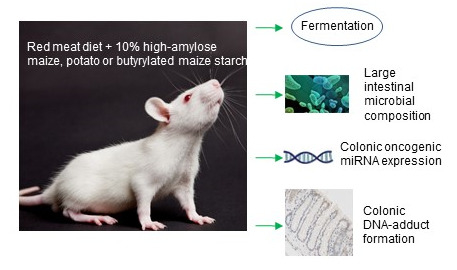
High-Amylose Maize, Potato, and Butyrylated Starch Modulate Large Intestinal Fermentation, Microbial Composition, and Oncogenic miRNA Expression in Rats Fed A High-Protein Meat Diet
Abstract
1. Introduction
2. Results
2.1. BW, Feed Intake, and Fermentation Products
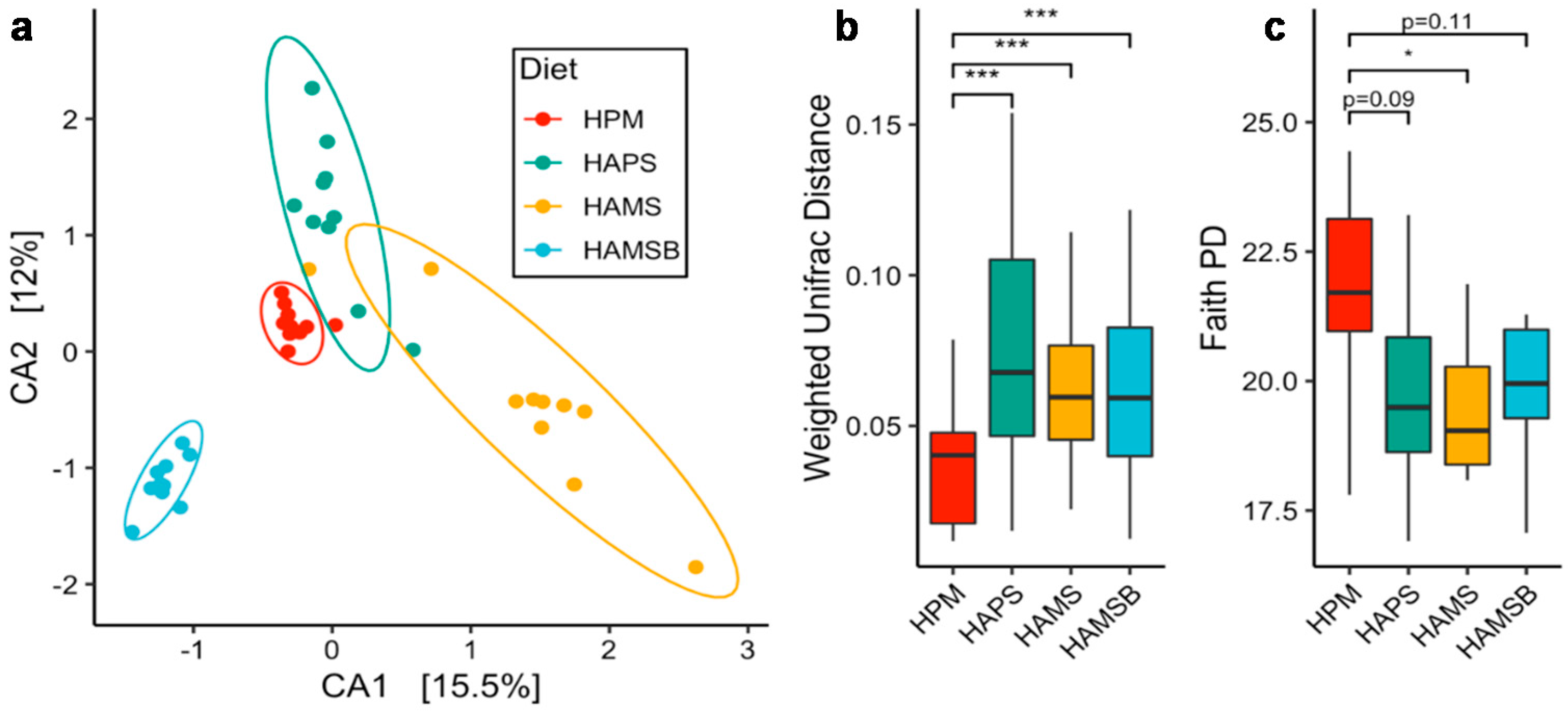
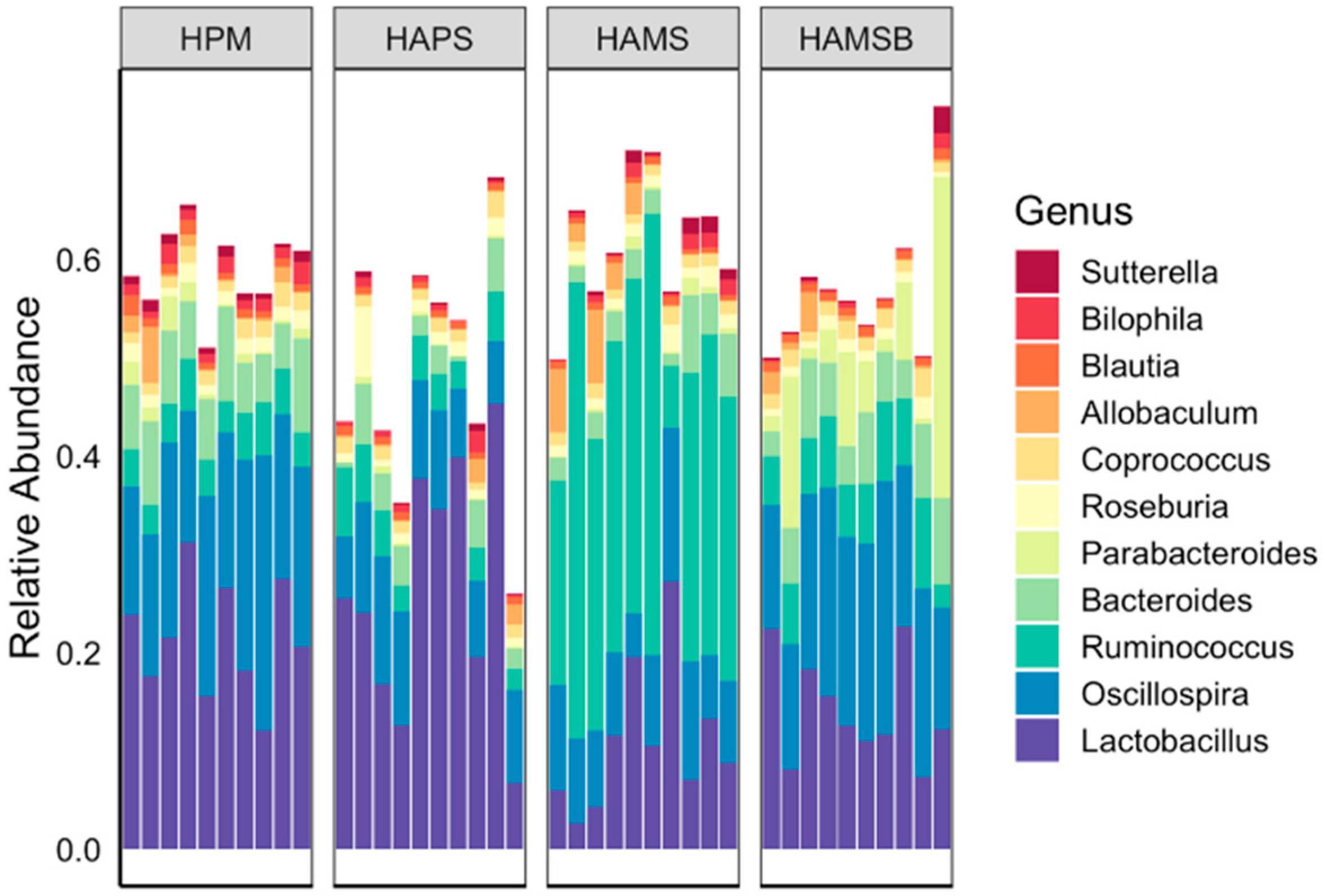
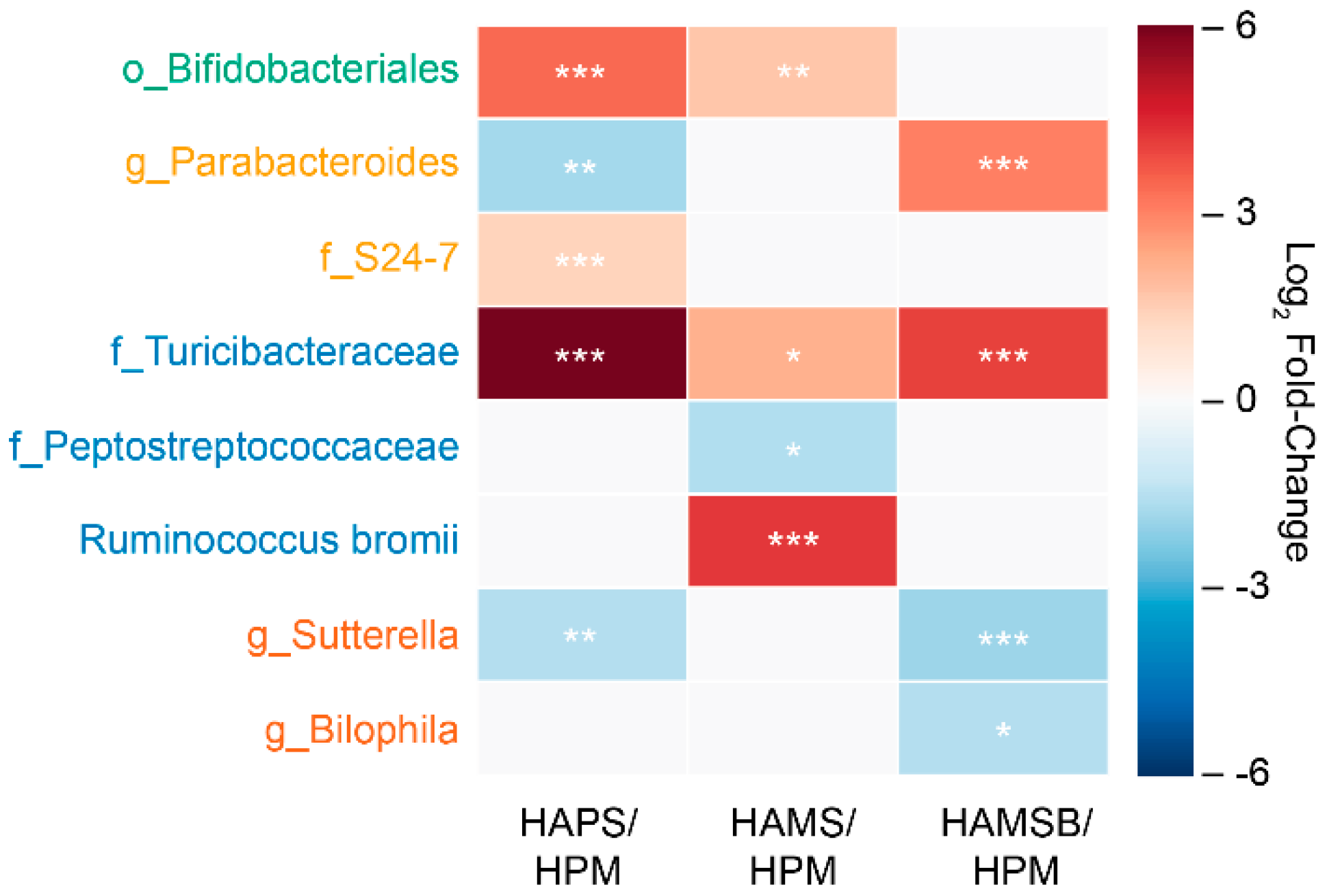
2.2. The Gut Microbiota is Influenced by the RS Type
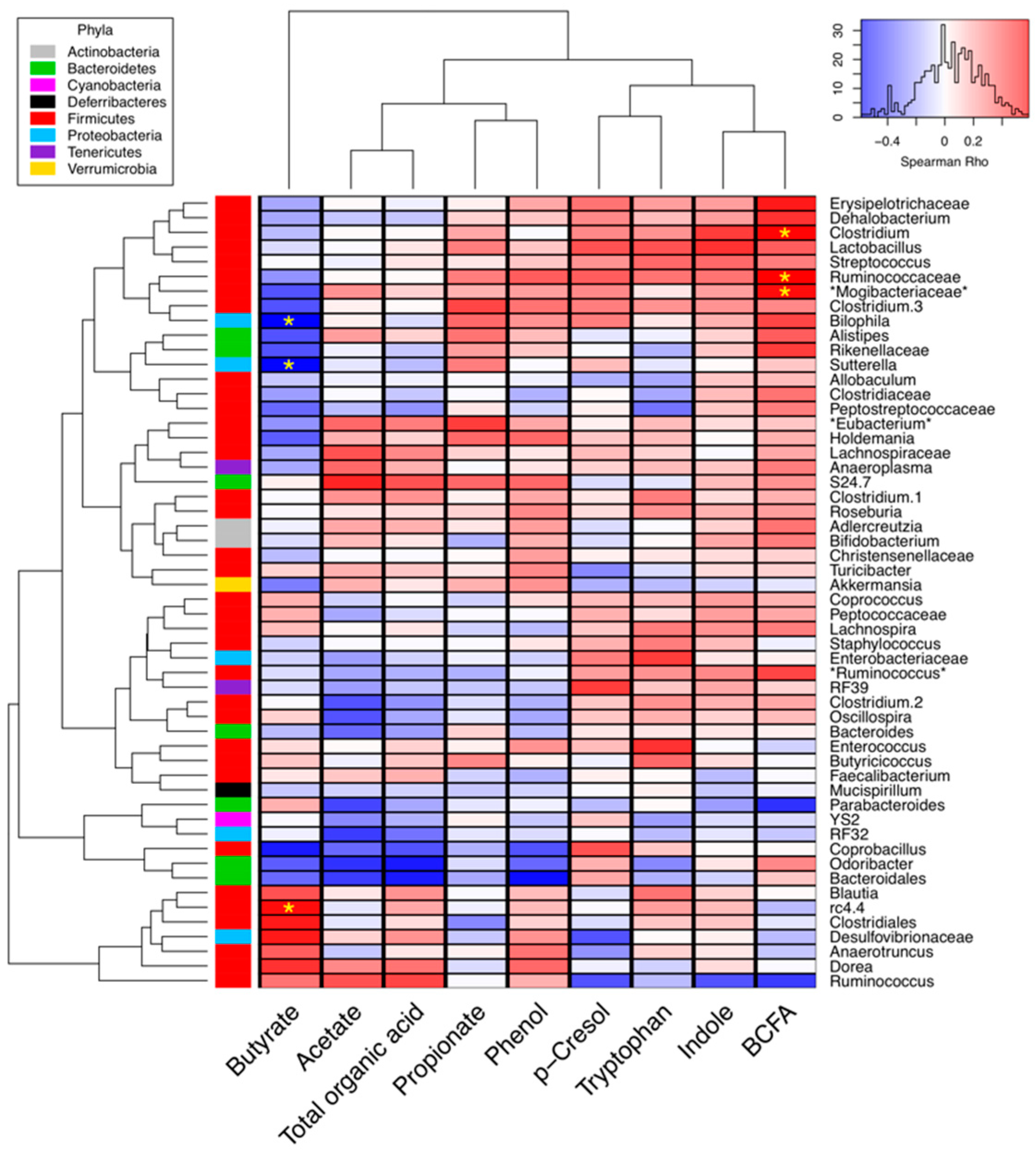
2.3. Correlations between Microbial Abundance and Cecal Metabolites
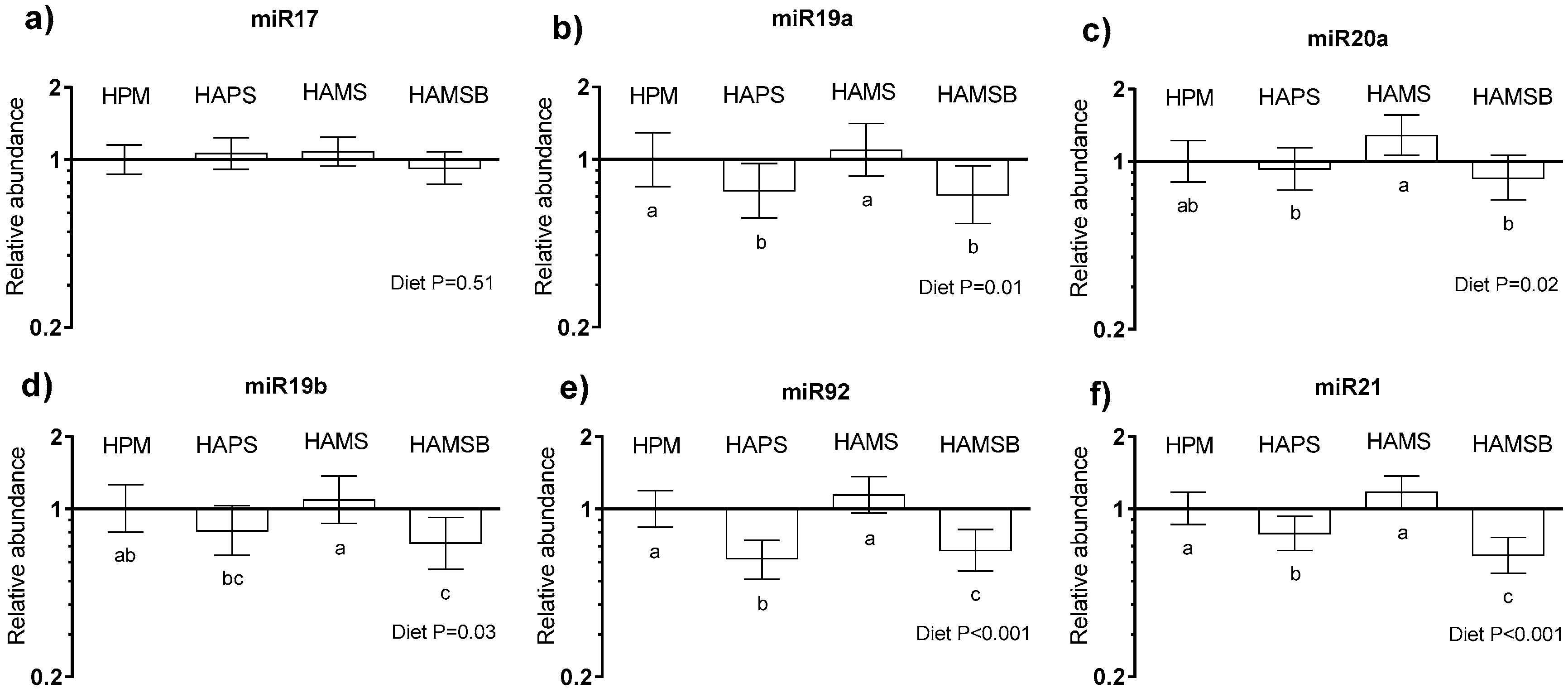
2.4. Colonic Oncogenic miRNA Expression
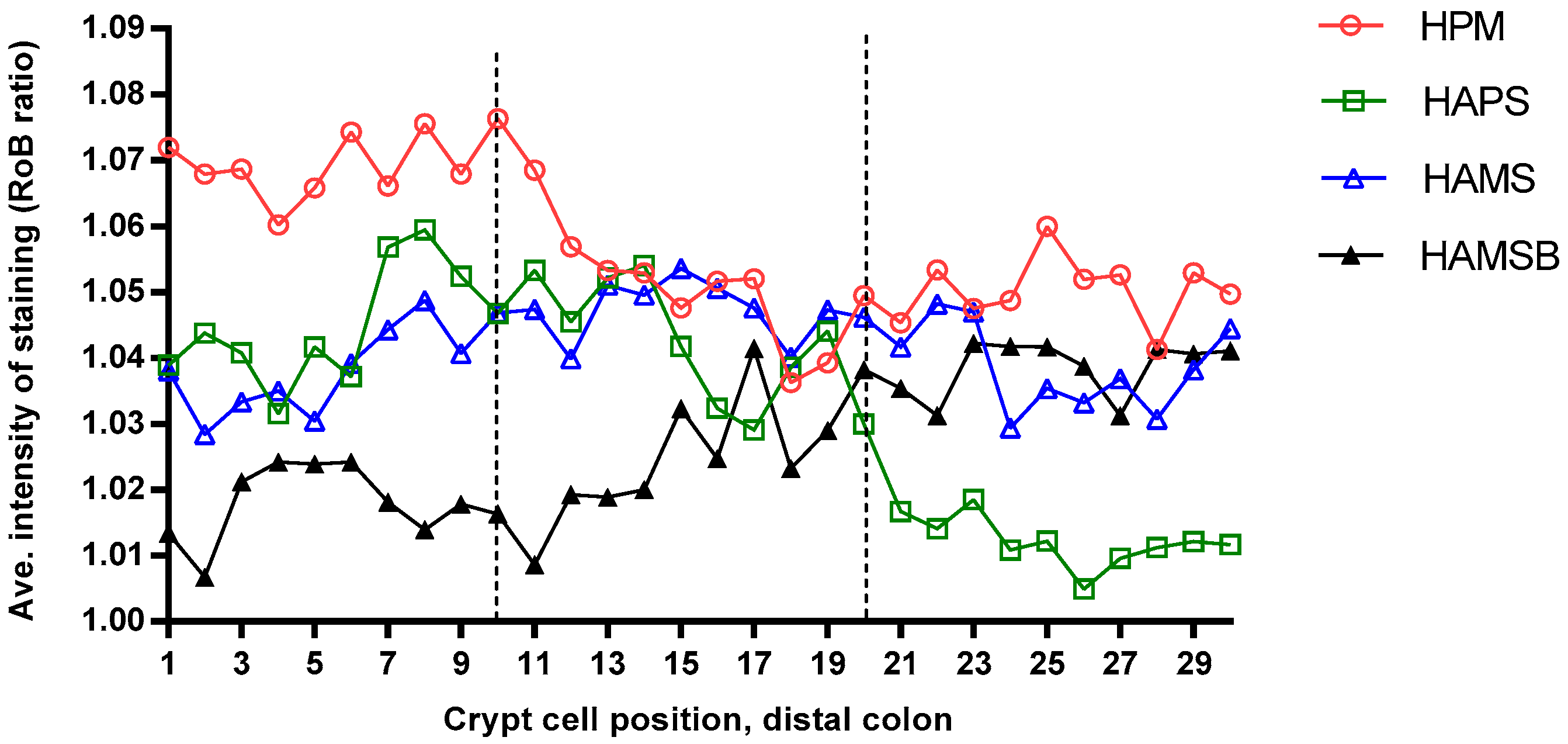
2.5. O6MeG Adduct Formation
3. Discussion
4. Materials and Methods
4.1. Diets
4.2. Animals
4.3. Sampling and Analysis
4.4. Microbial Composition in the Cecum Digesta
4.5. Oncogenic miRNA Expression Analysis
4.6. O6MeG DNA-Adduct Quantification
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Cancer Research Fund/American Institute for Cancer Research. Continous Update Project Report: Diet, Nutrition, Physical Activity and Colorectal Cancer. 2017. Available online: Wcrf.org/colorectal-cancer-2017 (accessed on 26 April 2019).
- Le Leu, R.K.; Brown, I.L.; Hu, Y.; Esterman, A.; Young, G.P. Suppression of azoxymethane-induced colon cancer development in rats by dietary resistant starch. Cancer Biol. Ther. 2007, 6, 1621–1626. [Google Scholar] [CrossRef]
- Bauer-Marinovic, M.; Florian, S.; Muller-Schmehl, K.; Glatt, H.; Jacobasch, G. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis 2006, 27, 1849–1859. [Google Scholar] [CrossRef][Green Version]
- Sakamoto, J.; Nakaji, S.; Sugawara, K.; Iwane, S.; Munakata, A. Comparison of resistant starch with cellulose diet on 1,2-dimethylhydrazine-induced colonic carcinogenesis in rats. Gastroenterol 1996, 110, 116–120. [Google Scholar] [CrossRef]
- Maziere, S.; Meflah, K.; Tavan, E.; Champ, M.; Narbonne, J.F.; Cassand, P. Effect of resistant starch and/or fat-soluble vitamins a and e on the initiation stage of aberrant crypts in rat colon. Nutr. Cancer 1998, 31, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Coelho, D.; Blachier, F. Review of the association between meat consumption and risk of colorectal cancer. Nutr. Res. 2013, 33, 983–994. [Google Scholar] [CrossRef] [PubMed]
- Lewin, M.H.; Bailey, N.; Bandaletova, T.; Bowman, R.; Cross, A.J.; Pollock, J.; Shuker, D.E.; Bingham, S.A. Red meat enhances the colonic formation of the DNA adduct o6-carboxymethyl guanine: Implications for colorectal cancer risk. Cancer Res. 2006, 66, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.; Nyskohus, L.; Young, G.P.; Hu, Y.; Conlon, M.A.; Bird, A.R.; Topping, D.L.; Le Leu, R.K. Inhibition by resistant starch of red meat-induced promutagenic adducts in mouse colon. Cancer Prev. Res. 2011, 4, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Le Leu, R.K.; Winter, J.M.; Christophersen, C.T.; Young, G.P.; Humphreys, K.J.; Hu, Y.; Gratz, S.W.; Miller, R.B.; Topping, D.L.; Bird, A.R.; et al. Butyrylated starch intake can prevent red meat-induced o6-methyl-2-deoxyguanosine adducts in human rectal tissue: A randomised clinical trial. Br. J. Nutr. 2015, 114, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, K.J.; Conlon, M.A.; Young, G.P.; Topping, D.L.; Hu, Y.; Winter, J.M.; Bird, A.R.; Cobiac, L.; Kennedy, N.A.; Michael, M.Z.; et al. Dietary manipulation of oncogenic microrna expression in human rectal mucosa: A randomized trial. Cancer Prev. Res. 2014, 7, 786–795. [Google Scholar] [CrossRef]
- Garzon, R.; Calin, G.A.; Croce, C.M. MicroRNAs in cancer. Ann. Rev. Med. 2009, 60, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Kunte, D.P.; DelaCruz, M.; Wali, R.K.; Menon, A.; Du, H.; Stypula, Y.; Patel, A.; Backman, V.; Roy, H.K. Dysregulation of microRNAs in colonic field carcinogenesis: Implications for screening. PLoS ONE 2012, 7, e45591. [Google Scholar] [CrossRef]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef] [PubMed]
- Fung, K.Y.; Cosgrove, L.; Lockett, T.; Head, R.; Topping, D.L. A review of the potential mechanisms for the lowering of colorectal oncogenesis by butyrate. Br. J. Nutr. 2012, 108, 820–831. [Google Scholar] [CrossRef]
- Humphreys, K.J.; Cobiac, L.; Le Leu, R.K.; Van der Hoek, M.B.; Michael, M.Z. Histone deacetylase inhibition in colorectal cancer cells reveals competing roles for members of the oncogenic mir-17-92 cluster. Mol. Carcinogenesis 2013, 52, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Bird, A.R.; Topping, D.L.; Conlon, M.A. Dose-dependent reduction of dietary protein-induced colonocyte DNA damage by resistant starch in rats correlates more highly with caecal butyrate than with other short chain fatty acids. Cancer Biol. Ther. 2007, 6, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Bird, A.R.; Topping, D.L.; Conlon, M.A. High red meat diets induce greater numbers of colonic DNA double-strand breaks than white meat in rats: Attenuation by high-amylose maize starch. Carcinogenesis 2007, 28, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, N.J.; Toden, S.; Bird, A.R.; Topping, D.L.; Fenech, M.; Conlon, M.A. Colonocyte telomere shortening is greater with dietary red meat than white meat and is attenuated by resistant starch. Clin. Nutr. 2012, 31, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Bajka, B.H.; Topping, D.L.; Cobiac, L.; Clarke, J.M. Butyrylated starch is less susceptible to enzymic hydrolysis and increases large-bowel butyrate more than high-amylose maize starch in the rat. Br. J. Nutr. 2006, 96, 276–282. [Google Scholar] [CrossRef]
- Clarke, J.M.; Topping, D.L.; Christophersen, C.T.; Bird, A.R.; Lange, K.; Saunders, I.; Cobiac, L. Butyrate esterified to starch is released in the human gastrointestinal tract. Am. J. Clin. Nutr. 2011, 94, 1276–1283. [Google Scholar] [CrossRef]
- Toden, S.; Lockett, T.J.; Topping, D.L.; Scherer, B.L.; Watson, E.J.; Southwood, J.G.; Clarke, J.M. Butyrylated starch affects colorectal cancer markers beneficially and dose-dependently in genotoxin-treated rats. Cancer Biol. Ther. 2014, 15, 1515–1523. [Google Scholar] [CrossRef]
- Abell, G.C.J.; Christophersen, C.T.; McOrist, A.L.; Clarke, J.M. Dietary resistant and butyrylated starches have different effects on the faecal bacterial flora of azoxymethane-treated rats. Br. J. Nutr. 2011, 105, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Sattari Najafabadi, Z.; Skau Nielsen, T.; Skou Hedemann, M. Dietary protein source and butyrylated high-amylose maize starch included in a high-protein diet determines the urinary metabolome of rats. Int. J. Food Sci. Nutr. 2018, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.S.; Canibe, N.; Larsen, F.H. Butyrylation of maize and potato starches and characterization of the products by nuclear magnetic resonance and in vitro fermentation. Foods 2018, 7, 79. [Google Scholar] [CrossRef]
- Tupa, M.; Maldonado, L.; Vazquez, A.; Foresti, M.L. Simple organocatalytic route for the synthesis of starch esters. Carbohydr. Polym. 2013, 98, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Tupa, M.V.; Avila Ramirez, J.A.; Vazquez, A.; Foresti, M.L. Organocatalytic acetylation of starch: Effect of reaction conditions on ds and characterisation of esterified granules. Food Chem. 2015, 170, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Diosdado, B.; van de Wiel, M.A.; Terhaar Sive Droste, J.S.; Mongera, S.; Postma, C.; Meijerink, W.J.; Carvalho, B.; Meijer, G.A. Mir-17-92 cluster is associated with 13q gain and c-myc expression during colorectal adenoma to adenocarcinoma progression. Br. J. Cancer 2009, 101, 707–714. [Google Scholar] [CrossRef]
- Conlon, M.A.; Kerr, C.A.; McSweeney, C.S.; Dunne, R.A.; Shaw, J.M.; Kang, S.; Bird, A.R.; Morell, M.K.; Lockett, T.J.; Molloy, P.L.; et al. Resistant starches protect against colonic DNA damage and alter microbiota and gene expression in rats fed a western diet. J. Nutr. 2012, 142, 832–840. [Google Scholar] [CrossRef]
- Bajka, B.H.; Clarke, J.M.; Cobiac, L.; Topping, D.L. Butyrylated starch protects colonocyte DNA against dietary protein-induced damage in rats. Carcinogenesis 2008, 29, 2169–2174. [Google Scholar] [CrossRef]
- Jackson-Thompson, J.; Ahmed, F.; German, R.R.; Lai, S.M.; Friedman, C. Descriptive epidemiology of colorectal cancer in the united states, 1998–2001. Cancer 2006, 107, 1103–1111. [Google Scholar] [CrossRef]
- Svihus, B.; Uhlen, A.K.; Harstad, O.M. Effect of starch granule structure, associated components and processing on nutritive value of cereal starch: A review. Anim. Feed Sci. Technol. 2005, 122, 303–320. [Google Scholar] [CrossRef]
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Lærke, H.N.; Agnholt, J.; Bach Knudsen, K.E.; Hermansen, K.; Marco, M.L.; Gregersen, S.; et al. Effects of arabinoxylan and resistant starch on intestinal microbiota and short-chain fatty acids in subjects with metabolic syndrome: A randomised crossover study. PLoS ONE 2016, 11, e0159223. [Google Scholar] [CrossRef]
- Barouei, J.; Bendiks, Z.; Martinic, A.; Mishchuk, D.; Heeney, D.; Hsieh, Y.H.; Kieffer, D.; Zaragoza, J.; Martin, R.; Slupsky, C.; et al. Microbiota, metabolome, and immune alterations in obese mice fed a high-fat diet containing type 2 resistant starch. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Warren, F.J.; Fukuma, N.M.; Mikkelsen, D.; Flanagan, B.M.; Williams, B.A.; Lisle, A.T.; Ó Cuív, P.; Morrison, M.; Gidley, M.J. Food starch structure impacts gut microbiome composition. mSphere 2018, 3, e00086-18. [Google Scholar] [CrossRef]
- Martinez, I.; Kim, J.; Duffy, P.R.; Schlegel, V.L.; Walter, J. Resistant starches types 2 and 4 have differential effects on the composition of the fecal microbiota in human subjects. PLoS ONE 2010, 5, e15046. [Google Scholar] [CrossRef]
- Cockburn, D.W.; Koropatkin, N.M. Polysaccharide degradation by the intestinal microbiota and its influence on human health and disease. J. Mol. Biol. 2016, 428, 3230–3252. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, L.; Fang, L.; Su, Y.; Zhu, W. Responses in colonic microbial community and gene expression of pigs to a long-term high resistant starch diet. Front. Microbiol. 2015, 6, 877. [Google Scholar] [CrossRef]
- Herrmann, E.; Young, W.; Reichert-Grimm, V.; Weis, S.; Riedel, C.U.; Rosendale, D.; Stoklosinski, H.; Hunt, M.; Egert, M. In vivo assessment of resistant starch degradation by the caecal microbiota of mice using rna-based stable isotope probing-a proof-of-principle study. Nutrients 2018, 10, E79. [Google Scholar] [CrossRef]
- Garrett, W.S.; Onderdonk, A.B. Bacteroides, prevotella, porphyromonas, and fusobacterium species (and other medically important anaerobic gram-negative bacilli). Douglas Bennett’s Princ. Pract. Infect. Dis. 2014, 2, 2773–2780. [Google Scholar]
- Brahma, S.; Martinez, I.; Walter, J.; Clarke, J.; Gonzalez, T.; Menon, R.; Rose, D.J. Impact of dietary pattern of the fecal donor on in vitro fermentation properties of whole grains and brans. J. Funct. Foods 2017, 29, 281–289. [Google Scholar] [CrossRef]
- Metzler-Zebeli, B.U.; Schmitz-Esser, S.; Mann, E.; Grull, D.; Molnar, T.; Zebeli, Q. Adaptation of the cecal bacterial microbiome of growing pigs in response to resistant starch type 4. Appl. Environ. Microbiol. 2015, 81, 8489–8499. [Google Scholar] [CrossRef]
- Hu, Y.; Le Leu, R.K.; Christophersen, C.T.; Somashekar, R.; Conlon, M.A.; Meng, X.Q.; Winter, J.M.; Woodman, R.J.; McKinnon, R.; Young, G.P. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 2016, 37, 366–375. [Google Scholar] [CrossRef]
- Slattery, M.L.; Wolff, E.; Hoffman, M.D.; Pellatt, D.F.; Milash, B.; Wolff, R.K. Micrornas and colon and rectal cancer: Differential expression by tumor location and subtype. Genes Chrom. Cancer 2011, 50, 196–206. [Google Scholar] [CrossRef]
- Slaby, O.; Svoboda, M.; Fabian, P.; Smerdova, T.; Knoflickova, D.; Bednarikova, M.; Nenutil, R.; Vyzula, R. Altered expression of mir-21, mir-31, mir-143 and mir-145 is related to clinicopathologic features of colorectal cancer. Oncology 2007, 72, 397–402. [Google Scholar] [CrossRef]
- Olive, V.; Bennett, M.J.; Walker, J.C.; Ma, C.; Jiang, I.; Cordon-Cardo, C.; Li, Q.J.; Lowe, S.W.; Hannon, G.J.; He, L. Mir-19 is a key oncogenic component of mir-17-92. Genes Dev. 2009, 23, 2839–2849. [Google Scholar] [CrossRef]
- Le Leu, R.K.; Scherer, B.L.; Mano, M.T.; Winter, J.M.; Lannagan, T.; Head, R.J.; Lockett, T.; Clarke, J.M. Dietary butyrylated high-amylose starch reduces azoxymethane-induced colonic o(6)-methylguanine adducts in rats as measured by immunohistochemistry and high-pressure liquid chromatography. Nutr. Res. 2016, 36, 982–988. [Google Scholar] [CrossRef]
- Povey, A.C.; Badawi, A.F.; Cooper, D.P.; Hall, C.N.; Harrison, K.L.; Jackson, P.E.; Lees, N.P.; O’Connor, P.J.; Margison, G.P. DNA alkylation and repair in the large bowel: Animal and human studies. J. Nutr. 2002, 132, 3518s–3521s. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Hansen, B. Determination of nitrogen as elementary n, and alternative to kjeldahl. Acta Agri. Scand. 1989, 39, 113–118. [Google Scholar] [CrossRef]
- Stoldt, W. Worslag zur verinheitlichung der fettbestimmung in lebensmitteln (suggestion to standardise the determination of fat in foodstuffs). F. Seif. Anst. 1952, 54, 206–207. [Google Scholar] [CrossRef]
- Bach-Knudsen, K.E. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim. Feed Sci. Technol. 1997, 67, 319–338. [Google Scholar] [CrossRef]
- Eggum, B.O. A study of certain factors influencing protein utilization in rats and pigs. In 406th Report from the National Institute of Animal Science; National Institute of Animal Science: Copenhagen, Denmark, 1973. [Google Scholar]
- Canibe, N.; Hojberg, O.; Badsberg, J.H.; Jensen, B.B. Effect of feeding fermented liquid feed and fermented grain on gastrointestinal ecology and growth performance in piglets. J. Anim. Sci. 2007, 85, 2959–2971. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Peterson, C.M.; Raggio, A.; Keenan, M.J.; Martin, R.J.; Ravussin, E.; Marco, M.L. Impact of different fecal processing methods on assessments of bacterial diversity in the human intestine. Front. Microbiol. 2016, 7, 1643. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Met. 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. Dada2: High-resolution sample inference from illumina amplicon data. Nat. Met. 2016, 13, 581–583. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Nyskohus, L.S.; Watson, A.J.; Margison, G.P.; Le Leu, R.K.; Kim, S.W.; Lockett, T.J.; Head, R.J.; Young, G.P.; Hu, Y. Repair and removal of azoxymethane-induced o6-methylguanine in rat colon by o6-methylguanine DNA methyltransferase and apoptosis. Mut. Res. 2013, 758, 80–86. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An r package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for rna-seq data with deseq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
| HPM | HAPS | HAMS | HAMSB | SEM | p | |
|---|---|---|---|---|---|---|
| Cecum, concentration | ||||||
| Acetate, µmol/g | 51.0 b | 60.4 a | 61.7 a | 47.7 b | 3.6 | <0.01 |
| Propionate, µmol/g | 11.2 | 11.5 | 10.2 | 9.8 | 0.6 | 0.22 |
| Butyrate, µmol/g | 12.1 d | 18.9 b | 15.1 c | 23.9 a | 1.5 | <0.001 |
| Total org. acids 1, µmol/g | 79.8 c | 97.5 a | 92.3 ab | 86.3 bc | 4.1 | 0.03 |
| BCFA 2, µmol/g | 2.2 a | 1.9 a | 1.5 b | 1.0 c | 0.1 | <0.001 |
| p-cresol, µg/g | 14.4 a | 10.0 ab | 7.7 b | 6.5 b | 1.7 | 0.02 |
| Phenol, µg/g | 8.1 a | 6.9 a | 1.6 b | 5.5 a | 1.4 | 0.01 |
| Indole, µg/g | 19.7 ab | 20.9 a | 13.5 c | 16.0 bc | 1.5 | <0.01 |
| Cecum, pool | ||||||
| Cecum digesta, g, wet weight | 1.8 b | 1.9 b | 2.4 a | 2.6 a | 0.1 | <0.001 |
| Acetate, µmol | 89 c | 113 b | 156 a | 125 b | 7 | <0.001 |
| Propionate, µmol | 20 | 21 | 24 | 26 | 2 | 0.12 |
| Butyrate, µmol | 21 c | 35 b | 36 b | 62 a | 3 | <0.001 |
| Total org. acids 1, µmol | 139 c | 181 b | 233 a | 226 a | 11 | <0.001 |
| BCFA 2, µmol | 3.8 a | 3.7 a | 3.4 ab | 2.5 b | 0.4 | 0.05 |
| p-cresol, µg | 26 | 18 | 17 | 17 | 3 | 0.19 |
| Phenol, µg | 14 a | 18 a | 4 b | 16 a | 3 | 0.03 |
| Indole, µg | 34 | 39 | 32 | 42 | 4 | 0.23 |
| Feces, concentration | ||||||
| Acetate, µmol/g | 31.7 b | 38.3 b | 61.3 a | 68.6 a | 4.8 | <0.001 |
| Propionate, µmol/g | 3.4 b | 3.1 b | 3.7 b | 7.7 a | 0.8 | <0.001 |
| Butyrate, µmol/g 4 | 0.78 b [0.51;1.19] | 0.73 b [0.48;1.09] | 1.1 b [0.78;1.67] | 16.0 a [0.48;1.09] | <0.001 | |
| Total org. acids 1, µmol/g | 43.5 b | 51.3 b | 138.4 a | 115.0 a | 8.5 | <0.001 |
| BCFA 1, µmol/g 4 | 0.94 ab [0.68;1.28] | 1.40 a [1.02;1.92] | 0.60 bc [0.39;0.91] | 0.54 c [0.39;0.76] | <0.01 | |
| p-cresol, µg/g | 25.7 a | 18.8 b | 5.2 c | 15.1 b | 1.6 | <0.001 |
| Phenol, µg/g 5 | 4.3 | 3.6 | 0.0 | 0.59 | 1.4 | 0.08 |
| Indole, µg/g | 19.2 a | 14.3 b | 1.5 d | 8.8 c | 0.8 | <0.001 |
| Feces pool | ||||||
| Feces 3, g wet weight/d | 2.3 c | 2.8 c | 3.4 b | 4.8 a | 0.1 | <0.001 |
| Acetate, µmol | 73 c | 103 c | 206 b | 329 a | 24 | <0.001 |
| Propionate, µmol 4 | 7 b [5.4;9.9] | 9 b [6.3;11.5] | 12 b [8.6;15.6] | 40 a [27.4;50.0] | <0.001 | |
| Butyrate, µmol 4 | 2 b [1.2;2.7] | 2 b [1.4;3.0] | 4 b [2.6;5.4] | 64 a [43.3;93.2] | <0.001 | |
| Total org. acids, µmol | 101 b | 137 b | 448 a | 547 a | 42 | <0.001 |
| BCFA 1, µmol 4 | 2.2 [1.52;3.05] | 3.8 [2.69;5.38] | 1.9 [1.21;3.09] | 2.5 [1.74;3.60] | 0.08 | |
| p-cresol, µg | 60 a | 50 a | 17 b | 64 a | 5 | <0.001 |
| Phenol, µg 5 | 16 | 10 | 0 | 3 | 5 | 0.16 |
| Indole, µg | 44 a | 39 a | 5 b | 41 a | 3 | <0.001 |
| HPM | HAPS | HAMS | HAMSB | |
|---|---|---|---|---|
| o_Bifidobacteriales | 0.001 ± 0.0001 1a | 0.017 ± 0.036 b | 0.0018 ± 0.003 bc | 0.001 ± 0.0001 a |
| g_Parabacteroides | 0.012 ± 0.011 a | 0.003 ± 0.003 c | 0.006 ± 0.005 ac | 0.078 ± 0.099 b |
| f_S24-7 | 0.033 ± 0.01 a | 0.052 ± 0.027 b | 0.040 ± 0.018 ab | 0.027 ± 0.012 a |
| f_Turicibacteraceae | 0.0001 ± 0.0001 a | 0.0070 ± 0.012 b | 0.0004 ± 0.0007 c | 0.0020 ± 0.003 bc |
| f_Peptostreptococcaceae | 0.037 ± 0.028 a | 0.014 ± 0.014 ab | 0.010 ± 0.008 b | 0.013 ± 0.009 ab |
| Ruminococcus bromii | 0.010 ± 0.005 a | 0.018 ± 0.014 a | 0.278 ± 0.123 b | 0.005 ± 0.001 a |
| f_Lactobacillaceae | 0.215 ± 0.0589 ab | 0.263 ± 0.127 a | 0.111 ± 0.075 b | 0.142 ± 0.054 b |
| g_Bilophila | 0.013 ± 0.005 a | 0.007 ± 0.006 ab | 0.008 ± 0.006 ab | 0.003 ± 0.005 b |
| g_Sutterella | 0.008 ± 0.003 a | 0.003 ± 0.003 b | 0.007 ± 0.007 ab | 0.004 ± 0.008 b |
| Diet | ||||
|---|---|---|---|---|
| HPM | HAPS | HAMS | HAMSB | |
| Ingredient (g/kg, as-fed basis) | ||||
| Maize starch | 283 | 183 | 183 | 183 |
| Sugar | 100 | 100 | 100 | 100 |
| Cellulose | 50 | 50 | 50 | 50 |
| AIN 93G Mineral Mix | 35 | 35 | 35 | 35 |
| AIN 93G Vitamin Mix | 10 | 10 | 10 | 10 |
| L-cysteine | 3.0 | 3.0 | 3.0 | 3.0 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 | 2.5 |
| t-Butylhydroquinone | 0.14 | 0.14 | 0.14 | 0.14 |
| Beef 1 | 517 | 517 | 517 | 517 |
| High-amylose potato starch (HAPS) 2 | 100 | |||
| High-amylose maize starch (HAMS) 3 | 100 | |||
| HAMS-butyrylated (HAMSB) 4 | 100 | |||
| Chemical composition (% of dry matter, DM) | ||||
| DM (%) | 96.5 | 96.6 | 96.9 | 96.6 |
| Protein (N × 6.25) | 32.5 | 31.2 | 32.6 | 30.8 |
| Starch | 30.9 | 23.9 | 25.3 | 26.8 |
| Fat | 20.8 | 19.0 | 19.1 | 18.6 |
| Ash | 3.9 | 4.1 | 4.0 | 4.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nielsen, T.S.; Bendiks, Z.; Thomsen, B.; Wright, M.E.; Theil, P.K.; Scherer, B.L.; Marco, M.L. High-Amylose Maize, Potato, and Butyrylated Starch Modulate Large Intestinal Fermentation, Microbial Composition, and Oncogenic miRNA Expression in Rats Fed A High-Protein Meat Diet. Int. J. Mol. Sci. 2019, 20, 2137. https://doi.org/10.3390/ijms20092137
Nielsen TS, Bendiks Z, Thomsen B, Wright ME, Theil PK, Scherer BL, Marco ML. High-Amylose Maize, Potato, and Butyrylated Starch Modulate Large Intestinal Fermentation, Microbial Composition, and Oncogenic miRNA Expression in Rats Fed A High-Protein Meat Diet. International Journal of Molecular Sciences. 2019; 20(9):2137. https://doi.org/10.3390/ijms20092137
Chicago/Turabian StyleNielsen, Tina S., Zach Bendiks, Bo Thomsen, Matthew E. Wright, Peter K. Theil, Benjamin L. Scherer, and Maria L. Marco. 2019. "High-Amylose Maize, Potato, and Butyrylated Starch Modulate Large Intestinal Fermentation, Microbial Composition, and Oncogenic miRNA Expression in Rats Fed A High-Protein Meat Diet" International Journal of Molecular Sciences 20, no. 9: 2137. https://doi.org/10.3390/ijms20092137
APA StyleNielsen, T. S., Bendiks, Z., Thomsen, B., Wright, M. E., Theil, P. K., Scherer, B. L., & Marco, M. L. (2019). High-Amylose Maize, Potato, and Butyrylated Starch Modulate Large Intestinal Fermentation, Microbial Composition, and Oncogenic miRNA Expression in Rats Fed A High-Protein Meat Diet. International Journal of Molecular Sciences, 20(9), 2137. https://doi.org/10.3390/ijms20092137