F]Aluminum Fluoride Chelation Chemistry. Front. Pharmacol. 2018, 13, 1274. [Google Scholar] [CrossRef] [PubMed]
- Possenti, R.; Muccioli, G.; Petrocchi, P.; Cero, C.; Cabassi, A.; Vulchanova, L.; Riedl, M.S.; Manieri, M.; Frontini, A.; Giordano, A.; et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: Role of VGF-derived peptide TLQP-21. Biochem. J. 2012, 441, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.; Brameld, J.M.; Jethwa, P.H. Neuroendocrine Role for VGF. Front. Endocrinol. 2015, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Sahu, B.S.; He, R.; Finan, B.; Cero, C.; Verardi, R.; Razzoli, M.; Veglia, G.; Di Marchi, R.D.; Miles, J.M.; et al. Clearance kinetics of the VGF-derived neuropeptide TLQP-21. Neuropeptides 2013, 97, 212–224. [Google Scholar] [CrossRef]
- Petrocchi Passeri, P.; Biondini, L.; Mongiardi, M.P.; Mordini, N.; Quaresima, S.; Frank, C.; Baratta, M.; Bartolomucci, A.; Levi, A.; Severini, C.; et al. Neuropeptide TLQP-21, a VGF internal fragment, modulates hormonal gene expression and secretion in GH3 cell line. Neuropeptides 2018, 71, 97–103. [Google Scholar] [CrossRef]
- Brancia, C.; Nicolussi, P.; Cappai, P.; La Corte, G.; Possenti, R.; Ferri, G.L. Differential expression and seasonal modulation of VGF peptides in sheep pituitary. J. Endocrinol. 2005, 186, 97–107. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Rigamonti, A.E.; Bulgarelli, I.; Torsello, A.; Locatelli, V.; Pavone, F.; Levi, A.; Possenti, R.; Muller, E.E.; Moles, A. Chronic intracerebroventricular TLQP-21 delivery does not modulate the GH/IGF-1-axis and muscle strength in mice. Growth Horm. IGF Res. 2007, 17, 342–345. [Google Scholar] [CrossRef]
- Ferri, G.L.; Gaudio, R.M.; Cossu, M.; Rinaldi, A.M.; Polak, J.M.; Berger, P.; Possenti, R. The VGF protein in rat adenohypophysis: Sex differences and changes during the estrous cycle and after gonadectomy. J. Endocrinol. 1995, 136, 2244–2251. [Google Scholar] [CrossRef]
- Choi, S.G.; Wang, Q.; Jia, J.; Chikina, M.; Pincas, H.; Dolios, G.; Sasaki, K.; Wang, R.; Minamino, N.; Salton, S.R.; et al. Characterization of Gonadotrope Secretoproteome Identifies Neurosecretory Protein VGF-derived Peptide Suppression of Follicle-stimulating Hormone Gene Expression. J. Biol. Chem. 2016, 291, 21322–21334. [Google Scholar] [CrossRef]
- Aguilar, E.; Pineda, R.; Gaytán, F.; Sánchez-Garrido, M.A.; Romero, M.; Romero-Ruiz, A.; Ruiz-Pino, F.; Tena-Sempere, M.; Pinilla, L. Characterization of the reproductive effects of the Vgf-derived peptide TLQP-21 in female rats: In vivo and in vitro studies. Neuropeptides 2013, 98, 38–50. [Google Scholar]
- Pinilla, L.; Pineda, R.; Gaytán, F.; Romero, M.; García-Galiano, D.; Sánchez-Garrido, M.A.; Ruiz-Pino, F.; Tena-Sempere, M.; Aguilar, E. Characterization of the reproductive effects of the anorexigenic VGF-derived peptide TLQP-21, in vivo and in vitro studies in male rats. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E837–E847. [Google Scholar] [CrossRef] [PubMed]
- Cocco, C.; Brancia, C.; Pirisi, I.; D’Amato, F.; Noli, B.; Possenti, R.; Ferri, G.L. VGF metabolic-related gene: Distribution of its derived peptides in mammalian pancreatic islets. J. Histochem. Cytochem. 2007, 55, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.; Edwards, R.J.; Sadahiro, M.; Lin, W.J.; Jiang, C.; Salton, S.R.; Newgard, C.B. The Prohormone VGF Regulates β Cell Function via Insulin Secretory Granule Biogenesis. Cell Rep. 2017, 20, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Stephens, S.B.; Schisler, J.C.; Hohmeier, H.E.; An, J.; Sun, A.Y.; Pitt, G.S.; Newgard, C.B. A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet β-cell survival and function. Cell Metab. 2012, 16, 33–43. [Google Scholar] [CrossRef]
- Christiansen, C.B.; Svendsen, B.; Holst, J.J. The VGF-Derived Neuropeptide TLQP-21 Shows No Impact on Hormone Secretion in the Isolated Perfused Rat Pancreas. Horm. Metab. Res. 2015, 47, 537–543. [Google Scholar] [CrossRef]
- Severini, C.; La Corte, G.; Improta, G.; Broccardo, M.; Agostini, S.; Petrella, C.; Sibilia, V.; Pagani, F.; Guidobono, F.; Bulgarelli, I.; et al. In vitro and in vivo pharmacological role of TLQP-21, a VGF-derived peptide, in the regulation of rat gastric motor functions. Br. J. Pharmacol. 2009, 157, 984–993. [Google Scholar] [CrossRef]
- Sibilia, V.; Pagani, F.; Bulgarelli, I.; Mrak, E.; Broccardo, M.; Improta, G.; Severini, C.; Possenti, R.; Guidobono, F. TLQP-21, a VGF-derived peptide, prevents ethanol-induced gastric lesions: Insights into its mode of action. Neuropeptides 2010, 92, 189–197. [Google Scholar] [CrossRef]
- Sibilia, V.; Pagani, F.; Bulgarelli, I.; Tulipano, G.; Possenti, R.; Guidobono, F. Characterization of the mechanisms involved in the gastric antisecretory effect of TLQP-21, a vgf-derived peptide, in rats. Amino Acids 2012, 42, 1261–1268. [Google Scholar] [CrossRef]
- Petrella, C.; Broccardo, M.; Possenti, R.; Severini, C.; Improta, G. TLQP-21, a VGF-derived peptide, stimulates exocrine pancreatic secretion in the rat. Peptides 2012, 36, 133–136. [Google Scholar] [CrossRef]
- Bartolomucci, A.; La Corte, G.; Possenti, R.; Locatelli, V.; Rigamonti, A.E.; Torsello, A.; Bresciani, E.; Bulgarelli, I.; Rizzi, R.; Pavone, F.; et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc. Natl. Acad. Sci. USA 2006, 103, 14584–14589. [Google Scholar] [CrossRef]
- Jethwa, P.H.; Warner, A.; Nilaweera, K.N.; Brameld, J.M.; Keyte, J.W.; Carter, W.G.; Bolton, N.; Bruggraber, M.; Morgan, P.J.; Barrett, P.; et al. VGF-derived peptide, TLQP-21, regulates food intake and body weight in Siberian hamsters. J. Endocrinol. 2007, 148, 4044–4055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lisci, C.; Lewis, J.E.; Daniel, Z.C.T.R.; Stevenson, T.J.; Monnier, C.; Marshall, H.J.; Fowler, M.; Ebling, F.J.P.; Ferri, G.L.; Cocco, C.; et al. Photoperiodic changes in adiposity increase sensitivity of female Siberian hamsters to systemic VGF derived peptide TLQP-21. PLoS ONE 2019, 14, e0221517. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.; Ross, A.W.; Balik, A.; Littlewood, P.A.; Mercer, J.G.; Moar, K.M.; Sallmen, T.; Kaslin, J.; Panula, P.; Schuhler, S.; et al. Photoperiodic regulation of histamine H3 receptor and VGF messenger ribonucleic acid in the arcuate nucleus of the Siberian hamster. J. Endocrinol. 2005, 146, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Ebling, F.J.; Barrett, P. The regulation of seasonal changes in food intake and body weight. J. Neuroendocrinol. 2008, 20, 827–833. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Possenti, R.; Mahata, S.K.; Fischer-Colbrie, R.; Loh, Y.P.; Salton, S.R. The extended granin family: Structure, function, and biomedical implications. Endocr. Rev. 2011, 32, 755–797. [Google Scholar] [CrossRef]
- Severini, C.; Ciotti, M.T.; Biondini, L.; Quaresima, S.; Rinaldi, A.M.; Levi, A.; Frank, C.; Possenti, R. TLQP-21, a neuroendocrine VGF-derived peptide, prevents cerebellar granule cells death induced by serum and potassium deprivation. J. Neurochem. 2008, 104, 534–544. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Shimazawa, M.; Ohuchi, K.; Kuse, Y.; Nakamura, S.; Hara, H. Impaired Cerebellar Development in Mice Overexpressing VGF. Neurochem. Res. 2019, 44, 374–387. [Google Scholar] [CrossRef]
- Young, K.G.; Yan, K.; Picketts, D.J. C3aR signaling and gliosis in response to neurodevelopmental damage in the cerebellum. J. Neuroinflamm. 2019, 16, 135. [Google Scholar] [CrossRef]
- Snyder, S.E.; Salton, S.R. Expression of VGF mRNA in the adult rat central nervous system. J. Comp. Neurol. 1998, 394, 91–105. [Google Scholar] [CrossRef]
- Lombardo, A.; Rabacchi, S.A.; Cremisi, F.; Pizzorusso, T.; Cenni, M.C.; Possenti, R.; Barsacchi, G.; Maffei, L. A developmentally regulated nerve growth factor-induced gene, VGF, is expressed in geniculocortical afferents during synaptogenesis. Neuroscience 1995, 65, 997–1008. [Google Scholar] [CrossRef]
- Snyder, S.E.; Pintar, J.E.; Salton, S.R. Developmental expression of VGF mRNA in the prenatal and postnatal rat. J. Comp. Neurol. 1998, 394, 64–90. [Google Scholar] [CrossRef]
- Mead, B.; Logan, A.; Berry, M.; Leadbeater, W.; Scheven, B.A. Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: Comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS ONE 2014, 9, e109305. [Google Scholar] [CrossRef] [PubMed]
- Brancia, C.; Noli, B.; Boido, M.; Pilleri, R.; Boi, A.; Puddu, R.; Marrosu, F.; Vercelli, A.; Bongioanni, P.; Ferri, G.L.; et al. TLQP Peptides in Amyotrophic Lateral Sclerosis: Possible Blood Biomarkers with a Neuroprotective Role. Neuroscience 2018, 380, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Bartolomucci, A.; Pasinetti, G.M.; Salton, S.R. Granins as disease-biomarkers: Translational potential for psychiatric and neurological disorders. Neuroscience 2010, 170, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Sesta, A.; Calabrese, F.; Nielsen, G.; Riva, M.A.; Gennarelli, M. The expression of VGF is reduced in leukocytes of depressed patients and it is restored by effective antidepressant treatment. Neuropsychopharmacology 2010, 35, 1423–1428. [Google Scholar] [CrossRef]
- Hunsberger, J.G.; Newton, S.S.; Bennett, A.H.; Duman, C.H.; Russell, D.S.; Salton, S.R.; Duman, R.S. Antidepressant actions of the exercise-regulated gene VGF. Nat. Med. 2007, 13, 1476–1482. [Google Scholar] [CrossRef]
- Thakker-Varia, S.; Krol, J.J.; Nettleton, J.; Bilimoria, P.M.; Bangasser, D.A.; Shors, T.J.; Black, I.B.; Alder, J. The neuropeptide VGF produces antidepressant-like behavioral effects and enhances proliferation in the hippocampus. J. Neurosci. 2007, 27, 12156–12167. [Google Scholar] [CrossRef]
- Razzoli, M.; Bo, E.; Pascucci, T.; Pavone, F.; D’Amato, F.R.; Cero, C.; Sanghez, V.; Dadomo, H.; Palanza, P.; Parmigiani, S.; et al. Implication of the VGF-derived peptide TLQP-21 in mouse acute and chronic stress responses. Behav. Brain Res. 2012, 229, 333–339. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flügge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Noda, Y.; Motoyama, S.; Nakamura, S.; Shimazawa, M.; Hara, H. Neuropeptide VGF-Derived Peptide LQEQ-19 has Neuroprotective Effects in an In Vitro Model of Amyotrophic Lateral Sclerosis. Neurochem. Res. 2019, 44, 897–904. [Google Scholar] [CrossRef]
- Toshinai, K.; Nakazato, M. Neuroendocrine regulatory peptide-1 and -2, novel bioactive peptides processed from VGF. Cell. Mol. Life Sci. 2009, 66, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, R.; Bartolomucci, A.; Moles, A.; D’Amato, F.; Sacerdote, P.; Levi, A.; La Corte, G.; Ciotti, M.T.; Possenti, R.; Pavone, F. The VGF-derived peptide TLQP-21, a new modulatory peptide for inflammatory pain. Neurosci. Lett. 2008, 441, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Pristerá, A.; Ayub, M.; Swanwick, R.S.; Karu, K.; Hamada, Y.; Rice, A.S.; Okuse, K. Identification of a receptor for neuropeptide VGF and its role in neuropathic pain. J. Biol. Chem. 2013, 288, 34638–34646. [Google Scholar] [CrossRef] [PubMed]
- Fairbanks, C.A.; Peterson, C.D.; Speltz, R.H.; Riedl, M.S.; Kitto, K.F.; Dykstra, J.A.; Braun, P.D.; Sadahiro, M.; Salton, S.R.; Vulchanova, L. The VGF-derived peptide TLQP-21 contributes to inflammatory and nerve injury-induced hypersensitivity. Pain 2014, 155, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Cassina, V.; Torsello, A.; Tempestini, A.; Salerno, D.; Brogioli, D.; Tamiazzo, L.; Bresciani, E.; Martinez, J.; Fehrentz, J.A.; Verdié, P.; et al. Biophysical characterization of a binding site for TLQP-21, a naturally occurring peptide which induces resistance to obesity. Biochim. Biophys. Acta 2013, 1828, 455–460. [Google Scholar] [CrossRef]
- Molteni, L.; Rizzi, L.; Bresciani, E.; Possenti, R.; Petrocchi Passeri, P.; Ghè, C.; Muccioli, G.; Fehrentz, J.A.; Verdié, P.; Martinez, J.; et al. Pharmacological and Biochemical Characterization of TLQP-21 Activation of a Binding Site on CHO Cells. Front. Pharmacol. 2017, 8, 167. [Google Scholar] [CrossRef]
- Rivolta, I.; Binda, A.; Molteni, L.; Rizzi, L.; Bresciani, E.; Possenti, R.; Fehrentz, J.A.; Verdié, P.; Martinez, J.; Omeljaniuk, R.J.; et al. JMV5656, A Novel Derivative of TLQP-21, Triggers the Activation of a Calcium-Dependent Potassium Outward Current in Microglial Cells. Front. Cell Neurosci. 2017, 11, 41. [Google Scholar] [CrossRef]
- Molteni, L.; Rizzi, L.; Bresciani, E.; Meanti, R.; Fehrentz, J.A.; Verdié, P.; Omeljaniuk, R.J.; Biagini, G.; Locatelli, V.; Torsello, A. STIM Proteins and Orai Ca2+ Channels Are Involved in the Intracellular Pathways Activated by TLQP-21 in RAW264.7 Macrophages. Front. Pharmacol. 2018, 9, 1386. [Google Scholar] [CrossRef]
- Hannedouche, S.; Beck, V.; Leighton-Davies, J.; Beibel, M.; Roma, G.; Oakeley, E.J.; Lannoy, V.; Bernard, J.; Hamon, J.; Barbieri, S.; et al. Identification of the C3a receptor (C3AR1) as the target of the VGF-derived peptide TLQP-21 in rodent cells. J. Biol. Chem. 2013, 288, 27434–27443. [Google Scholar] [CrossRef]
- Klos, A.; Wende, E.; Wareham, K.J.; Monk, P.N. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXVII. Complement peptide C5a, C4a, and C3a receptors. Pharmacol. Rev. 2013, 65, 500–543. [Google Scholar] [CrossRef]
- Ricklin, D.; Reis, E.S.; Mastellos, D.C.; Gros, P.; Lambris, J.D. Complement component C3—The Swiss Army Knife of innate immunity and host defense. Immunol. Rev. 2016, 274, 33–58. [Google Scholar] [CrossRef] [PubMed]
- Opstal-van Winden, A.W.; Vermeulen, R.C.; Peeters, P.H.; Beijnen, J.H.; van Gils, C.H. Early diagnostic protein biomarkers for breast cancer: How far have we come? Breast Cancer Res. Treat. 2012, 134, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Klos, A.; Tenner, A.J.; Johswich, K.O.; Ager, R.R.; Reis, E.S.; Köhl, J. The role of the anaphylatoxins in health and disease. Mol. Immunol. 2009, 46, 2753–2766. [Google Scholar] [CrossRef] [PubMed]
- Mamane, Y.; Chung Chan, C.; Lavallee, G.; Morin, N.; Xu, L.J.; Huang, J.; Gordon, R.; Thomas, W.; Lamb, J.; Schadt, E.E.; et al. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes 2009, 58, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Francis, K.; Lewis, B.M.; Akatsu, H.; Monk, P.N.; Cain, S.A.; Scanlon, M.F.; Morgan, B.P.; Ham, J.; Gasque, P. Complement C3a receptors in the pituitary gland: A novel pathway by which an innate immune molecule releases hormones involved in the control of inflammation. FASEB J. 2003, 17, 2266–2268. [Google Scholar] [CrossRef]
- Cero, C.; Razzoli, M.; Han, R.; Sahu, B.S.; Patricelli, J.; Guo, Z.; Zaidman, N.A.; Miles, J.M.; O’Grady, S.M.; Bartolomucci, A. The neuropeptide TLQP-21 opposes obesity via C3aR1-mediated enhancement of adrenergic-induced lipolysis. Mol. Metab. 2016, 6, 148–158. [Google Scholar] [CrossRef]
- Sahu, B.S.; Rodriguez, P.; Nguyen, M.E.; Han, R.; Cero, C.; Razzoli, M.; Piaggi, P.; Laskowski, L.J.; Pavlicev, M.; Muglia, L.; et al. Peptide/Receptor Co-evolution Explains the Lipolytic Function of the Neuropeptide TLQP-21. Cell Rep. 2019, 28, 2567–2580. [Google Scholar] [CrossRef]
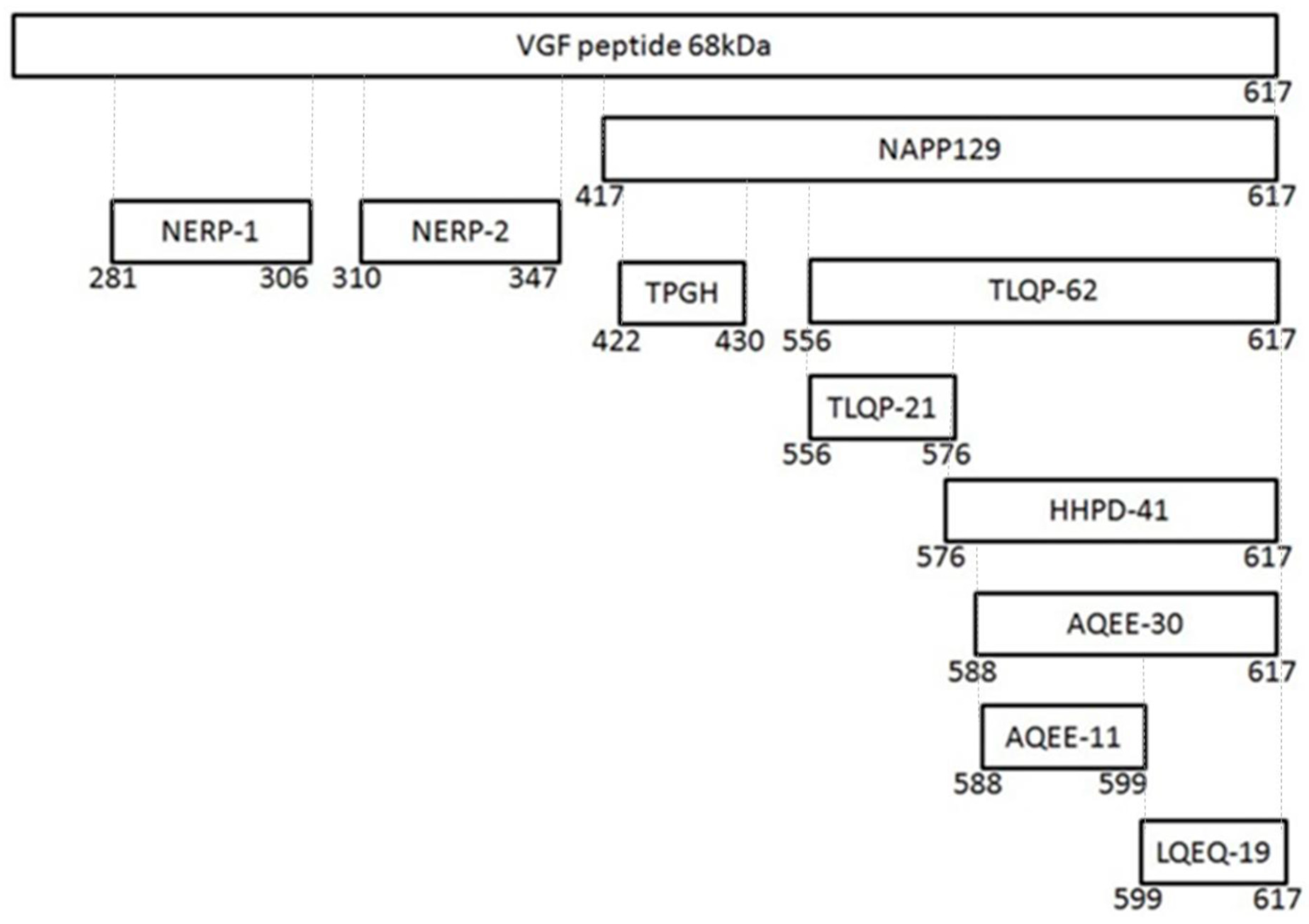
| Name | Fragment | Espression | Potential Role | Reference |
|---|---|---|---|---|
| VGF | 0–617 | Hypothalamus Hippocampus Amygdala Thalamus Cerebral Cortex Pituitary Adrenal Medulla Gut Pancreas | Energy balance Reproduction Momory Learning Depression | Lewis J.E.; et al.; 2015 |
| NERP-1 | 281–306 | Hypothalamus Thyroid Gastric Antrum | Inhibitory modulators of vasopressin relaease | Toshinai K.; et al.; 2009 |
| NERP-2 | 310–347 | Hypothalamus Thyroid Pancreas Gastric antrum | Inhibitory modulators of vasopressin relaease Stimulator offeeding behavior | Toshinai K.; et al.; 2009 |
| Enhancer of glucose-stimulated insulinsecretion | Moin A.S.; 2012 | |||
| Increased gastric acid secretion and gastric emptying | Namkoong C.;et al.; 2017 | |||
| TLQP-62 | 556–617 | Hypothalamus Hippocampus | Enhanced synaptic activity | Alder J.; et al.; 2003 |
| Effects on spontaneous excitability of superficial dorsal horn neurons | Moss A.; 2008 | |||
| Antidepressant effects | Hunsberger J.G.; et al.; 2007 | |||
| Spinal plasticity | Skorput A.G.J.; et al.; 2018;. | |||
| Long-term memory formation | Lin W.-J. et al.; 2015 | |||
| AQUEE-30 | 588–617 | Pituitary | In vitro neuroprotective effects | Noda Y.; et al.; 2019 |
| Enhanced synaptic activity | Alder J.; et al.; 2003 | |||
| Antidepressant effects | Humsberger J.G.; et al.; 2007 | |||
| Pro-nociceptive and hyperalgesic functions | Riedl M.S.; et al.; 2009 | |||
| Thermal hyperalgesia | Riedl M.S.; et al.; 2009 | |||
| LQEQ-19 | 599–617 | Thalamus cerebral cortex | In vitro neuroprotective effects | Noda Y.; et al.; 2019 |
| Pro-nociceptive and hyperalgestc functions | Riedl M.S.; 2009 | |||
| Thermal hyperalgesia | Riedl M.S.; 2009 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bresciani, E.; Possenti, R.; Coco, S.; Rizzi, L.; Meanti, R.; Molteni, L.; Locatelli, V.; Torsello, A. TLQP-21, A VGF-Derived Peptide Endowed of Endocrine and Extraendocrine Properties: Focus on In Vitro Calcium Signaling. Int. J. Mol. Sci. 2020, 21, 130. https://doi.org/10.3390/ijms21010130
Bresciani E, Possenti R, Coco S, Rizzi L, Meanti R, Molteni L, Locatelli V, Torsello A. TLQP-21, A VGF-Derived Peptide Endowed of Endocrine and Extraendocrine Properties: Focus on In Vitro Calcium Signaling. International Journal of Molecular Sciences. 2020; 21(1):130. https://doi.org/10.3390/ijms21010130
Chicago/Turabian StyleBresciani, Elena, Roberta Possenti, Silvia Coco, Laura Rizzi, Ramona Meanti, Laura Molteni, Vittorio Locatelli, and Antonio Torsello. 2020. "TLQP-21, A VGF-Derived Peptide Endowed of Endocrine and Extraendocrine Properties: Focus on In Vitro Calcium Signaling" International Journal of Molecular Sciences 21, no. 1: 130. https://doi.org/10.3390/ijms21010130
APA StyleBresciani, E., Possenti, R., Coco, S., Rizzi, L., Meanti, R., Molteni, L., Locatelli, V., & Torsello, A. (2020). TLQP-21, A VGF-Derived Peptide Endowed of Endocrine and Extraendocrine Properties: Focus on In Vitro Calcium Signaling. International Journal of Molecular Sciences, 21(1), 130. https://doi.org/10.3390/ijms21010130