Abstract
The INDETERMINATE DOMAIN (IDD) genes comprise a conserved transcription factor family that regulates a variety of developmental and physiological processes in plants. Many recent studies have focused on the genetic characterization of IDD family members and revealed various biological functions, including modulation of sugar metabolism and floral transition, cold stress response, seed development, plant architecture, regulation of hormone signaling, and ammonium metabolism. In this review, we summarize the functions and working mechanisms of the IDD gene family in the regulatory network of metabolism and developmental processes.
1. Introduction
The Cys2His2 zinc-finger domain (C2H2) transcription factor family is one of the largest in plants. Analysis of 176 zinc finger proteins (ZFPs) from Arabidopsis revealed that 81% (143 ZFPs) were plant-specific (Arabidopsis thaliana, Zea mays, and Solanum tuberosum); only 19% (33 ZFPs) were conserved in other eukaryotes (Protozoa and animals) [1,2]. Extensive duplication has led to an expanded C2H2 family in plants [3]. Its subfamily INDETERMINATE DOMAIN (IDD), a conserved group across plants, contains functional genes that encode putative nuclear proteins with four zinc finger domains [4]. Since the report of the first plant IDD gene, PCP1, which is involved in sucrose uptake via an unknown mechanism in potatoes [5], many IDD genes have been functionally characterized. In general, IDDs form extensive protein interaction networks to ensure precise transcriptional control and thereby tissue- and/or cell-fate specification and hormonal signaling to control various aspects of plant growth and development [6,7,8,9].
Here, we provide a new understanding of the biological functions of IDD genes and their working mechanisms. We mainly focus on the role of IDDs in the linkage between sugar metabolism and developmental processes in plants.
2. Structure and Phylogenetic Analysis of IDD Proteins
The IDD genes encode putative proteins containing four zinc finger motifs (ZF1-C2H2, ZF2-C2H2, ZF3-C2HC, and ZF4-C2HC) that bind zinc atoms, forming the core structure [4,10] (Figure 1). ZF1, ZF2, and ZF3 are important for DNA binding [11], whereas C2HC is required for RNA binding [12,13]. Amino acid sequence alignment showed that ZF1, ZF2, ZF3, and ZF4 motifs are conserved in many plant species (Figure 2 and Table S1). Evolutionary relationships of IDD genes in many plant species were investigated by constructing a phylogenetic tree using 74 IDD proteins from Arabidopsis (16), potato (1), maize (22), rice (15), barley (1), sorghum (5), conifers (5), ferns (1), mosses (7), and freshwater green algae (1). Three major clades were identified. Clade I included IDDs from mosses, conifers, maize, barley, rice, and Arabidopsis. Clade II contained IDDs of freshwater green algae, mosses, conifers, maize, rice, sorghum, and Arabidopsis. Clade III mainly contained IDDs identified as being from flowering plants, known as angiosperms, such as Arabidopsis, potato, maize, rice, and sorghum (Figure 3). This evolutionary relationship demonstrates the conserved biological function of IDDs in many plant species.
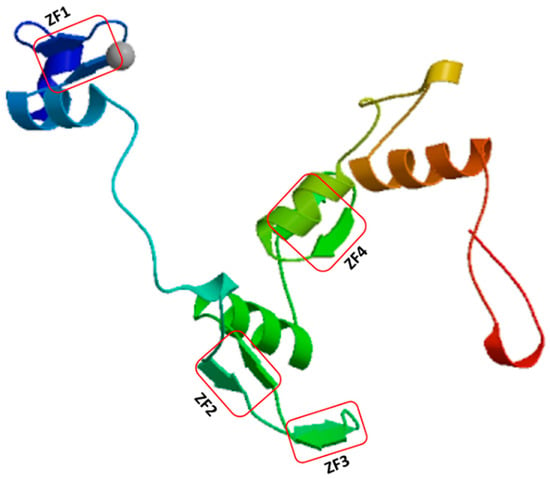
Figure 1.
Predicted secondary structure of Arabidopsis thaliana AtIDD11 with functional zinc finger domains. It was generated by SWISS-MODEL (https://swissmodel.expasy.org/). The model predicts the monomeric protein chain binding to zinc atoms (grey circle). The red rectangles indicate the position of the four zinc finger motifs.
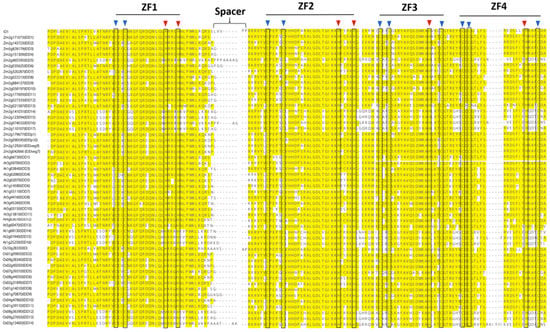
Figure 2.
Comparative amino acid sequence alignment of INDETERMINATE DOMAIN (IDD) genes that shows motifs or domain that are conserved in different species. Alignment includes IDDs from Arabidopsis thaliana (AtIDD), Oryza sativa (OsIDD), and Zea mays (ZmIDD). Black boxes mark the position of cysteines (C, in blue triangles) and histidines (H, red triangles) characterized for each zinc finger.
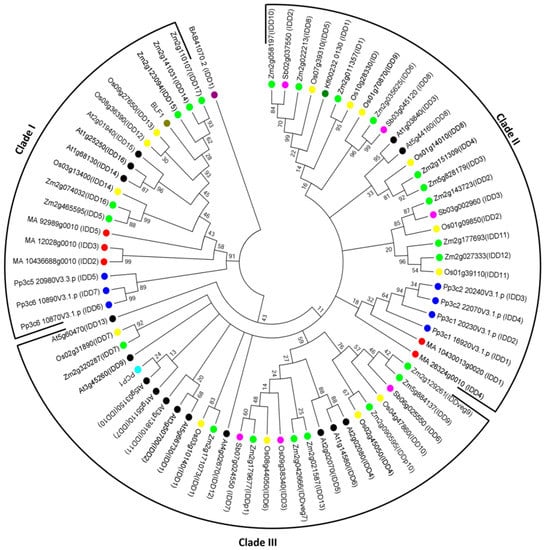
Figure 3.
Phylogenetic analysis of IDDs from various plants generated using MEGA7 software. IDD amino acid sequences were collected by finding best hits using protein–protein BLAST at the NCBI [14], and from PlantTFDB database (http://planttfdb.cbi.pku.edu.cn/). The phylogenetic tree was made by using the neighbor-joining method, based on the JTT matrix-based model [15] with 1000 bootstrap replicates after amino acid sequences were aligned by Clustal W. Bootstrap values less than 10 were cut off. The tree is drawn to scale, with branch lengths measured in the number of substitutions per site. The phylogenetic tree includes 74 protein sequences with 17 dicot IDDs: 16 Arabidopsis thaliana (At, black), and 1 Solanum tuberosum, (Potato couch potato1 (PCP1), aqua); 43 monocots IDDs: 15 Oryza sativa (Os, yellow), 22 Zea mays (ID1 and ZmIDD, green), 5 Sorghum bicolor (Sb, fuchsia), and 1 Hordeum vulgare (BLF1, olive); 1 freshwater green algae IDD: Klebsormidium flaccidum (Kfl, teal); 5 Conifer IDDs: Picea abies (MA, red); 1 Fern IDD: Ceratopteris reichardii (BAB, purple), and 7 Moss IDDs: Physcomitrella patens (Pp, blue).
3. Biological Functions of IDDs
3.1. Modulation of Sugar Metabolism and Floral Transition
ID1 regulates floral transition in maize [4,16,17]. Structural studies of ID1, along with other IDD proteins revealed the unique DNA-binding properties of two out of four zinc finger motifs [18], indicating that maize ID1 acts as a unique transcriptional regulator in the control of the floral transition. The id1 mutant displays a prolonged vegetative phase without other developmental defects. In support, various genes involved in flowering significantly changed their expression in the id1 mutant, including ZCN8 and ZMM4 [19,20]. The ID1 ortholog in rice, INDETERMINATE1 (OsID1)/Early Heading Date2 (Ehd2), Rice Indeterminate1 (RID1) is also involved in flowering [20,21,22,23]. Ehd2 acts as a floral activator by upregulating Ehd1 and the downstream floral activator genes, Heading date 3a (Hd3a) and Rft1 (Hd3b) genes in a unique regulatory network of photoperiodic flowering [20,21,22,23].
As a source of energy and carbon, sugar is the most important nutrient for growth and development of nearly all living organisms. Sugar metabolism is most likely associated with the floral transition, and IDDs are core members involved in the crosstalk. PCP1, an IDD gene of Solanum tuberosum, was shown to activate the silent endogenous sucrose uptake system in yeast [5]. The yeast strain SUSY7, which lacks an endogenous invertase gene, is unable to grow on sucrose-containing medium, but can be rescued by complementation of PCP1. Moreover, expression of PCP1 also rescues the yeast mutant strains that have defects in sucrose synthase and sucrose transport, although the underlying molecular mechanism remains unclear [5].
Several Arabidopsis IDD members function as transcriptional regulators of floral transition, possibly through the control of sucrose signaling [24]. In Arabidopsis, AtIDD8 has been reported to function in sugar metabolism and contribute to photoperiodic flowering [25]. Expression of Sucrose Transporter genes (SUC2, SUC6, SUC7, and SUC8) and Sucrose Synthase genes (SUS1 and SUS4) are affected by IDD8 activity. IDD8-SUS4 module-regulated sugar metabolism is associated with photoperiod flowering [25,26]. AtIDD8 is further regulated through phosphorylation at two positions, Ser-178 and Ser-182, which is catalyzed by the catalytic α-subunit of Sucrose-non-fermenting1 (Snf1)-related kinase 1 (SnRK1)/AKIN10 [27]. Phosphorylation of AtIDD8 significantly reduced its transcriptional activation activity [27]. Consistently, atidd8 mutants and plants overexpressing AKIN10 display a delayed-flowering phenotype. This pathway can be referred to as a gatekeeping mechanism for plants to regulate floral transition in a malnourished, low sugar level state (Figure 4).
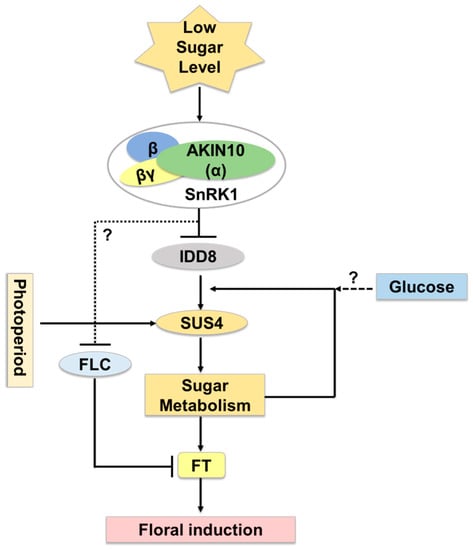
Figure 4.
AtIDD8-SUS4 module functioning in flowering time regulation in Arabidopsis thaliana. Under sugar deprived conditions, AKIN10, the α catalytic subunit of SnRK1 kinase, blocks IDD8 activity. IDD8 is phosphorylated at ser-178 and ser-182 positions to decrease its transcriptional activation activity, and thus, consequently, SUS4 expression. Additionally, endogenous sugar levels give feedback regulation to control the expression of SUS4. AKIN10 also has a role in the negative regulation of FLOWERING LOCUS C (FLC), which acts to suppress FLOWERING LOCUS T (FT), a floral activator in Arabidopsis.
At the pre-flowering stage, maize id1 mutant leaves have a significantly lower ratio of sucrose formation from starch [28]. Variations in sucrose and starch levels in id1 suggest an ID1 role in promoting carbohydrate export to the shoot apex upon flowering [28]. Another study reported the involvement of the sugar signaling molecule trehalose 6-phosphate (T6P) in developmental growth, including flowering [29]. Phloem-specific induction of the Arabidopsis florigen FLOWERING LOCUS T (FT) can rescue lines that have a late-flowering phenotype because of reduced expression of TREHALOSE-6-PHOSPHATE SYNTHASE1 (TPS1) [30,31]. These results indicate that sugar status linked to T6P signaling is vital for flowering [31]. In rice, OsIDD1 and OsIDD6 overexpression rescue the late-flowering phenotype of rice Ehd2, illustrating that IDD family genes might have a functional redundancy in sugar metabolism and the control of flowering time [32]. These reports suggest that multiple IDD genes modulate sugar metabolism, and some of them have a direct or indirect link with flowering regulation (Figure 5).
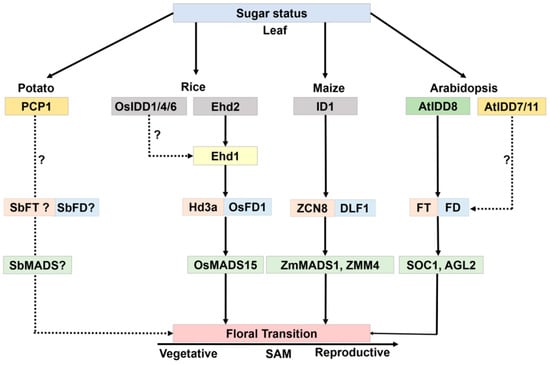
Figure 5.
Schematic representation of IDD members that might be involved in sugar transport and floral transition in the plant. Same color boxes are orthologous IDD genes. Dotted lines represent an unknown pathway. Genes with a question mark are still to be studied for floral transition.
3.2. Starch Accumulation and Cold Response
Abiotic stress, including salinity, drought, and cold, adversely affects plant growth and development. Cold stress is a significant environmental challenge, and plants have evolved various strategies to ensure plant fitness [33]. In Arabidopsis, two splice variants of AtIDD14 (AtIDD14α and β) directly regulate starch metabolism in response to cold via regulation of Qua-quine starch (QQS) expression. QQS represses starch accumulation [34]. The functional AtIDD14 form (AtIDD14α) binds to the QQS promoter and activates its expression, promoting starch degradation. The non-functional AtIDD14β form, which is produced mainly under cold conditions (4 °C), lacks a functional DNA-binding domain, but can form a heterodimer complex with AtIDD14α. Thus, the AtIDD14β isoform acts as a competitive inhibitor to repress DNA binding activity of AtIDD14α. Therefore, QQS is repressed by cold stress via the self-regulatory module provided by cold-induced alternative splicing. Competitive inhibition of AtIDD14α activity by AtIDD14β would serve as a cold adaptation strategy, helping plants maintain an appropriate level of starch accumulation during the dark period; this might be required to tolerate low temperatures during the light period [35].
In rice, an IDD gene encoding the ROC1 protein binds the CBF1 promoter directly to regulate cold tolerance [36]. Transcription activator CBF1 contains an AP2 domain, and it controls many cold-responsive genes [37]. In rice, MYB15 also controls the expression of CBF1 gene in cold stress. These results indicate that there might be some complex system that helps CBF1 regulate cold stress response, along with ROC1 and MYB15 [36,38,39,40]. The function of IDD in low temperature is remarkable. However, which endogenous signal activates ROC1 or AtIDD14 is less understood. Previous papers have shown that cold stress induces the alternative intracellular auxin gradient via auxin transporter gene (YUC, PIN) to regulate plant growth and development. Auxin and other phytohormones-responsive genes also respond to cold stress [41]. On the other hand, both AtIDD14 and ROC1 are reported to be involved in auxin signaling [6,36]. This suggests that auxin might induce IDD activity under cold-stress conditions.
3.3. Regulation of Seed Development
Seed development and maturation is a crucial process in the life cycle of a plant. IDDs are involved in the regulation of seed development. In maize, duplicated genes ZmIDDveg9/NAKED ENDOSPERM (NKD1) and ZmIDD9/NKD2 are involved in seed maturation, cell differentiation, thick walls, and accumulation of anthocyanin pigments [42]. These genes are required for aleurone cell fate and cell differentiation. Genetic mutations of the IDD genes Zmiddveg9 and Zmidd9 lead to naked endosperm phenotypes, decreases in germination rates, starch accumulation, delayed anthesis, less seed weight, and a propensity for vivipary [42,43]. NKD1 and NKD2 can directly regulate transcription and activate viviparous1 and opaque2 genes. Further, NKD2 functions as a negative regulator of NKD1 [42,43].
In Arabidopsis, AtIDD1 acts as either an activator or a repressor of germination, depending on the absence or presence of gibberellic acid, respectively. GID1b encoding a GA receptor is the target of GAF1/AtIDD1. Ectopic expression of IDD1/ENY under CaMV35S leads to disrupted seed development, delayed endosperm depletion, testa senescence, and an impaired maturation program. Subsequently, mature 2x35S:ENY seeds have high endosperm-specific fatty acids, starch retention, and defective mucilage extrusion with low expression of GID1b [4,5]. Studying the molecular mechanisms of IDD function, including transcriptional regulation of downstream gene networks, will provide a better understanding of regulated seed development, and the knowledge attained can be expanded to important work on cereal grain quality.
3.4. Modulation of Plant Architecture, Shoot Gravitropism, and Secondary Cell Wall Formation
Plant architecture influences plant fitness and productivity. IDDs play a role in organ development, and thereby plant architecture. In rice, secondary cell wall formation is negatively regulated by OsIDD2 [44]. Transgenic plants overexpressing OsIDD2 display dwarfism, fragile leaves, and decreased lignin content [45], whereas an osidd2 knockdown mutant produced by the CRISPR/Cas9 technique showed high lignin content. In particular, OsIDD2 downregulates genes involved in lignin biosynthesis and sucrose metabolism [44]. The Loose Plant Architecture1 (LPA1) gene, a functional ortholog of AtIDD15/SGR5 in rice, also affects the plant architecture, especially shoot gravitropism [46,47]. The lpa1 mutant coleoptile exhibits slower sedimentation rate of amyloplasts compared to wild-type [47]. The coleoptile of the lpa1 mutant exhibits negative gravitropism, indicating that signal transduction or gravity sensing is disturbed in the mutant. LPA1 also blocks auxin signaling through its interaction with C-22-hydroxylated and 6-deoxo brassinosteroids (BRs), which in turn regulate lamina inclination [46,47]. lpa1 mutants display indole-3-acetic acid (IAA) hypersensitivity during the lamina inclination response, which can be suppressed by brassinazole (Brz) (an inhibitor of C-22 hydroxylase involved in BR synthesis). Roles of LPA1 in OsPIN gene expression (OsPIN1a, OsPIN1c, and OsPIN3a) further indicate that the LPA1-mediated lamina inclination in rice might be due to auxin flux [46,47].
In barley, IDD protein BLF1 acts as a regulator of the leaf-width growth [48]. The blf1-1 mutation leads to wider but slightly shorter leaves than wild-type, because of a perturbation in the longitudinal cell numbers in leaves. A BLF1-vYFP fusion protein indicates BLF1 expression in the shoot apical meristem, epidermis, and prospective veins of leaf primordia. Given the economic and agronomical value of leaf traits in crop plants [49,50], BLF1 might be an ideal candidate for optimizing crop architecture.
In Arabidopsis, some IDD genes are associated with cellular patterning. Among them, AtIDD14-A (a spliced variant of AtIDD14), AtIDD15, and AtIDD16 regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport [6]. Since IDDs are also involved in starch metabolism, coordination between auxin accumulation and starch metabolism may underlie plant development. For example, the zinc finger transcription factor, SHOOT GRAVITROPISM5 (SGR5)/AtIDD15, has a crucial role in the early events of gravitropic responses in the inflorescence. The SGR5 gene has two splice variants: the truncated SGR5β form that lacks the functional ZF motifs and the full-size SGR5α transcription factor [51]. A truncated form of SGR5β inhibits SGR5α function, possibly by forming non-functional complex heterodimers. High temperatures might accelerate the alternative splicing of SGR5, resulting in a high level of accumulation of SGR5β proteins. SGR5β over-expression plants exhibit reduced gravitropic response of the inflorescence stem, similar to that of the atsgr5-1 phenotype [52].
JACKDAW (JKD/AtIDD10) and MAGPIE (MGP/AtIDD3) modulate the expression of SHORT-ROOT (SHR) and SCARECROW (SCR) in the root apex [53,54,55] (Figure 6). SCR and SHR are two GRAS family transcription factors, which are required for quiescent center and ground tissue formation in roots. JKD directly regulates SCR and MGP expression in cooperation with SHR [54]. SHR is a crucial regulator that directly activates the expression of SCL23 and SCR. In the SHR-SCR-SCL23 complex, SHR level is modulated by SCL23. The SHR-SCR-SCL23 complex plays a crucial role in endodermis formation in the hypocotyl [56]. JKD also modulates the repression of SCM that leads to the activity of the GLABRA3 (GL3)/ENHANCER OF GLABRA3 (EGL3)/TRANSPARENT TESTA GLABRA1 (TTG1) complex. This complex depends on the relative abundance of an MYB transcription factor, WEREWOLF (WER). WER triggers the trichoblasts (T cell) to inhibit GL2 and atrichoblasts (A cell) to lead cell division [53,57].
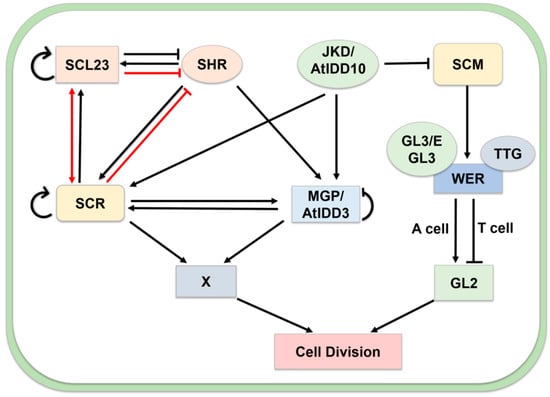
Figure 6.
Role of the MGP/AtIDD3 and JKD/AtIDD10 in cell division. JKD regulates the formation cells through two pathways: (1) Complex SHR-MGP-SCR-JKD with putative target (X) genes. SHR enhances expression of SCL23 and SCR and MGP. SHR negatively regulated via protein–protein interaction to SCL23 and SCR. Both SCR, MGP, and SCL23 can self-control their transcription. JKD directly regulates SCR and MGP expression in cooperation with MGP, SCR, and SHR. (2) JKD modulates the repression of SCM that leads to the activity of the GL3/EGL3/TTG1 complex, which depends on the relative abundance of an MYB transcription factor, WEREWOLF (WER). WER triggers the trichoblasts (T cell) to inhibit GL2 and atrichoblasts (A cell) to lead cell division. Arrows and bars represent positive regulation and negative regulation, respectively. Transcriptional controls are depicted with black arrows, protein–protein interactions are described with red arrows. The distinction of box color and shape describes different genes in this network.
3.5. Regulation of Hormonal Signaling
Hormone signaling has diverse and crucial roles in plant development. Hormone interactions control the formation of all organs in the plant by regulating meristem function. Gibberellins (GA), auxin, cytokinin (CK), brassinosteroids (BRs), and strigolactones (SLs) play vital roles during plant development, from embryogenesis to senescence [58]. DELLAs, the GRAS transcriptional regulators containing a GRAS domain at the C terminus and a DELLA/TVHYNP motif at the N terminus [9], act as key players in the regulation of GA responses. They lack a DNA binding domain, and act as transcriptional coregulators with other DNA-binding factors. Notably, five IDD members, AtIDD3, AtIDD4, AtIDD5, AtIDD9, and AtIDD10, interact with DELLA and regulate the GA-positive regulator, SCARECROW-LIKE3 (SCL3) [8,9,59,60]. Further experiments have indicated that DELLA and SCL3 act as coregulators and require IDDs as transcriptional scaffolds for DNA binding to check GA feedback regulation. IDD binding to DNA represents the balance of the SCL3 and DELLA protein levels to regulate downstream gene expression.
Auxin is another essential plant hormone that has a crucial role in controlling plant development processes, including embryogenesis, gametogenesis, patterning, lateral organ formation, tropic responses, and branching [61,62]. Auxin-mediated developmental and growth events are shaped by auxin biosynthesis and intercellular polar transport [61,63]. Some IDDs regulate auxin biosynthesis and transport. The epinastic leaves in plants overexpressing IDDs (IDD14, IDD15, and IDD16) are similar to those in auxin overproduction plants [6,64,65,66,67]. The IDD proteins directly bind to the promoter regions of TAA1, PIN1, and YUC5 and activate their expression. IDD-regulated auxin signaling might be further regulated by ZAT6 [68]. Further study of crosstalk between hormone metabolism and the surrounding environment will lead to a better understanding of the role of IDD proteins in the regulation of hormonal signaling.
3.6. Ammonium Metabolism
In the roots of higher plants, ammonium and nitrates are the primary sources of nitrogen (N). Asparagine and glutamine are the primary forms of organic N, and are transported to the shoots from the roots via the xylem [69]. Many reports have suggested a possible role of N in the various developmental and metabolic processes [70,71,72,73,74]. In rice, OsIDD10 directly activates the transcription of AMT1;2 (ammonium transporter) and GDH2 (glutamate dehydrogenase, which degrades glutamate to ammonia and alpha-ketoglutarate). Further, OsIDD10 also upregulates genes involved in N-linked metabolism, including nitrite reductases, glutamine synthetase 2, and trehalose 6-phosphate synthase [10,75]. Notably, OsIDD10 plays an essential role in the interaction between NH4+ and auxin signaling in rice roots [76]. The gravity response was delayed in osidd10 roots and accelerated in OsIDD10 overexpression (IDD10-OX) roots in the absence and presence of NH4+, respectively [69,76]. However, treatment with 1-N-naphthylphthalamic acid (NPA), a polar auxin transport inhibitor, suppressed the NH4+-induced root specific phenotype of the osidd10. The expression of NH4+-mediated auxin-related genes is affected in osidd10 and OsIDD10 overexpression plants. Phenotypes and expression patterns triggered by NH4+ are influenced by the actions of auxin during root development, suggesting a regulatory circuit in rice between NH4+ and auxin signaling that functions in root development [76]. The fact that IDD10 induces the expression of genes for trehalose-6-phosphate synthase, aminotransferase, and cytokinin dehydrogenase further strengthens the possibility of functional involvement of the gene in N-linked metabolism [10]. Therefore, it will be essential to perform a metabolite analysis to determine the possible agricultural benefits of manipulating IDD10 to enhance the efficiency of N metabolism in crop plants.
4. Conclusions
The IDD protein family comprises plant-specific transcription factors that have primary functions in inflorescence, leaf architecture, root architecture, seed development, and sugar homeostasis [77]. Most IDD genes have been mainly characterized in Arabidopsis; however, a few have been functionally characterized in other plants (Table 1). They are involved in seed maturation and germination, GA signaling, root development, sugar metabolism, leaf polarity, starch metabolism, cold-stress signaling, auxin biosynthesis and transport, flowering, plant architecture, shoot gravitropism, ammonium uptake, and endosperm development (Figure 7) [4,5,7,8,9,25,32,36,42,43,44,47,48,53,54,55,75,77,78,79,80,81,82]. IDD activity regulates many traits that have a direct or indirect impact on crop yield. Specifically, traits such as leaf angle contribute to overall plant architecture. Sugar metabolism and flowering time contribute to the allocation of carbon and grain yield, and endosperm development to seed maturation. Based on the fact that IDDs govern nearly all aspects of plant growth and development, information about IDDs will provide invaluable insights into the genetic programs underlying signaling networks in the regulation of plant development and metabolism with connections to external environmental fluctuations.

Table 1.
List of IDD genes described in this study.
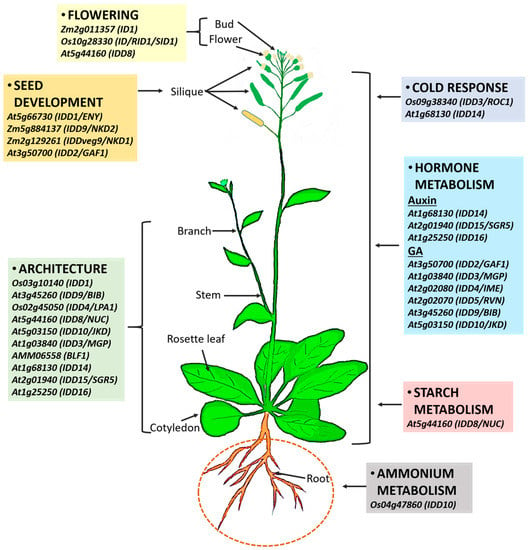
Figure 7.
Overview of multiple functions of IDDs in plant growth and development based on previous reports. IDDs control flowering transition, regulate seed development, metabolism of starch, hormones, and ammonium, and are involved in responsiveness to cold stress.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/20/9/2286/s1.
Author Contributions
M.K. and D.T.L. wrote the manuscript; S.H., P.J.S., and H.U.K. finalized the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Next Generation BioGreen 21 Program of the Rural Development Administration, Korea [grant number PJ013185]; the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET), Korea [grant number 316087-4]; and the Mid-Career Researcher Program of the National Research Foundation of Korea [grant number NRF-2017R1A2B4007096].
Conflicts of Interest
None of the authors has any conflict of interest to disclose.
Abbreviations
| IDD | INDETERMINATE DOMAIN |
| LPA | Loose Plant Architecture |
| TPS | Trehalose-6-phosphate synthase |
| FT | Flowering Locus T |
| RID | Rice Indeterminate |
| Ehd | Early heading date |
| SUS | Sucrose Synthase |
| BLF | Broadleaf |
| AMT | Ammonium Transporter |
| NKD | Naked Endosperm |
| ENY | Enhydrous |
| HD | Heading Date |
| Rft1 | Rice Flowering Locus T1 |
References
- Pabo, C.O.; Sauer, R.T. Transcription factors: Structural families and principles of DNA recognition. Annu. Rev. Biochem. 1992, 61, 1053–1095. [Google Scholar] [CrossRef] [PubMed]
- Englbrecht, C.C.; Schoof, H.; Bohm, S. Conservation, diversification and expansion of C2H2 zinc finger proteins in the Arabidopsis thaliana genome. BMC Genom. 2004, 5, 39. [Google Scholar] [CrossRef]
- Liu, Q.G.; Wang, Z.C.; Xu, X.M.; Zhang, H.Z.; Li, C.H. Genome-Wide Analysis of C2H2 Zinc-Finger Family Transcription Factors and Their Responses to Abiotic Stresses in Poplar (Populus trichocarpa). PLoS ONE 2015, 10, e0134753. [Google Scholar] [CrossRef]
- Colasanti, J.; Tremblay, R.; Wong, A.Y.; Coneva, V.; Kozaki, A.; Mable, B.K. The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genom. 2006, 7, 158. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.; Frommer, W.B. A novel zinc finger protein encoded by a couch potato homologue from Solanum tuberosum enables a sucrose transport-deficient yeast strain to grow on sucrose. Mol. Gen. Genet. 1995, 247, 759–763. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, J.; Jing, Y.; Fan, M.; Liu, J.; Wang, Z.; Xin, W.; Hu, Y. The arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013, 9, e1003759. [Google Scholar] [CrossRef]
- Feurtado, J.A.; Huang, D.; Wicki-Stordeur, L.; Hemstock, L.E.; Potentier, M.S.; Tsang, E.W.; Cutler, A.J. The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell 2011, 23, 1772–1794. [Google Scholar] [CrossRef]
- Fukazawa, J.; Teramura, H.; Murakoshi, S.; Nasuno, K.; Nishida, N.; Ito, T.; Yoshida, M.; Kamiya, Y.; Yamaguchi, S.; Takahashi, Y. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell 2014, 26, 2920–2938. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Hirano, K.; Sato, T.; Mitsuda, N.; Nomoto, M.; Maeo, K.; Koketsu, E.; Mitani, R.; Kawamura, M.; Ishiguro, S.; et al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc. Natl. Acad. Sci. USA 2014, 111, 7861–7866. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.H.; Priatama, R.A.; Huang, J.; Je, B.I.; Liu, J.M.; Park, S.J.; Piao, H.L.; Son, D.Y.; Lee, J.J.; Park, S.H.; et al. Indeterminate domain 10 regulates ammonium-mediated gene expression in rice roots. New Phytol. 2013, 197, 791–804. [Google Scholar] [CrossRef]
- Hirano, Y.; Nakagawa, M.; Suyama, T.; Murase, K.; Shirakawa, M.; Takayama, S.; Sun, T.P.; Hakoshima, T. Structure of the SHR-SCR heterodimer bound to the BIRD/IDD transcriptional factor JKD. Nat. Plants 2017, 3, 17010. [Google Scholar] [CrossRef]
- Clemens, K.R.; Wolf, V.; Mcbryant, S.J.; Zhang, P.H.; Liao, X.B.; Wright, P.E.; Gottesfeld, J.M. Molecular-Basis for Specific Recognition of Both Rna and DNA by a Zinc Finger Protein. Science 1993, 260, 530–533. [Google Scholar] [CrossRef]
- Searles, M.A.; Lu, D.; Klug, A. The role of the central zinc fingers of transcription factor IIIA in binding to 5 S RNA. J. Mol. Biol. 2000, 301, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The Rapid Generation of Mutation Data Matrices from Protein Sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Singleton, W.R. Inheritance of indeterminate growth in maize. J. Hered. 1946, 37, 61–64. [Google Scholar] [CrossRef]
- Wong, A.Y.; Colasanti, J. Maize floral regulator protein INDETERMINATE1 is localized to developing leaves and is not altered by light or the sink/source transition. J. Exp. Bot. 2007, 58, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, A.; Hake, S.; Colasanti, J. The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res. 2004, 32, 1710–1720. [Google Scholar] [CrossRef]
- Muszynski, M.G.; Dam, T.; Li, B.; Shirbroun, D.M.; Hou, Z.; Bruggemann, E.; Archibald, R.; Ananiev, E.V.; Danilevskaya, O.N. Delayed flowering1 encodes a basic leucine zipper protein that mediates floral inductive signals at the shoot apex in maize. Plant Physiol. 2006, 142, 1523–1536. [Google Scholar] [CrossRef]
- Meng, X.; Muszynski, M.G.; Danilevskaya, O.N. The FT-like ZCN8 Gene Functions as a Floral Activator and Is Involved in Photoperiod Sensitivity in Maize. Plant Cell 2011, 23, 942–960. [Google Scholar] [CrossRef]
- Matsubara, K.; Yamanouchi, U.; Wang, Z.X.; Minobe, Y.; Izawa, T.; Yano, M. Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol. 2008, 148, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, S.L.; Lee, S.; Je, B.I.; Piao, H.L.; Park, S.H.; Kim, C.M.; Ryu, C.H.; Park, S.H.; Xuan, Y.H.; et al. Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J. 2008, 56, 1018–1029. [Google Scholar] [CrossRef]
- Wu, C.; You, C.; Li, C.; Long, T.; Chen, G.; Byrne, M.E.; Zhang, Q. RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc. Natl. Acad. Sci. USA 2008, 105, 12915–12920. [Google Scholar] [CrossRef]
- Bernier, G.; Havelange, A.; Houssa, C.; Petitjean, A.; Lejeune, P. Physiological Signals That Induce Flowering. Plant Cell 1993, 5, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Seo, P.J.; Ryu, J.; Kang, S.K.; Park, C.M. Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J. 2011, 65, 418–429. [Google Scholar] [CrossRef] [PubMed]
- King, R.W.; Hisamatsu, T.; Goldschmidt, E.E.; Blundell, C. The nature of floral signals in Arabidopsis. I. Photosynthesis and a far-red photoresponse independently regulate flowering by increasing expression of FLOWERING LOCUS T (FT). J. Exp. Bot. 2008, 59, 3811–3820. [Google Scholar] [CrossRef]
- Jeong, E.Y.; Seo, P.J.; Woo, J.C.; Park, C.M. AKIN10 delays flowering by inactivating IDD8 transcription factor through protein phosphorylation in Arabidopsis. BMC Plant Biol. 2015, 15, 110. [Google Scholar] [CrossRef]
- Coneva, V.; Guevara, D.; Rothstein, S.J.; Colasanti, J. Transcript and metabolite signature of maize source leaves suggests a link between transitory starch to sucrose balance and the autonomous floral transition. J. Exp. Bot. 2012, 63, 5079–5092. [Google Scholar] [CrossRef]
- Figueroa, C.M.; Lunn, J.E. A Tale of Two Sugars: Trehalose 6-Phosphate and Sucrose. Plant Physiol. 2016, 172, 7–27. [Google Scholar] [CrossRef]
- Gomez-Ariza, J.; Galbiati, F.; Goretti, D.; Brambilla, V.; Shrestha, R.; Pappolla, A.; Courtois, B.; Fornara, F. Loss of floral repressor function adapts rice to higher latitudes in Europe. J. Exp. Bot. 2015, 66, 2027–2039. [Google Scholar] [CrossRef]
- Wahl, V.; Ponnu, J.; Schlereth, A.; Arrivault, S.; Langenecker, T.; Franke, A.; Feil, R.; Lunn, J.E.; Stitt, M.; Schmid, M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 2013, 339, 704–707. [Google Scholar] [CrossRef]
- Deng, L.; Li, L.; Zhang, S.; Shen, J.; Li, S.; Hu, S.; Peng, Q.; Xiao, J.; Wu, C. Suppressor of rid1 (SID1) shares common targets with RID1 on florigen genes to initiate floral transition in rice. PLoS Genet. 2017, 13, e1006642. [Google Scholar] [CrossRef]
- Thomashow, M.F. Role of cold-responsive genes in plant freezing tolerance. Plant Physiol. 1998, 118, 1–8. [Google Scholar] [CrossRef]
- Li, L.; Foster, C.M.; Gan, Q.; Nettleton, D.; James, M.G.; Myers, A.M.; Wurtele, E.S. Identification of the novel protein QQS as a component of the starch metabolic network in Arabidopsis leaves. Plant J. 2009, 58, 485–498. [Google Scholar] [CrossRef]
- Seo, P.J.; Kim, M.J.; Ryu, J.Y.; Jeong, E.Y.; Park, C.M. Two splice variants of the IDD14 transcription factor competitively form nonfunctional heterodimers which may regulate starch metabolism. Nat. Commun. 2011, 2, 303. [Google Scholar] [CrossRef]
- Dou, M.; Cheng, S.; Zhao, B.; Xuan, Y.; Shao, M. The Indeterminate Domain Protein ROC1 Regulates Chilling Tolerance via Activation of DREB1B/CBF1 in Rice. Int. J. Mol. Sci. 2016, 17, 233. [Google Scholar] [CrossRef]
- Jaglo-Ottosen, K.R.; Gilmour, S.J.; Zarka, D.G.; Schabenberger, O.; Thomashow, M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science 1998, 280, 104–106. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.H.; Fujii, H.; Zheng, X.; Zhu, J.K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.K.; Sunkar, R. Gene regulation during cold stress acclimation in plants. Methods Mol. Biol. 2010, 639, 39–55. [Google Scholar]
- Dong, C.H.; Agarwal, M.; Zhang, Y.; Xie, Q.; Zhu, J.K. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 2006, 103, 8281–8286. [Google Scholar] [CrossRef]
- Shibasaki, K.; Uemura, M.; Tsurumi, S.; Rahman, A. Auxin response in Arabidopsis under cold stress: Underlying molecular mechanisms. Plant Cell 2009, 21, 3823–3838. [Google Scholar] [CrossRef]
- Yi, G.; Neelakandan, A.K.; Gontarek, B.C.; Vollbrecht, E.; Becraft, P.W. The naked endosperm genes encode duplicate INDETERMINATE domain transcription factors required for maize endosperm cell patterning and differentiation. Plant Physiol. 2015, 167, 443–456. [Google Scholar] [CrossRef]
- Gontarek, B.C.; Neelakandan, A.K.; Wu, H.; Becraft, P.W. NKD Transcription Factors Are Central Regulators of Maize Endosperm Development. Plant Cell 2016, 28, 2916–2936. [Google Scholar] [CrossRef]
- Huang, P.; Yoshida, H.; Yano, K.; Kinoshita, S.; Kawai, K.; Koketsu, E.; Hattori, M.; Takehara, S.; Huang, J.; Hirano, K.; et al. OsIDD2, a zinc finger and INDETERMINATE DOMAIN protein, regulates secondary cell wall formation. J. Integr. Plant Biol. 2018, 60, 130–143. [Google Scholar] [CrossRef]
- Hirano, K.; Aya, K.; Morinaka, Y.; Nagamatsu, S.; Sato, Y.; Antonio, B.A.; Namiki, N.; Nagamura, Y.; Matsuoka, M. Survey of genes involved in rice secondary cell wall formation through a co-expression network. Plant Cell Physiol. 2013, 54, 1803–1821. [Google Scholar] [CrossRef]
- Liu, J.M.; Park, S.J.; Huang, J.; Lee, E.J.; Xuan, Y.H.; Je, B.I.; Kumar, V.; Priatama, R.A.; Raj, K.V.; Kim, S.H.; et al. Loose Plant Architecture1 (LPA1) determines lamina joint bending by suppressing auxin signalling that interacts with C-22-hydroxylated and 6-deoxo brassinosteroids in rice. J. Exp. Bot. 2016, 67, 1883–1895. [Google Scholar] [CrossRef]
- Wu, X.; Tang, D.; Li, M.; Wang, K.; Cheng, Z. Loose Plant Architecture1, an INDETERMINATE DOMAIN protein involved in shoot gravitropism, regulates plant architecture in rice. Plant Physiol. 2013, 161, 317–329. [Google Scholar] [CrossRef]
- Jost, M.; Hensel, G.; Kappel, C.; Druka, A.; Sicard, A.; Hohmann, U.; Beier, S.; Himmelbach, A.; Waugh, R.; Kumlehn, J.; et al. The INDETERMINATE DOMAIN Protein BROAD LEAF1 Limits Barley Leaf Width by Restricting Lateral Proliferation. Curr. Biol. 2016, 26, 903–909. [Google Scholar] [CrossRef]
- Richards, R.A. Selectable traits to increase crop photosynthesis and yield of grain crops. J. Exp. Bot. 2000, 51, 447–458. [Google Scholar] [CrossRef]
- Peng, S.; Khush, G.S.; Virk, P.; Tang, Q.; Zou, Y. Progress in ideotype breeding to increase rice yield potential. Field Crop. Res. 2008, 108, 32–38. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ryu, J.Y.; Baek, K.; Park, C.M. High temperature attenuates the gravitropism of inflorescence stems by inducing SHOOT GRAVITROPISM 5 alternative splicing in Arabidopsis. New Phytol. 2016, 209, 265–279. [Google Scholar] [CrossRef]
- Ryu, J.Y.; Kim, J.Y.; Park, C.M. Adaptive thermal control of stem gravitropism through alternative RNA splicing in Arabidopsis. Plant Signal Behav. 2015, 10, e1093715. [Google Scholar] [CrossRef]
- Hassan, H.; Scheres, B.; Blilou, I. JACKDAW controls epidermal patterning in the Arabidopsis root meristem through a non-cell-autonomous mechanism. Development 2010, 137, 1523–1529. [Google Scholar] [CrossRef]
- Ogasawara, H.; Kaimi, R.; Colasanti, J.; Kozaki, A. Activity of transcription factor JACKDAW is essential for SHR/SCR-dependent activation of SCARECROW and MAGPIE and is modulated by reciprocal interactions with MAGPIE, SCARECROW and SHORT ROOT. Plant Mol. Biol. 2011, 77, 489–499. [Google Scholar] [CrossRef]
- Welch, D.; Hassan, H.; Blilou, I.; Immink, R.; Heidstra, R.; Scheres, B. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev. 2007, 21, 2196–2204. [Google Scholar] [CrossRef]
- Yoon, E.K.; Dhar, S.; Lee, M.H.; Song, J.H.; Lee, S.A.; Kim, G.; Jang, S.; Choi, J.W.; Choe, J.E.; Kim, J.H.; et al. Conservation and Diversification of the SHR-SCR-SCL23 Regulatory Network in the Development of the Functional Endodermis in Arabidopsis Shoots. Mol. Plant 2016, 9, 1197–1209. [Google Scholar] [CrossRef]
- Salazar-Henao, J.E.; Velez-Bermudez, I.C.; Schmidt, W. The regulation and plasticity of root hair patterning and morphogenesis. Development 2016, 143, 1848–1858. [Google Scholar] [CrossRef]
- Durbak, A.; Yao, H.; McSteen, P. Hormone signaling in plant development. Curr. Opin. Plant Biol. 2012, 15, 92–96. [Google Scholar] [CrossRef]
- Zentella, R.; Zhang, Z.L.; Park, M.; Thomas, S.G.; Endo, A.; Murase, K.; Fleet, C.M.; Jikumaru, Y.; Nambara, E.; Kamiya, Y.; et al. Global analysis of della direct targets in early gibberellin signaling in Arabidopsis. Plant Cell 2007, 19, 3037–3057. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Ogawa, M.; Fleet, C.M.; Zentella, R.; Hu, J.; Heo, J.O.; Lim, J.; Kamiya, Y.; Yamaguchi, S.; Sun, T.P. Scarecrow-like 3 promotes gibberellin signaling by antagonizing master growth repressor DELLA in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 2160–2165. [Google Scholar] [CrossRef]
- Vanneste, S.; Friml, J. Auxin: A trigger for change in plant development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Woodward, A.W.; Bartel, B. Auxin: Regulation, action, and interaction. Ann. Bot. 2005, 95, 707–735. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Cheng, Y.; Dai, X.; Zhao, Y. Auxin biosynthesis by the YUCCA flavin monooxygenases controls the formation of floral organs and vascular tissues in Arabidopsis. Genes Dev. 2006, 20, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Murphy, A.S.; Baek, D.; Lee, S.W.; Yun, D.J.; Bressan, R.A.; Narasimhan, M.L. YUCCA6 over-expression demonstrates auxin function in delaying leaf senescence in Arabidopsis thaliana. J. Exp. Bot. 2011, 62, 3981–3992. [Google Scholar] [CrossRef]
- Kim, J.I.; Sharkhuu, A.; Jin, J.B.; Li, P.; Jeong, J.C.; Baek, D.; Lee, S.Y.; Blakeslee, J.J.; Murphy, A.S.; Bohnert, H.J.; et al. yucca6, a dominant mutation in Arabidopsis, affects auxin accumulation and auxin-related phenotypes. Plant Physiol. 2007, 145, 722–735. [Google Scholar] [CrossRef]
- Zhao, Y.; Christensen, S.K.; Fankhauser, C.; Cashman, J.R.; Cohen, J.D.; Weigel, D.; Chory, J. A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 2001, 291, 306–309. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, S.; Lin, D.; Wei, Y.; Yan, Y.; Liu, G.; Reiter, R.J.; Chan, Z. Zinc finger of Arabidopsis thaliana 6 is involved in melatonin-mediated auxin signaling through interacting INDETERMINATE DOMAIN15 and INDOLE-3-ACETIC ACID 17. J. Pineal Res. 2018, 65, e12494. [Google Scholar] [CrossRef]
- Fukumorita, T.; Chino, M. Sugar, Amino Acid and Inorganic Contents in Rice Phloem Sap. Plant Cell Physiol. 1982, 23, 273–283. [Google Scholar]
- Gutierrez, R.A.; Stokes, T.L.; Thum, K.; Xu, X.; Obertello, M.; Katari, M.S.; Tanurdzic, M.; Dean, A.; Nero, D.C.; McClung, C.R.; et al. Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc. Natl. Acad. Sci. USA 2008, 105, 4939–4944. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Cakmak, T.; Cooper, A.; Lager, I.; Rasmusson, A.G.; Escobar, M.A. Distinct signalling pathways and transcriptome response signatures differentiate ammonium- and nitrate-supplied plants. Plant Cell Environ. 2010, 33, 1486–1501. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.; Walters, L.A.; Cooper, A.M.; Olvera, J.G.; Rosas, M.A.; Rasmusson, A.G.; Escobar, M.A. Nitrate-Regulated Glutaredoxins Control Arabidopsis Primary Root Growth. Plant Physiol. 2016, 170, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Guegler, K.; LaBrie, S.T.; Crawford, N.M. Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell 2000, 12, 1491–1509. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tischner, R.; Gutierrez, R.A.; Hoffman, M.; Xing, X.; Chen, M.; Coruzzi, G.; Crawford, N.M. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004, 136, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.H.; Priatama, R.A.; Kumar, V.; Han, C.D. Regulatory role of indeterminate domain 10 (IDD10) in ammonium-dependent gene expression in rice roots. Plant Signal Behav. 2013, 8, e24139. [Google Scholar] [CrossRef][Green Version]
- Xuan, Y.H.; Kumar, V.; Zhu, X.F.; Je, B.I.; Kim, C.M.; Huang, J.; Cho, J.H.; Yi, G.; Han, C.D. IDD10 is Involved in the Interaction between NH4+ and Auxin Signaling in Rice Roots. J. Plant Biol. 2018, 61, 72–79. [Google Scholar] [CrossRef]
- Coelho, C.P.; Huang, P.; Lee, D.Y.; Brutnell, T.P. Making Roots, Shoots, and Seeds: IDD Gene Family Diversification in Plants. Trends Plant Sci. 2018, 23, 66–78. [Google Scholar] [CrossRef]
- Liu, T.; Reinhart, B.J.; Magnani, E.; Huang, T.; Kerstetter, R.; Barton, M.K. Of blades and branches: Understanding and expanding the Arabidopsis ad/abaxial regulatory network through target gene identification. Cold Spring Harb. Symp. Quant. Biol. 2012, 77, 31–45. [Google Scholar] [CrossRef]
- Moreno-Risueno, M.A.; Sozzani, R.; Yardimci, G.G.; Petricka, J.J.; Vernoux, T.; Blilou, I.; Alonso, J.; Winter, C.M.; Ohler, U.; Scheres, B.; et al. Transcriptional control of tissue formation throughout root development. Science 2015, 350, 426–430. [Google Scholar] [CrossRef]
- Morita, M.T.; Sakaguchi, K.; Kiyose, S.; Taira, K.; Kato, T.; Nakamura, M.; Tasaka, M. A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J. 2006, 47, 619–628. [Google Scholar] [CrossRef]
- Reinhart, B.J.; Liu, T.; Newell, N.R.; Magnani, E.; Huang, T.; Kerstetter, R.; Michaels, S.; Barton, M.K. Establishing a framework for the Ad/abaxial regulatory network of Arabidopsis: Ascertaining targets of class III homeodomain leucine zipper and KANADI regulation. Plant Cell 2013, 25, 3228–3249. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, M.; Tremblay, R.; Colasanti, J. Altered gravitropic response, amyloplast sedimentation and circumnutation in the Arabidopsis shoot gravitropism 5 mutant are associated with reduced starch levels. Plant Mol. Biol. 2008, 67, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Levesque, M.P.; Vernoux, T.; Busch, W.; Cui, H.C.; Wang, J.Y.; Blilou, I.; Hassan, H.; Nakajima, K.; Matsumoto, N.; Lohmann, J.U.; et al. Whole-genome analysis of the SHORT-ROOT developmental pathway in Arabidopsis (vol 4, pg 739, 2006). PLoS Biol. 2006, 4, 1284. [Google Scholar]
- Ingkasuwan, P.; Netrphan, S.; Prasitwattanaseree, S.; Tanticharoen, M.; Bhumiratana, S.; Meechai, A.; Chaijaruwanich, J.; Takahashi, H.; Cheevadhanarak, S. Inferring transcriptional gene regulation network of starch metabolism in Arabidopsis thaliana leaves using graphical Gaussian model. BMC Syst. Biol. 2012, 6, 100. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Avendano, E.; Ibanez, S.; Sanz, O.; Barros, J.A.S.; Gude, I.; Perianez-Rodriguez, J.; Micol, J.L.; Del Pozo, J.C.; Moreno-Risueno, M.A.; Perez-Perez, J.M. Regulation of Hormonal Control, Cell Reprogramming, and Patterning during De Novo Root Organogenesis. Plant Physiol. 2018, 176, 1709–1727. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Goedhart, J.; Schneijderberg, M.; Terpstra, I.; Shimotohno, A.; Bouchet, B.P.; Akhmanova, A.; Gadella, T.W., Jr.; Heidstra, R.; Scheres, B.; et al. SCARECROW-LIKE23 and SCARECROW jointly specify endodermal cell fate but distinctly control SHORT-ROOT movement. Plant J. 2015, 84, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.C.; Smet, W.; Cruz-Ramirez, A.; Castelijns, B.; de Jonge, W.; Mahonen, A.P.; Bouchet, B.P.; Perez, G.S.; Akhmanova, A.; Scheres, B.; et al. Arabidopsis BIRD Zinc Finger Proteins Jointly Stabilize Tissue Boundaries by Confining the Cell Fate Regulator SHORT-ROOT and Contributing to Fate Specification. Plant Cell 2015, 27, 1185–1199. [Google Scholar] [CrossRef]
- Long, Y.; Stahl, Y.; Weidtkamp-Peters, S.; Postma, M.; Zhou, W.; Goedhart, J.; Sanchez-Perez, M.I.; Gadella, T.W.J.; Simon, R.; Scheres, B.; et al. In vivo FRET-FLIM reveals cell-type-specific protein interactions in Arabidopsis roots. Nature 2017, 548, 97–102. [Google Scholar] [CrossRef]
- Colasanti, J.; Yuan, Z.; Sundaresan, V. The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 1998, 93, 593–603. [Google Scholar] [CrossRef]
- Galinat, W.C.; Naylor, A.W. Relation of Photoperiod to Inflorescence proliferation in Zea Mays L. Am. J. Bot. 1951, 38, 38–47. [Google Scholar] [CrossRef]
- Becraft, P.W.; Asuncion-Crabb, Y. Positional cues specify and maintain aleurone cell fate in maize endosperm development. Development 2000, 127, 4039–4048. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).