Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions
Abstract
1. Overview
2. Molecular Opinion
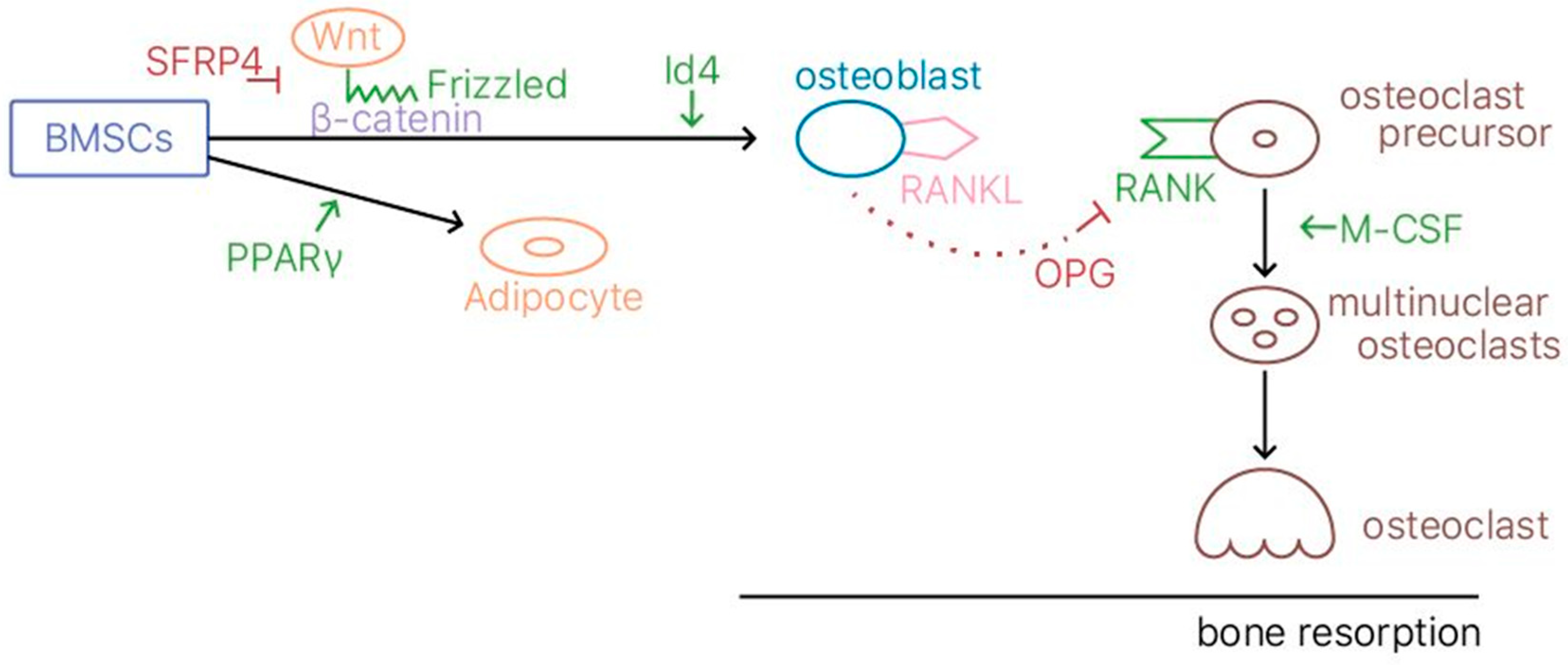
2.1. Bone Remodeling
2.2. Animal Model
2.3. Estrogen and SERMs
2.4. TSECs
2.5. Biphosphonates
2.6. Gene Expression
3. Clinical Opinion
3.1. Introduction
3.2. Pharmacological Treatment
3.2.1. Estrogen Therapy (ET)/Hormone Replacement Therapy (HRT)
3.2.2. Bisphosphonates
3.2.3. Adverse Effects of Bisphosphonates
3.2.4. SERMs
3.2.5. PTH Peptide: Teriparatide
3.3. Nutrition—Intake of Calcium and Vitamin D
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 27HC | 27-hydroxycholesterol |
| ASBMR | American Society for Bone and Mineral Research |
| bHLH | basic helix-loop-helix |
| BMD | bone mineral density |
| BMP2 | bone morphogenetic protein 2 |
| BMP-7 | bone morphogenetic protein 7 |
| BMSC | bone marrow stromal cell |
| CACNG1 | voltage-dependent calcium channel γ1 |
| CaMKII | calcium calmodulin-mediated kinase II |
| CCR3 | C-C motif chemokine receptor 3 |
| CNV | copy number variation |
| COL1A1 | collagen, type I, alpha 1 |
| CpG | 5′—C—Phosphate—G—3 |
| DEG | differentially expressed gene |
| Dkk1 | Dickkopf-1 |
| DXA | dual-energy X-ray absorptiometry |
| E2 | estradiol |
| ER | estrogen receptor |
| ERα | estrogen receptor alpha |
| ERβ | estrogen receptor beta |
| ESR1 | estrogen receptor 1 |
| ET | estrogen therapy |
| FDA | US Food and Drug Administration |
| GCR | glucocorticoid receptor |
| GI | gastrointestinal |
| GWAS | genome-wide association studies |
| HDC | histidine decarboxylase |
| HRT | hormone replacement therapy |
| Id4 | Inhibitor of differentiation 4 |
| IGF-1 | Insulin-like growth factor 1 |
| JNK | c-Jun N-terminal kinase |
| LRP5 | Low-density lipoprotein receptor-related protein 5 |
| M-CSF | macrophage colony stimulating factor |
| MSC | mesenchymal stem cell |
| mTECs | medullary thymic epithelial cells |
| NFAT | nuclear factor of activated T cells |
| ONJ | osteonecrosis of the jaw |
| OPG | osteoprotegerin |
| PEG | polyethylene glycol |
| Pi | phosphate |
| PO | postmenopausal osteoporosis |
| PPARγ | peroxisome proliferator activator gamma |
| PPI | protein-protein interaction |
| PTH | parathyroid hormone |
| QTL | quantitative trait locus |
| RANK | receptor activator of nuclear factor kappa-B |
| RANKL | receptor activator of nuclear factor kappa-B ligand |
| RCT | randomized controlled trial |
| Runx2 | runt-related transcription factor 2 |
| RXR | retinoid X receptor |
| SAMP6 | Senescence-accelerated mouse prone 6 |
| SERMs | Selective Estrogen Receptor Modulators |
| SFRP | secreted frizzled-related protein |
| SFRP4 | secreted frizzled-related protein 4 |
| SGMS2 | sphingomyelin synthase 2 gene |
| SMAD4 | Mothers against decapentaplegic homolog 4 |
| SNP | single-nucleotide polymorphism |
| TF | transcription factor |
| TRACP-5b | tartrate-resistant acid phosphatase 5b |
| TSECs | Tissue Selective Estrogen Receptor Complexes |
| UGT2B17 | UDP Glucuronosyl transferase Family 2 Member B17 |
| VDR | vitamin D receptor |
| WHI | Women’s Health Initiative |
| WHO | World Health Organization |
| Wnt | Wingless/Integrated |
| ZA | Zoledronic acid |
References
- Gallagher, J.; Sai, A. Molecular biology of bone remodeling: Implications for new therapeutic targets for osteoporosis. Maturitas 2010, 65, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, M.; Sawamoto, K.; Alméciga-Díaz, C.; Mackenzie, W.; Mason, R.; Orii, T.; Tomatsu, S. Development of Bone Targeting Drugs. Int. J. Mol. Sci. 2017, 18, 1345. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.; Wardell, S.; McDonnell, D. The molecular mechanisms underlying the pharmacological actions of estrogens, SERMs and oxysterols: Implications for the treatment and prevention of osteoporosis. Bone 2013, 53, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Yang, N.; Wu, S.; Lv, Y.; Xu, L. In silico analysis of the molecular mechanism of postmenopausal osteoporosis. Mol. Med. 2015, 12, 6584–6590. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, X.; Dong, S.; Guo, Y.; Yang, T.; Lei, S.; Papasian, C.; Zhao, M.; Deng, H. Molecular Genetic Studies of Gene Identification for Osteoporosis: The 2009 Update. Endocr. Rev. 2010, 31, 447–505. [Google Scholar] [CrossRef]
- Yuan, R.; Ma, S.; Zhu, X.; Li, J.; Liang, Y.; Liu, T.; Zhu, Y.; Zhang, B.; Tan, S.; Guo, H.; et al. Core level regulatory network of osteoblast as molecular mechanism for osteoporosis and treatment. Oncotarget 2016, 7, 3692–3701. [Google Scholar] [CrossRef]
- Azuma, K.; Zhou, Q.; Kubo, K. Morphological and molecular characterization of the senile osteoporosis in senescence-accelerated mouse prone 6 (SAMP6). Med. Mol. Morphol. 2018, 51, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Recker, R.; Deng, H. Molecular studies of identification of genes for osteoporosis: The 2002 update. J. Endocrinol. 2003, 177, 147–196. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Shen, H.; Xiao, P.; Xiong, D.; Li, L.; Recker, R.; Deng, H. Molecular Genetic Studies of Gene Identification for Osteoporosis: A 2004 Update. J Bone Miner Res. 2006, 21, 1511–1535. [Google Scholar] [CrossRef]
- Tokuzawa, Y.; Yagi, K.; Yamashita, Y.; Nakachi, Y.; Nikaido, I.; Bono, H.; Ninomiya, Y.; Kanesaki-Yatsuka, Y.; Akita, M.; Motegi, H.; et al. Id4, a New Candidate Gene for Senile Osteoporosis, Acts as a Molecular Switch Promoting Osteoblast Differentiation. PLoS Genet. 2010, 6. [Google Scholar] [CrossRef]
- Caccamo, D.; Ricca, S.; Currò, M.; Ientile, R. Health Risks of Hypovitaminosis D: A Review of New Molecular Insights. Int. J. Mol. Sci. 2018, 19, 892. [Google Scholar] [CrossRef]
- Jacome-Galarza, C.; Percin, G.; Muller, J.; Mass, E.; Lazarov, T.; Eitler, J.; Rauner, M.; Yadav, V.; Crozet, L.; Bohm, M.; et al. Developmental origin, functional maintenance and genetic rescue of osteoclasts. Nature 2019. [CrossRef] [PubMed]
- Ng, P.; Ribet, A.; Pavlos, N. Membrane trafficking in osteoclasts and implications for osteoporosis. Biochem. Soc. Trans. 2019. [Google Scholar] [CrossRef] [PubMed]
- Colditz, J.; Thiele, S.; Baschant, U.; Garbe, AI.; Niehrs, C.; Hofbauer, L.; Rauner, M. Osteogenic Dkk1 Mediates Glucocorticoid-Induced but Not Arthritis-Induced Bone Loss. J. Bone Miner. Res. 2019, 3702, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Amjadi-Moheb, F.; Akhavan-Niaki, H. Wnt signaling pathway in osteoporosis: Epigenetic regulation, interaction with other signaling pathways, and therapeutic promises. J. Cell Physiol. 2019, 28207, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Menale, C.; Villa, A. The RANKL-RANK Axis: A Bone to Thymus Round Trip. Front. Immunol. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Beck-Cormier, S.; Lelliott, C.; Logan, J.; Lafont, D.; Merametdjian, L.; Leitch, V.; Butterfield, N.; Protheroe, H.; Croucher, P.; Baldock, P.; et al. Slc20a2, Encoding the Phosphate Transporter PiT2, Is an Important Genetic Determinant of Bone Quality and Strength. J. Bone Miner. Res. 2019, 3691, 1–14. [Google Scholar] [CrossRef]
- Morris, J.; Kemp, J.; Youlten, S.; Laurent, L.; Logan, J.; Chai, R.; Vulpescu, N.; Forgetta, V.; Kleinman, A.; Mohanty, S.; et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019, 2, 258–266. [Google Scholar] [CrossRef]
- Raje, M.; Ashma, R. Epigenetic regulation of BMP2 gene in osteoporosis: A DNA methylation study. Mol. Biol. Rep. 2019, 46, 1667–1674. [Google Scholar] [CrossRef]
- Pekkinen, M.; Terhal, P.; Botto, L.; Henning, P.; Mäkitie, R.; Roschger, P.; Jain, A.; Kol, M.; Kjellberg, M.; Paschalis, E.; et al. Osteoporosis and skeletal dysplasia caused by pathogenic variants in SGMS2. JCI Insight. 2019, 4, 126180. [Google Scholar] [CrossRef]
- Trajanoska, K.; Rivadeneira, F. The genetic architecture of osteoporosis and fracture risk. Bone 2019. accepted; pii: S8756-3282(19)30133-4. [Google Scholar] [CrossRef]
- Roca-Ayats, N.; Martínez-Gil, N.; Cozar, M.; Gerousi, M.; Garcia-Giralt, N.; Ovejero, D.; Mellibovsky, L.; Nogués, X.; Díez-Pérez, A.; Grinberg, D.; et al. Functional characterization of the C7ORF76 genomic region, a prominent GWAS signal for osteoporosis in 7q21.3. Bone 2019, 123, 39–47. [Google Scholar] [CrossRef]
- Leboime, A.; Confavreux, C.B.; Mehsen, N.; Paccou, J.; David, C.; Roux, C. Osteoporosis and mortality. Joint Bone Spine 2010, 77 (Suppl. 2), S107–S112. [Google Scholar] [CrossRef]
- Center, J.R.; Bliuc, D.; Nguyen, N.D.; Eisman, J.A. Osteoporosis medication and reduced mortality risk in elderly women and men. J. Clin. Endocrinol. Metab. 2011, 96, 1006–1014. [Google Scholar] [CrossRef]
- Kanis, J.A.; Melton, L.J.; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141. [Google Scholar] [CrossRef]
- Root, A.; Diamond, F.; Sperling, M. Disorders of mineral homeostasis in the newborn, infant, child and adolescent. In Pediatric Endocrinology, 3rd ed.; Saunders: Philadelphia, PA, USA, 2008; pp. 686–768. [Google Scholar]
- Bagger, Y.Z.; Tanko, L.B.; Alexandersen, P.; Hansen, H.B.; Møllgaard, A.; Ravn, P.; Qvist, P.; Kanis, J.A.; Christiansen, C. Two to three years of hormone replacement treatment in healthy women have long-term preventive effects on bone mass and osteoporotic fractures: The PERF study. Bone 2004, 34, 728–735. [Google Scholar] [CrossRef]
- National Osteoporosis Foundation. America’s Bone Health: The State of Osteoporosis and Low Bone Mass in Our Nation; National Osteoporosis Foundation: Washington, DC, USA, 2002. [Google Scholar]
- National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis; National Osteoporosis Foundation: Washington, DC, USA, 2010. [Google Scholar]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar]
- Body, J.J.; Bergmann, P.; Boonen, S.; Boutsen, Y.; Devogelaer, J.P.; Goemaere, S.; Kaufman, J.M.; Rozenberg, S.; Reginster, J.Y. Evidence-based guidelines for the pharmacological treatment of postmenopausal osteoporosis: A consensus document by the belgian bone club. Osteoporos. Int. 2010, 21, 1657–1680. [Google Scholar] [CrossRef]
- Cummings, S.R.; Ettinger, B.; Delmas, P.D.; Kenemans, P.; Stathopoulos, V.; Verweij, P.; Mol-Arts, M.; Kloosterboer, L.; Mosca, L.; Christiansen, C.; et al. The effects of tibolone in older postmenopausal women. N. Engl. J. Med. 2008, 359, 697–708. [Google Scholar] [CrossRef]
- Alexandersen, P.; Toussaint, A.; Christiansen, C.; Devogelaer, J.P.; Roux, C.; Fechtenbaum, J.; Gennari, C.; Reginster, J.Y. Ipriflavone in the treatment of postmenopausal osteoporosis: A randomized controlled trial. JAMA 2001, 285, 1482–1488. [Google Scholar] [CrossRef]
- Alekel, D.L.; Germain, A.S.; Peterson, C.T.; Hanson, K.B.; Stewart, J.W.; Toda, T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am. J. Clin. Nutr. 2000, 72, 844–852. [Google Scholar] [CrossRef]
- Nikander, E.; Metsä-Heikkilä, M.; Ylikorkala, O.; Tiitinen, A. Effects of phytoestrogens on bone turnover in postmenopausal women with a history of breast cancer. J. Clin. Endocrinol. Metab. 2004, 89, 1207–1212. [Google Scholar] [CrossRef][Green Version]
- Noble, J.; Greene, H.L. Textbook of Primary Care Medicine, 3rd ed.; Mosby: MO, USA, 2001; pp. 387–397. [Google Scholar]
- Watts, N.B.; Diab, D.L. Long-term use of bisphosphonates in osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95, 1555–1565. [Google Scholar] [CrossRef]
- Karaguzel, G.; Holick, M.F. Diagnosis and treatment of osteopenia. Rev. Endocr. Metab. Disord. 2010, 11, 237–251. [Google Scholar] [CrossRef]
- Schousboe, J.T.; Nyman, J.A.; Kane, R.L.; Ensrud, K.E. Cost-effectiveness of alendronate therapy for osteopenic postmenopausal women. Ann. Intern. Med. 2005, 142, 734–741. [Google Scholar] [CrossRef]
- Ivergård, M.; Ström, O.; Borgström, F.; Burge, R.T.; Tosteson, A.N.; Kanis, J. Identifying cost-effective treatment with raloxifene in postmenopausal women using risk algorithms for fractures and invasive breast cancer. Bone 2010, 47, 966–974. [Google Scholar] [CrossRef]
- Stevenson, M.; Jones, M.L.; De Nigris, E.; Brewer, N.; Davis, S.; Oakley, J. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol. Assess. 2005, 9, 1–160. [Google Scholar] [CrossRef]
- Boskey, A.; Spevak, L.; Ma, Y.; Wang, H.; Bauer, D.; Black, D.; Schwartz, A. Insights into the bisphosphonate holiday: A preliminary FTIRI study. Osteoporos. Int. 2018, 3, 699–705. [Google Scholar] [CrossRef]
- Wright, N.; Foster, P.; Mudano, A.; Melnick, J.; Lewiecki, E.; Shergy, W.; Curtis, J.; Cutter, G.; Danila, M.; Kilgore, M.; et al. Assessing the Feasibility of The Effectiveness of Discontinuing bisphosphonates Trial: A Pilot Study. Osteoporos. Int. 2017, 28, 2495–2503. [Google Scholar] [CrossRef]
- Saag, K.; Petersen, J.; Brandi, M.; Karaplis, A.; Lorentzon, M.; Thomas, T.; Maddox, J.; Fan, M.; Meisner, P.; Grauer, A. Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N. Engl. J. Med. 2017, 377, 1417–1427. [Google Scholar] [CrossRef]
- Maricic, M. The role of zoledronic acid in the management of osteoporosis. Clin. Rheumatol. 2010, 29, 1079–1084. [Google Scholar] [CrossRef]
- Mathavan, N.; Turunen, M.; Guizar-Sicairos, M.; Bech, M.; Schaf, M.; Tägil, M.; Isaksson, H. The compositional and nano-structural basis of fracture healing in healthy and osteoporotic bone. Nature 2018, 8, 1591. [Google Scholar] [CrossRef]
- Reid, I.; Horne, A.; Mihov, B.; Stewart, A.; Garratt, E.; Wong, S.; Wiessing, K.; Bolland, M.; Bastin, S.; Gamble, G. Fracture Prevention with Zoledronate in Older Women with Osteopenia. N. Engl. J. Med. 2018, 379, 2407–2416. [Google Scholar] [CrossRef]
- Shiraki, M.; Tanaka, S.; Suzuki, H.; Ueda, S.; Nakamura, T. Safety, pharmacokinetics, and changes in bone metabolism associated with zoledronic acid treatment in Japanese patients with primary osteoporosis. J. Bone Miner. Metab. 2017, 35, 675–684. [Google Scholar] [CrossRef]
- Fujimoto, K.; Inage, K.; Orita, S.; Yamashita, M.; Abe, K.; Yamagata, M.; Sainoh, T.; Akazawa, T.; Kinoshita, T.; Nemoto, T.; et al. The nature of osteoporotic low back pain without acute vertebral fracture: A prospective multicenter study on the analgesic effect of monthly minodronic acid hydrate. J. Orthop. Sci. 2017, 4, 613–617. [Google Scholar] [CrossRef]
- Mori, Y.; Kasai, H.; Ose, A.; Serada, M.; Ishiguro, M.; Shiraki, M.; Tanigawara, Y. Modeling and simulation of bone mineral density in Japanese osteoporosis patients treated with zoledronic acid using tartrate-resistant acid phosphatase 5b, a bone resorption marker. Osteoporos. Int. 2018, 5, 1155–1163. [Google Scholar] [CrossRef]
- Recker, R.R.; Lewiecki, E.M.; Miller, P.D.; Reiffel, J. Safety of bisphosphonates in the treatment of osteoporosis. Am. J. Med. 2009, 122, S22–S32. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the american society for bone and mineral research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef]
- Khan, A.A.; Sándor, G.K.; Dore, E.; Morrison, A.D.; Alsahli, M.; Amin, F.; Peters, E.; Hanley, D.A.; Chaudry, S.R.; Lentle, B.; et al. Bisphosphonate associated osteonecrosis of the jaw. J. Rheumatol. 2009, 36, 478–490. [Google Scholar] [CrossRef]
- Allen, M.R.; Burr, D.B. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: So many hypotheses, so few data. J. Oral Maxillofac. Surg. 2009, 67, 61–70. [Google Scholar] [CrossRef]
- Lenart, B.A.; Lorich, D.G.; Lane, J.M. Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N. Engl. J. Med. 2008, 358, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.P. Bisphosphonates and low-impact femoral fractures: Current evidence on alendronate-fracture risk. Geriatrics 2009, 64, 18–23. [Google Scholar] [PubMed]
- Abrahamsen, B.; Eiken, P.; Eastell, R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: A register-based national cohort study. J. Bone Miner. Res. 2009, 24, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Shane, E.; Burr, D.; Ebeling, P.R.; Abrahamsen, B.; Adler, R.A.; Brown, T.D.; Cheung, A.M.; Cosman, F.; Curtis, J.R.; Dell, R.; et al. Atypical subtrochanteric and diaphyseal femoral fractures: Report of a task force of the american society for bone and mineral research. J. Bone Miner. Res. 2010, 25, 2267–2294. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, D.P. Mining the complexities of the estrogen signaling pathways for novel therapeutics. Endocrinology 2003, 144, 4237–4240. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S. Update on estrogens and the skeleton. J. Clin. Endocrinol. Metab. 2010, 95, 3569–3577. [Google Scholar] [CrossRef]
- Ettinger, B.; Black, D.M.; Mitlak, B.H.; Knickerbocker, R.K.; Nickelsen, T.; Genant, H.K.; Christiansen, C.; Delmas, P.D.; Zanchetta, J.R.; Stakkestad, J.; et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: Results from a 3-year randomized clinical trial. Multiple outcomes of raloxifene evaluation (more) investigators. JAMA 1999, 282, 637–645. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; Black, D.M.; Downs, R.W., Jr.; Sarkar, S.; Fuerst, T.; Secrest, R.J.; Pavo, I. Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: A reanalysis of the multiple outcomes of raloxifene evaluation trial. Bone 2003, 33, 293–300. [Google Scholar] [CrossRef]
- Pleiner-Duxneuner, J.; Zwettler, E.; Paschalis, E.; Roschger, P.; Nell-Duxneuner, V.; Klaushofer, K. Treatment of osteoporosis with parathyroid hormone and teriparatide. Calcif. Tissue Int. 2009, 84, 159–170. [Google Scholar] [CrossRef]
- Canalis, E.; Giustina, A.; Bilezikian, J.P. Mechanisms of anabolic therapies for osteoporosis. N. Engl. J. Med. 2007, 357, 905–916. [Google Scholar] [CrossRef]
- Leder, B.; Tsai, J.; Jiang, L.; Lee, H. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: The Denosumab and Teriparatide Follow-up study (DATA-Follow-up). Bone 2017, 98, 54–58. [Google Scholar] [CrossRef]
- Nakatoh, S. Effect of osteoporosis medication on changes in bone mineral density and bone turnover markers after 24-month administration of daily teriparatide: Comparison among minodronate, raloxifene, and eldecalcitol. J. Bone Miner. Metab. 2018, 36, 221–228. [Google Scholar] [CrossRef]
- Cumming, R.G.; Nevitt, M.C. Calcium for prevention of osteoporotic fractures in postmenopausal women. J. Bone Miner. Res. 1997, 12, 1321–1329. [Google Scholar] [CrossRef]
- Chel, V.G.; Ooms, M.E.; Popp-Snijders, C.; Pavel, S.; Schothorst, A.A.; Meulemans, C.C.; Lips, P. Ultraviolet irradiation corrects vitamin d deficiency and suppresses secondary hyperparathyroidism in the elderly. J. Bone Miner. Res. 1998, 13, 1238–1242. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin d deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Chen, L.R.; Wen, Y.T.; Kuo, C.L.; Chen, K.H. Calcium and vitamin D supplementation on bone health: Current evidence and recommendations. Int. J. Gerontol. 2014, 8, 183–188. [Google Scholar] [CrossRef]
- Lips, P.; Cashman, K.; Lamberg-Allardt, C.; Bischoff-Ferrari, H.; Obermayer-Pietsch, B.; Bianchi, M.; Stepan, J.; El-Hajj Fuleihan, G.; Bouillon, R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society. Eur. J. Endocrinol. 2019, 180, 23–54. [Google Scholar] [CrossRef]
- Liao, E.; Zhang, Z.; Xia, W.; Lin, H.; Cheng, Q.; Wang, L.; Hao, Y.; Chen, D.; Tang, H.; Peng, Y.; et al. Calcifediol (25-hydroxyvitamin D) improvement and calcium-phosphate metabolism of alendronate sodium/vitamin D3 combination in Chinese women with postmenopausal osteoporosis: A post hoc efficacy analysis and safety reappraisal. BMC Musculoskelet. Disord. 2018, 19, 210. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. https://doi.org/10.3390/ijms20092213
Chen L-R, Ko N-Y, Chen K-H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. International Journal of Molecular Sciences. 2019; 20(9):2213. https://doi.org/10.3390/ijms20092213
Chicago/Turabian StyleChen, Li-Ru, Nai-Yu Ko, and Kuo-Hu Chen. 2019. "Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions" International Journal of Molecular Sciences 20, no. 9: 2213. https://doi.org/10.3390/ijms20092213
APA StyleChen, L.-R., Ko, N.-Y., & Chen, K.-H. (2019). Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. International Journal of Molecular Sciences, 20(9), 2213. https://doi.org/10.3390/ijms20092213