Neurochemical Characterization of Neurons Expressing Estrogen Receptor β in the Hypothalamic Nuclei of Rats Using in Situ Hybridization and Immunofluorescence
Abstract
1. Introduction
2. Results
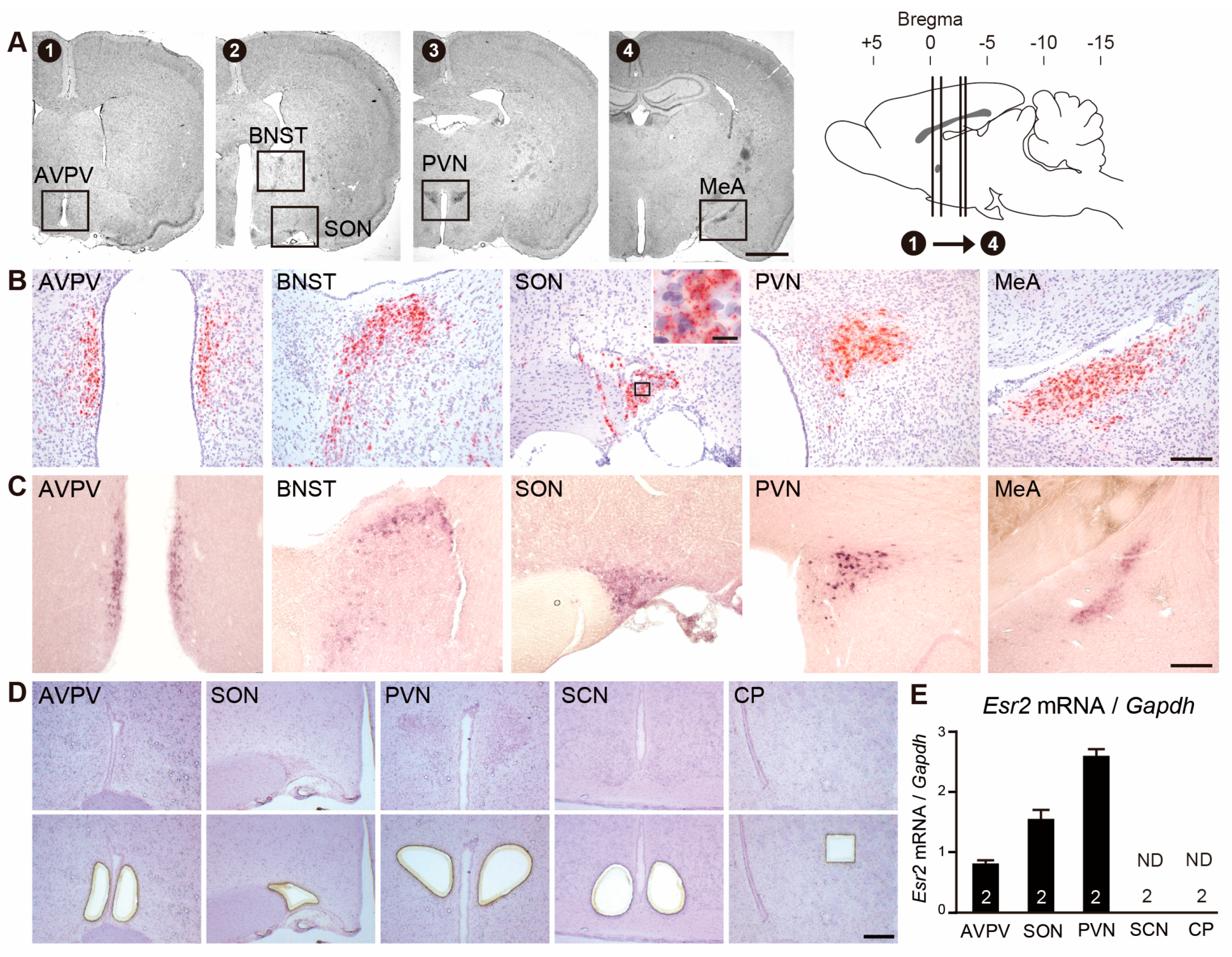
2.1. Validation of RNAscope Signals for Esr2
2.2. Co-Expression of Esr2 and Neuropeptides
2.2.1. Co-Expression of Esr2 in Kisspeptin Neurons in the AVPV and ARC
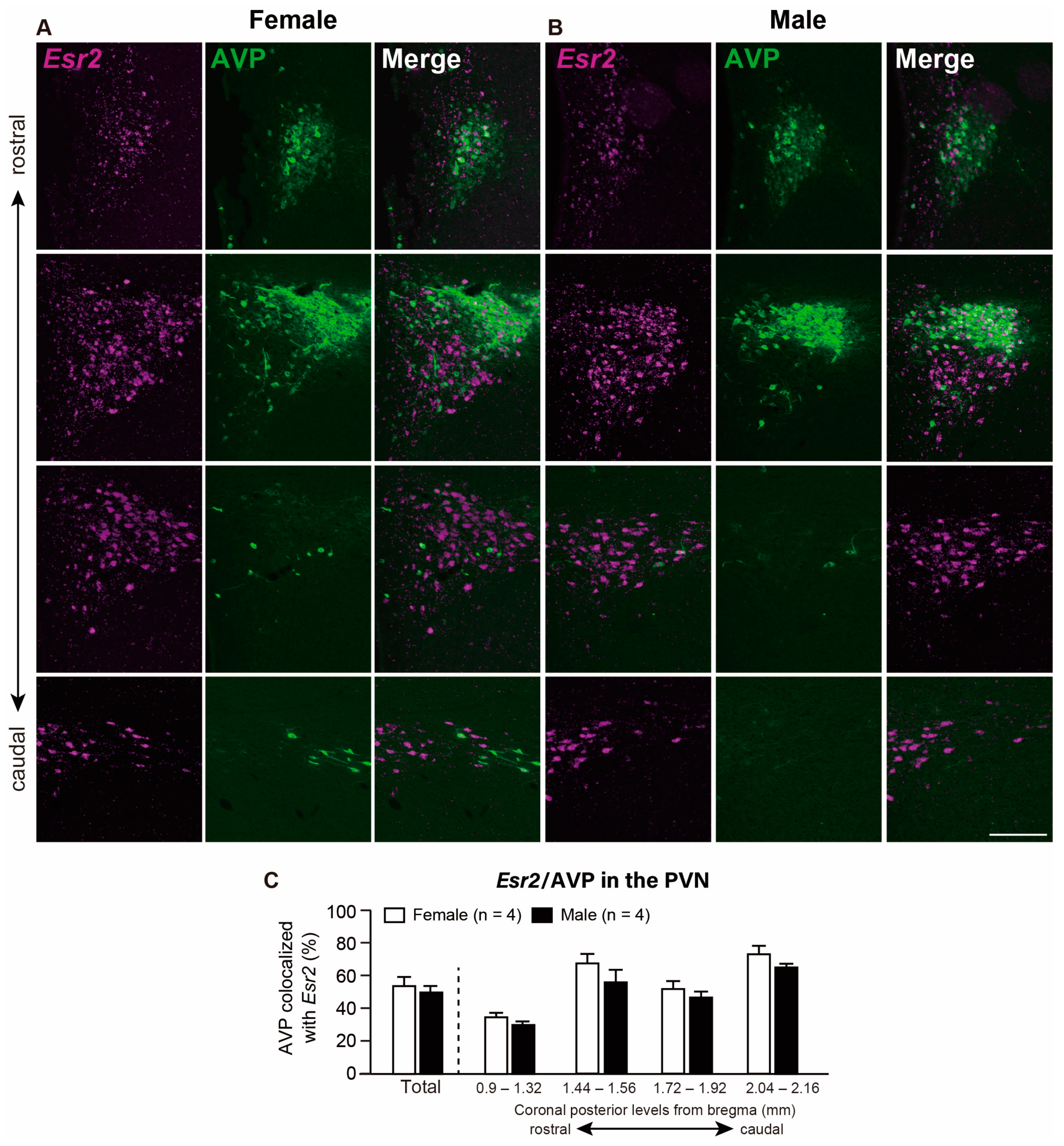
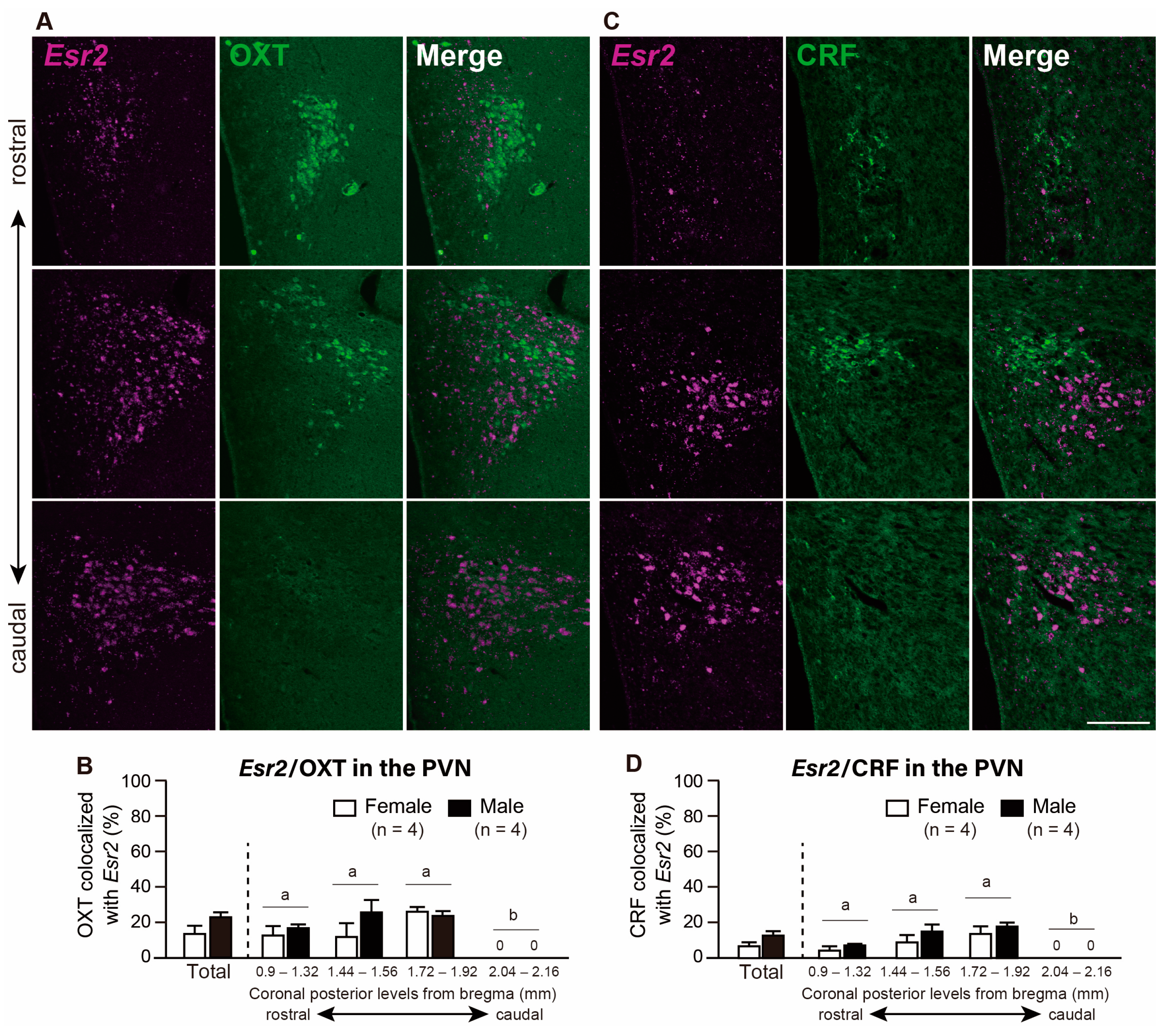
2.2.2. Co-Expression of Esr2 in the AVP, OXT, and CRF Neurons in the PVN
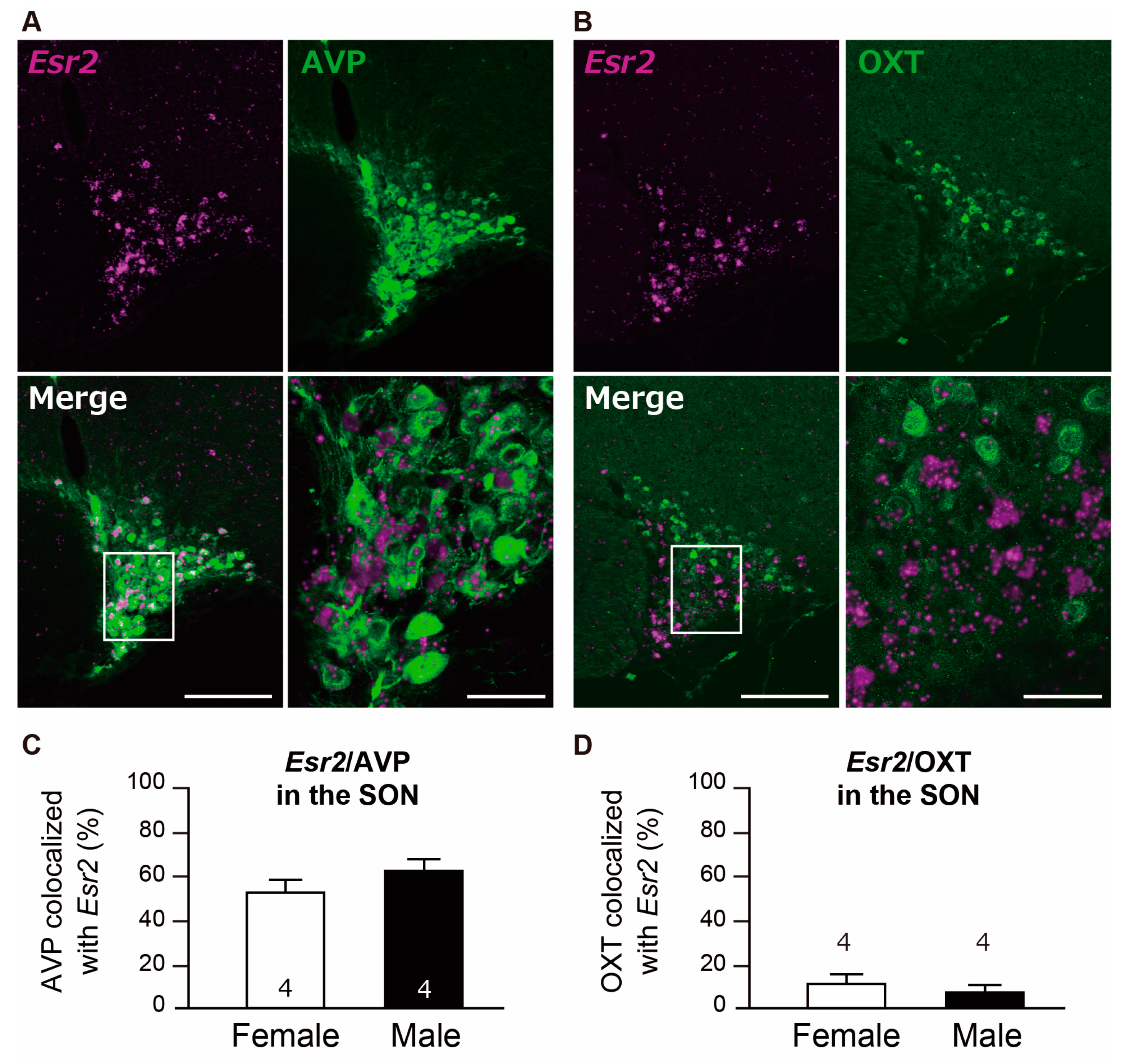
2.2.3. Col-Expression of Esr2/AVP and Esr2/OXT in the SON
3. Discussion
3.1. Effectiveness of RNAscope in Detecting Esr2 mRNA in the Rat Brain
3.2. Kisspeptin Neurons Colocalizes with Esr2 in a Region-Specific and Sex-Specific Manner
3.3. Majority of AVP Neurons Co-Express Esr2 in the PVN and SON in Both Sexes
3.4. OXT Neurons Co-Expressing Esr2 were Present at Low Levels in the PVN and SON in Both Sexes
3.5. CRF Co-Expressing Esr2 mRNA Neurons Are almost Absent in the PVN
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.2.1. Experiment 1: Validation of Esr2 mRNA Signals in RNAscope ISH
4.2.2. Experiment 2: Characterization of Esr2 mRNA-Expressing Neurons by a Combination of RNAscope ISH and Immunofluorescence
4.3. General Procedure
4.3.1. Tissue Preparation
4.3.2. Single RNAscope ISH for Esr2 mRNA
4.3.3. Conventional ISH for Esr2 mRNA Using a DIG-Labeled Probe
4.3.4. Analysis of Esr2 mRNA Expression in the Nuclei Isolated by Laser Microdissection
4.3.5. RNAscope ISH and Immunofluorescence
4.3.6. Images and Data Analysis
4.3.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ARC | Arcuate nucleus |
| AVP | Arginine vasopressin |
| AVPV | Anteroventral periventricular nucleus |
| BNST | Bed nucleus stria terminalis |
| CP | Corpus striatum |
| CRF | Corticotropin-releasing factor |
| ERα | Estrogen receptor α |
| ERβ | Estrogen receptor β |
| Esr2 | Estrogen receptor gene |
| MeA | Medial amygdala |
| OVLT | Organum vasculosum laminae terminalis |
| OXT | Oxytocin |
| PVN | Hypothalamic paraventricular nucleus |
| SCN | Suprachiasmatic nucleus |
| SON | Supraoptic nucleus |
| vlh | Ventrolateral hypothalamic tract |
| VMHvl | Ventrolateral parts of ventromedial hypothalamus |
References
- Lindzey, J.; Wetsel, W.C.; Couse, J.F.; Stoker, T.; Cooper, R.; Korach, K.S. Effects of castration and chronic steroid treatments on hypothalamic gonadotropin-releasing hormone content and pituitary gonadotropins in male wild-type and estrogen receptor-alpha knockout mice. Endocrinology 1998, 139, 4092–4101. [Google Scholar] [CrossRef] [PubMed]
- Wersinger, S.R.; Sannen, K.; Villalba, C.; Lubahn, D.B.; Rissman, E.F.; De Vries, G.J. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm. Behav. 1997, 32, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Rissman, E.F.; Heck, A.L.; Leonard, J.E.; Shupnik, M.A.; Gustafsson, J.A. Disruption of estrogen receptor beta gene impairs spatial learning in female mice. Proc. Natl. Acad. Sci. USA 2002, 99, 3996–4001. [Google Scholar] [CrossRef] [PubMed]
- Jacome, L.F.; Gautreaux, C.; Inagaki, T.; Mohan, G.; Alves, S.; Lubbers, L.S.; Luine, V. Estradiol and ERbeta agonists enhance recognition memory, and DPN, an ERbeta agonist, alters brain monoamines. Neurobiol. Learn. Mem. 2010, 94, 488–498. [Google Scholar] [CrossRef]
- Lund, T.D.; Rovis, T.; Chung, W.C.; Handa, R.J. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology 2005, 146, 797–807. [Google Scholar] [CrossRef]
- Oyola, M.G.; Portillo, W.; Reyna, A.; Foradori, C.D.; Kudwa, A.; Hinds, L.; Handa, R.J.; Mani, S.K. Anxiolytic effects and neuroanatomical targets of estrogen receptor-β (ERbeta) activation by a selective ERbeta agonist in female mice. Endocrinology 2012, 153, 837–846. [Google Scholar] [CrossRef]
- Weiser, M.J.; Wu, T.J.; Handa, R.J. Estrogen receptor-beta agonist diarylpropionitrile: Biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology 2009, 150, 1817–1825. [Google Scholar] [CrossRef]
- Laflamme, N.; Nappi, R.E.; Drolet, G.; Labrie, C.; Rivest, S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: Anatomical evidence of distinct roles of each subtype. J. Neurobiol. 1998, 36, 357–378. [Google Scholar] [CrossRef]
- Shughrue, P.J.; Merchenthaler, I. Distribution of estrogen receptor beta immunoreactivity in the rat central nervous system. J. Comp. Neurol. 2001, 436, 64–81. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Carlsson, B.; Grandien, K.; Enmark, E.; Haggblad, J.; Nilsson, S.; Gustafsson, J.A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Smith, J.T.; Popa, S.M.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 2006, 26, 6687–6694. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shi, H. Regulation of Estrogen Receptor alpha Expression in the Hypothalamus by Sex Steroids: Implication in the Regulation of Energy Homeostasis. Int. J. Endocrinol. 2015, 2015, 949085. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.A.; Smejkalova, T.; Forlano, P.M.; Woolley, C.S. Multiple ERbeta antisera label in ERbeta knockout and null mouse tissues. J. Neurosci. Methods 2010, 188, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Hrabovszky, E.; Kallo, I.; Steinhauser, A.; Merchenthaler, I.; Coen, C.W.; Petersen, S.L.; Liposits, Z. Estrogen receptor-beta in oxytocin and vasopressin neurons of the rat and human hypothalamus: Immunocytochemical and in situ hybridization studies. J. Comp. Neurol. 2004, 473, 315–333. [Google Scholar] [CrossRef] [PubMed]
- Handa, R.J.; Weiser, M.J. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 2014, 35, 197–220. [Google Scholar] [CrossRef]
- Orikasa, C.; Kondo, Y.; Hayashi, S.; McEwen, B.S.; Sakuma, Y. Sexually dimorphic expression of estrogen receptor beta in the anteroventral periventricular nucleus of the rat preoptic area: Implication in luteinizing hormone surge. Proc. Natl. Acad. Sci. USA 2002, 99, 3306–3311. [Google Scholar] [CrossRef]
- Clarkson, J.; d’Anglemont de Tassigny, X.; Moreno, A.S.; Colledge, W.H.; Herbison, A.E. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci. 2008, 28, 8691–8697. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, L.; Lin, N.; Pan, X.; Zhu, Y.; Chen, X. Aging-related changes in RP3V kisspeptin neurons predate the reduced activation of GnRH neurons during the early reproductive decline in female mice. Neurobiol. Aging 2014, 35, 655–668. [Google Scholar] [CrossRef]
- Smith, J.T.; Cunningham, M.J.; Rissman, E.F.; Clifton, D.K.; Steiner, R.A. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005, 146, 3686–3692. [Google Scholar] [CrossRef]
- Smith, J.T.; Dungan, H.M.; Stoll, E.A.; Gottsch, M.L.; Braun, R.E.; Eacker, S.M.; Clifton, D.K.; Steiner, R.A. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 2005, 146, 2976–2984. [Google Scholar] [CrossRef]
- Grabinski, T.M.; Kneynsberg, A.; Manfredsson, F.P.; Kanaan, N.M. A method for combining RNAscope in situ hybridization with immunohistochemistry in thick free-floating brain sections and primary neuronal cultures. PLoS ONE 2015, 10, e0120120. [Google Scholar] [CrossRef] [PubMed]
- Weiser, M.J.; Foradori, C.D.; Handa, R.J. Estrogen receptor beta in the brain: From form to function. Brain Res. Rev. 2008, 57, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Handa, R.J.; Ogawa, S.; Wang, J.M.; Herbison, A.E. Roles for oestrogen receptor beta in adult brain function. J. Neuroendocrinol. 2012, 24, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Polston, E.K.; Simerly, R.B. Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. J. Comp. Neurol. 2006, 495, 122–132. [Google Scholar] [CrossRef]
- Navarro, V.M.; Castellano, J.M.; McConkey, S.M.; Pineda, R.; Ruiz-Pino, F.; Pinilla, L.; Clifton, D.K.; Tena-Sempere, M.; Steiner, R.A. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E202–E210. [Google Scholar] [CrossRef]
- Krege, J.H.; Hodgin, J.B.; Couse, J.F.; Enmark, E.; Warner, M.; Mahler, J.F.; Sar, M.; Korach, K.S.; Gustafsson, J.A.; Smithies, O. Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc. Natl. Acad. Sci. USA 1998, 95, 15677–15682. [Google Scholar] [CrossRef]
- Wang, L.; Vanacker, C.; Burger, L.L.; Barnes, T.; Shah, Y.M.; Myers, M.G.; Moenter, S.M. Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife 2019, 8. [Google Scholar] [CrossRef]
- Takumi, K.; Iijima, N.; Ozawa, H. Developmental changes in the expression of kisspeptin mRNA in rat hypothalamus. J. Mol. Neurosci. 2011, 43, 138–145. [Google Scholar] [CrossRef]
- Takumi, K.; Iijima, N.; Iwata, K.; Higo, S.; Ozawa, H. The effects of gonadal steroid manipulation on the expression of Kiss1 mRNA in rat arcuate nucleus during postnatal development. J. Physiol. Sci. 2012, 62, 453–460. [Google Scholar] [CrossRef]
- Kauffman, A.S.; Gottsch, M.L.; Roa, J.; Byquist, A.C.; Crown, A.; Clifton, D.K.; Hoffman, G.E.; Steiner, R.A.; Tena-Sempere, M. Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 2007, 148, 1774–1783. [Google Scholar] [CrossRef]
- Homma, T.; Sakakibara, M.; Yamada, S.; Kinoshita, M.; Iwata, K.; Tomikawa, J.; Kanazawa, T.; Matsui, H.; Takatsu, Y.; Ohtaki, T.; et al. Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular kisspeptin neurons and the GnRH/LH surge system in male rats. Biol. Reprod. 2009, 81, 1216–1225. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Handa, R.J. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: Comparison with other neuropeptides. J. Comp. Neurol. 2005, 484, 28–42. [Google Scholar] [CrossRef]
- Simonian, S.X.; Herbison, A.E. Differential expression of estrogen receptor alpha and beta immunoreactivity by oxytocin neurons of rat paraventricular nucleus. J. Neuroendocrinol. 1997, 9, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.E.; Lopez, V.; McEwen, B.S.; Weiland, N.G. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: An immunocytochemical study. Proc. Natl. Acad. Sci. USA 1998, 95, 3281–3286. [Google Scholar] [CrossRef]
- Andersson, S.; Sundberg, M.; Pristovsek, N.; Ibrahim, A.; Jonsson, P.; Katona, B.; Clausson, C.M.; Zieba, A.; Ramstrom, M.; Soderberg, O.; et al. Insufficient antibody validation challenges oestrogen receptor beta research. Nat. Commun. 2017, 8, 15840. [Google Scholar] [CrossRef]
- Oyola, M.G.; Thompson, M.K.; Handa, A.Z.; Handa, R.J. Distribution and chemical composition of estrogen receptor beta neurons in the paraventricular nucleus of the female and male mouse hypothalamus. J. Comp. Neurol. 2017, 525, 3666–3682. [Google Scholar] [CrossRef] [PubMed]
- Biag, J.; Huang, Y.; Gou, L.; Hintiryan, H.; Askarinam, A.; Hahn, J.D.; Toga, A.W.; Dong, H.W. Cyto- and chemoarchitecture of the hypothalamic paraventricular nucleus in the C57BL/6J male mouse: A study of immunostaining and multiple fluorescent tract tracing. J. Comp. Neurol. 2012, 520, 6–33. [Google Scholar] [CrossRef]
- Shapiro, R.A.; Xu, C.; Dorsa, D.M. Differential transcriptional regulation of rat vasopressin gene expression by estrogen receptor alpha and beta. Endocrinology 2000, 141, 4056–4064. [Google Scholar] [CrossRef]
- Barron, W.M.; Schreiber, J.; Lindheimer, M.D. Effect of ovarian sex steroids on osmoregulation and vasopressin secretion in the rat. Am. J. Physiol. 1986, 250, E352–E361. [Google Scholar] [CrossRef]
- Stachenfeld, N.S.; DiPietro, L.; Palter, S.F.; Nadel, E.R. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am. J. Physiol. 1998, 274, R187–R195. [Google Scholar] [CrossRef]
- Gillies, G.E.; Linton, E.A.; Lowry, P.J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature 1982, 299, 355–357. [Google Scholar] [CrossRef] [PubMed]
- Rittmaster, R.S.; Cutler, G.B., Jr.; Gold, P.W.; Brandon, D.D.; Tomai, T.; Loriaux, D.L.; Chrousos, G.P. The relationship of saline-induced changes in vasopressin secretion to basal and corticotropin-releasing hormone-stimulated adrenocorticotropin and cortisol secretion in man. J. Clin. Endocrinol. Metab. 1987, 64, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Handa, R.J.; Burgess, L.H.; Kerr, J.E.; O’Keefe, J.A. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm. Behav. 1994, 28, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Shughrue, P.J.; Lane, M.V.; Merchenthaler, I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J. Comp. Neurol. 1997, 388, 507–525. [Google Scholar] [CrossRef]
- Kohl, J.; Babayan, B.M.; Rubinstein, N.D.; Autry, A.E.; Marin-Rodriguez, B.; Kapoor, V.; Miyamishi, K.; Zweifel, L.S.; Luo, L.; Uchida, N.; et al. Functional circuit architecture underlying parental behaviour. Nature 2018, 556, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Amico, J.A.; Crowley, R.S.; Insel, T.R.; Thomas, A.; O’Keefe, J.A. Effect of gonadal steroids upon hypothalamic oxytocin expression. Adv. Exp. Med. Biol. 1995, 395, 23–35. [Google Scholar]
- Thomas, A.; Crowley, R.S.; Amico, J.A. Effect of progesterone on hypothalamic oxytocin messenger ribonucleic acid levels in the lactating rat. Endocrinology 1995, 136, 4188–4194. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, R.; Lacagnina, A.F.; Hinds, L.R.; Carbone, D.G.; Uht, R.M.; Handa, R.J. The androgen metabolite, 5α-androstane-3β,17β-diol (3beta-diol), activates the oxytocin promoter through an estrogen receptor-β pathway. Endocrinology 2013, 154, 1802–1812. [Google Scholar] [CrossRef]
- Neumann, I.D.; Wigger, A.; Torner, L.; Holsboer, F.; Landgraf, R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: Partial action within the paraventricular nucleus. J. Neuroendocrinol. 2000, 12, 235–243. [Google Scholar] [CrossRef]
- Kudwa, A.E.; McGivern, R.F.; Handa, R.J. Estrogen receptor beta and oxytocin interact to modulate anxiety-like behavior and neuroendocrine stress reactivity in adult male and female rats. Physiol. Behav. 2014, 129, 287–296. [Google Scholar] [CrossRef]
- Russell, J.A.; Leng, G. Sex, parturition and motherhood without oxytocin? J. Endocrinol. 1998, 157, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.F.; Hou, G. Commentary: Oxytocin Enables Maternal Behavior by Balancing Cortical Inhibition. Front. Behav. Neurosci. 2015, 9, 311. [Google Scholar] [CrossRef] [PubMed]
- Knobloch, H.S.; Charlet, A.; Hoffmann, L.C.; Eliava, M.; Khrulev, S.; Cetin, A.H.; Osten, P.; Schwarz, M.K.; Seeburg, P.H.; Stoop, R.; et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 2012, 73, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.W.; Kuypers, H.G. The paraventricular nucleus of the hypothalamus: Cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J. Comp. Neurol. 1980, 194, 555–570. [Google Scholar] [CrossRef]
- Chen, Q.H.; Toney, G.M. Identification and characterization of two functionally distinct groups of spinal cord-projecting paraventricular nucleus neurons with sympathetic-related activity. Neuroscience 2003, 118, 797–807. [Google Scholar] [CrossRef]
- Bohler, H.C., Jr.; Zoeller, R.T.; King, J.C.; Rubin, B.S.; Weber, R.; Merriam, G.R. Corticotropin releasing hormone mRNA is elevated on the afternoon of proestrus in the parvocellular paraventricular nuclei of the female rat. Brain Res. Mol. Brain Res. 1990, 8, 259–262. [Google Scholar] [CrossRef]
- Patchev, V.K.; Hayashi, S.; Orikasa, C.; Almeida, O.F. Implications of estrogen-dependent brain organization for gender differences in hypothalamo-pituitary-adrenal regulation. FASEB J. 1995, 9, 419–423. [Google Scholar] [CrossRef]
- Herman, J.P.; Figueiredo, H.; Mueller, N.K.; Ulrich-Lai, Y.; Ostrander, M.M.; Choi, D.C.; Cullinan, W.E. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 2003, 24, 151–180. [Google Scholar] [CrossRef]
- Whitnall, M.H.; Mezey, E.; Gainer, H. Co-localization of corticotropin-releasing factor and vasopressin in median eminence neurosecretory vesicles. Nature 1985, 317, 248–250. [Google Scholar] [CrossRef]
- Adachi, S.; Yamada, S.; Takatsu, Y.; Matsui, H.; Kinoshita, M.; Takase, K.; Sugiura, H.; Ohtaki, T.; Matsumoto, H.; Uenoyama, Y.; et al. Involvement of anteroventral periventricular metastin/kisspeptin neurons in estrogen positive feedback action on luteinizing hormone release in female rats. J. Reprod. Dev. 2007, 53, 367–378. [Google Scholar] [CrossRef]
- Meloni, E.G.; Jackson, A.V.; Cohen, B.M.; Carlezon, W.A., Jr. Corticotropin-releasing factor from the rat brain measured by protein immunoblot. Peptides 2005, 26, 2252–2256. [Google Scholar] [CrossRef] [PubMed]
- Lehman, M.N.; Hileman, S.M.; Goodman, R.L. Neuroanatomy of the kisspeptin signaling system in mammals: Comparative and developmental aspects. Adv. Exp. Med. Biol. 2013, 784, 27–62. [Google Scholar] [PubMed]
- O’Brien, M.L.; Park, K.; In, Y.; Park-Sarge, O.K. Characterization of estrogen receptor-beta (ERbeta) messenger ribonucleic acid and protein expression in rat granulosa cells. Endocrinology 1999, 140, 4530–4541. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saunders, P.T.; Maguire, S.M.; Gaughan, J.; Millar, M.R. Expression of oestrogen receptor β (ER beta) in multiple rat tissues visualised by immunohistochemistry. J. Endocrinol. 1997, 154, R13–R16. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Marmer, M.; Woodham, C.; Chang, W.; Kuiper, G.; Gustafsson, J.A.; Birch, L. Estrogen receptor-beta messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology 1998, 139, 874–883. [Google Scholar] [CrossRef]
- Kanaya, M.; Morishita, M.; Tsukahara, S. Temporal Expression Patterns of Genes Related to Sex Steroid Action in Sexually Dimorphic Nuclei During Puberty. Front. Endocrinol. (Lausanne) 2018, 9, 213. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain, 6th ed.; Elsevier Academic Press: London, UK, 2007. [Google Scholar]
- Samineni, V.K.; Grajales-Reyes, J.G.; Sundaram, S.S.; Yoo, J.J.; Gereau, R.W. Cell type-specific modulation of sensory and affective components of itch in the periaqueductal gray. Nat. Commun. 2019, 10, 4356. [Google Scholar] [CrossRef]
- Steinkellner, T.; Zell, V.; Farino, Z.J.; Sonders, M.S.; Villeneuve, M.; Freyberg, R.J.; Przedborski, S.; Lu, W.; Freyberg, Z.; Hnasko, T.S. Role for VGLUT2 in selective vulnerability of midbrain dopamine neurons. J. Clin. Investig. 2018, 128, 774–788. [Google Scholar] [CrossRef]
- Lanfranco, M.F.; Mocchetti, I.; Burns, M.P.; Villapol, S. Glial- and Neuronal-Specific Expression of CCL5 mRNA in the Rat Brain. Front. Neuroanat. 2017, 11, 137. [Google Scholar] [CrossRef]
- Swanson, L.W.; Sawchenko, P.E. Paraventricular nucleus: A site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology 1980, 31, 410–417. [Google Scholar] [CrossRef]

| Target | Primary Antibody | Manufacturer, Catalog # or Source | Species Raised in Monoclonal or Polyclonal | Secondary Antibody | Manufacturer, Catalog # or Source |
|---|---|---|---|---|---|
| Kisspeptin | Mouse monoclonal kisspeptin antibody | Takeda Pharmaceutical Co., Ltd. | Mouse; monoclonal | Donkey Alexa 488 anti-mouse | Abcam, ab150105 |
| Arginine-vasopressin | Guinea Pig anti-(Arg8)-Vasopressin | Peninsula, T5048 | Guinea pig; polyclonal | Donkey Dy Light 488 anti-guinea pig | Jackson, 706-485-148 |
| Oxytocin | Anti-Oxytocin Antibody | Merck Millipore, AB911 | Rabbit; polyclonal | Donkey Alexa 488 anti-rabbit | Abcam, ab150073 |
| Corticotropin-releasing factor | Rabbit anti- Corticotropin-releasing factor | Peninsula, T4037 | Rabbit; polyclonal | Donkey Alexa 488 anti-rabbit | Abcam, ab150073 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanaya, M.; Higo, S.; Ozawa, H. Neurochemical Characterization of Neurons Expressing Estrogen Receptor β in the Hypothalamic Nuclei of Rats Using in Situ Hybridization and Immunofluorescence. Int. J. Mol. Sci. 2020, 21, 115. https://doi.org/10.3390/ijms21010115
Kanaya M, Higo S, Ozawa H. Neurochemical Characterization of Neurons Expressing Estrogen Receptor β in the Hypothalamic Nuclei of Rats Using in Situ Hybridization and Immunofluorescence. International Journal of Molecular Sciences. 2020; 21(1):115. https://doi.org/10.3390/ijms21010115
Chicago/Turabian StyleKanaya, Moeko, Shimpei Higo, and Hitoshi Ozawa. 2020. "Neurochemical Characterization of Neurons Expressing Estrogen Receptor β in the Hypothalamic Nuclei of Rats Using in Situ Hybridization and Immunofluorescence" International Journal of Molecular Sciences 21, no. 1: 115. https://doi.org/10.3390/ijms21010115
APA StyleKanaya, M., Higo, S., & Ozawa, H. (2020). Neurochemical Characterization of Neurons Expressing Estrogen Receptor β in the Hypothalamic Nuclei of Rats Using in Situ Hybridization and Immunofluorescence. International Journal of Molecular Sciences, 21(1), 115. https://doi.org/10.3390/ijms21010115





