Single-Domain Antibodies Represent Novel Alternatives to Monoclonal Antibodies as Targeting Agents against the Human Papillomavirus 16 E6 Protein
Abstract
1. Introduction
2. Results
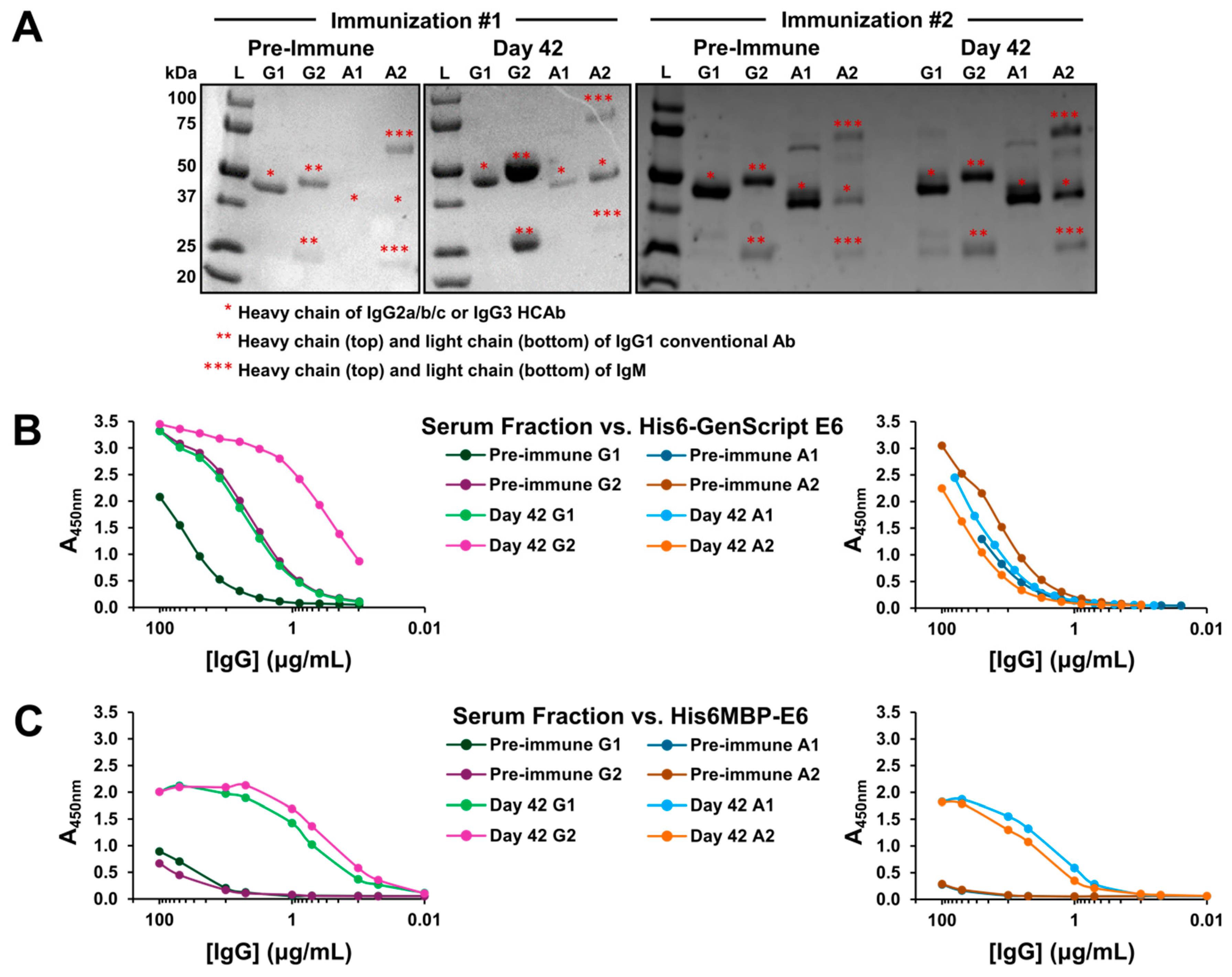
2.1. A Specific Heavy-Chain IgG Response Was Induced Following Each Llama Immunization
2.2. VHH Phage Display Libraries Corresponding to Each Llama Immunization Were Successfully Constructed and Enriched for Antigen-Specific Binders Using Subtractive Panning
2.3. The Eluted VHH-Displaying Phage Were Further Characterized Using Phage ELISA
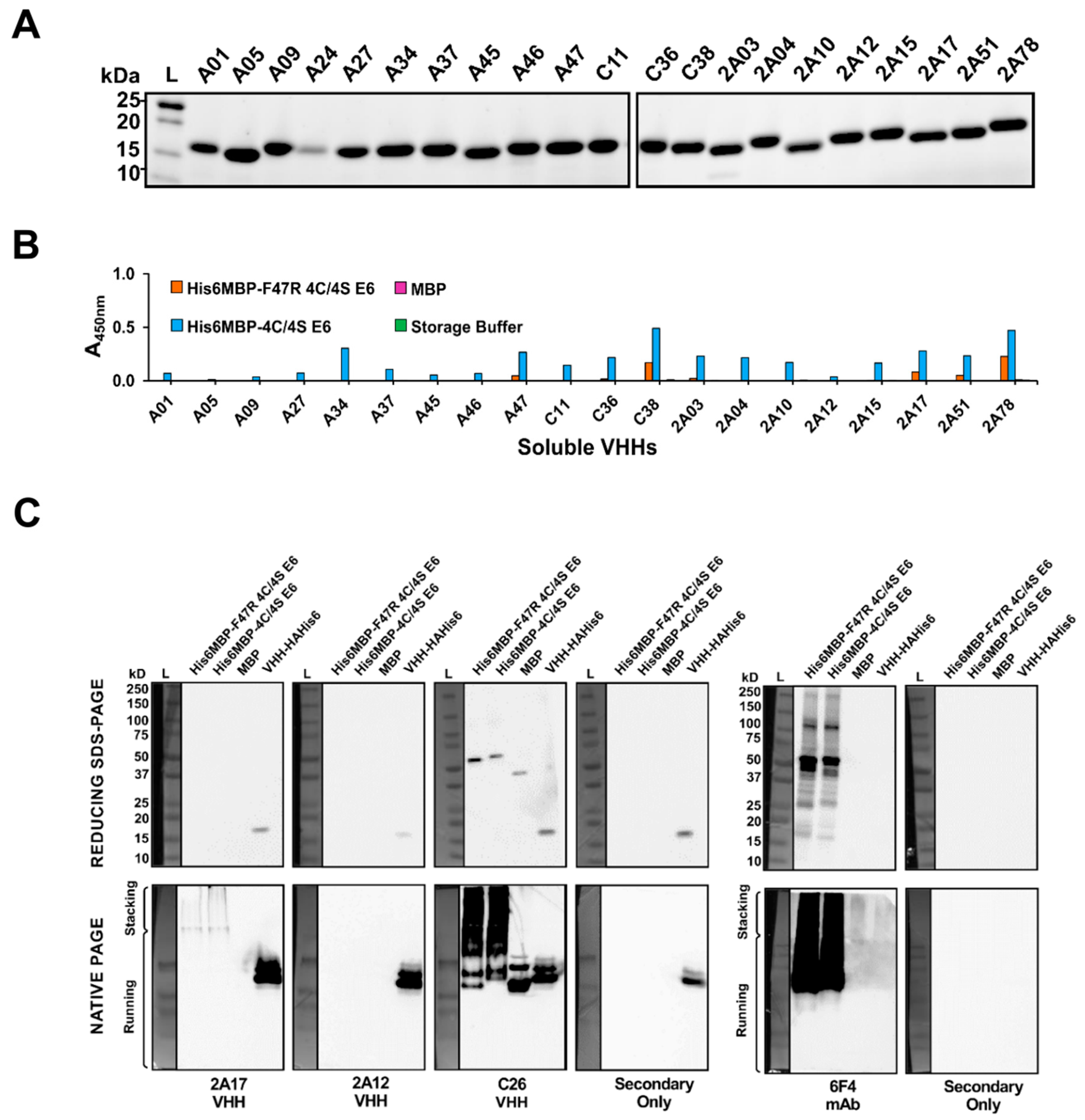
2.4. VHHs Were Expressed and Characterized Using Soluble ELISA
2.5. Characterization of VHHs Using Western Blotting under Denaturing and Native Conditions
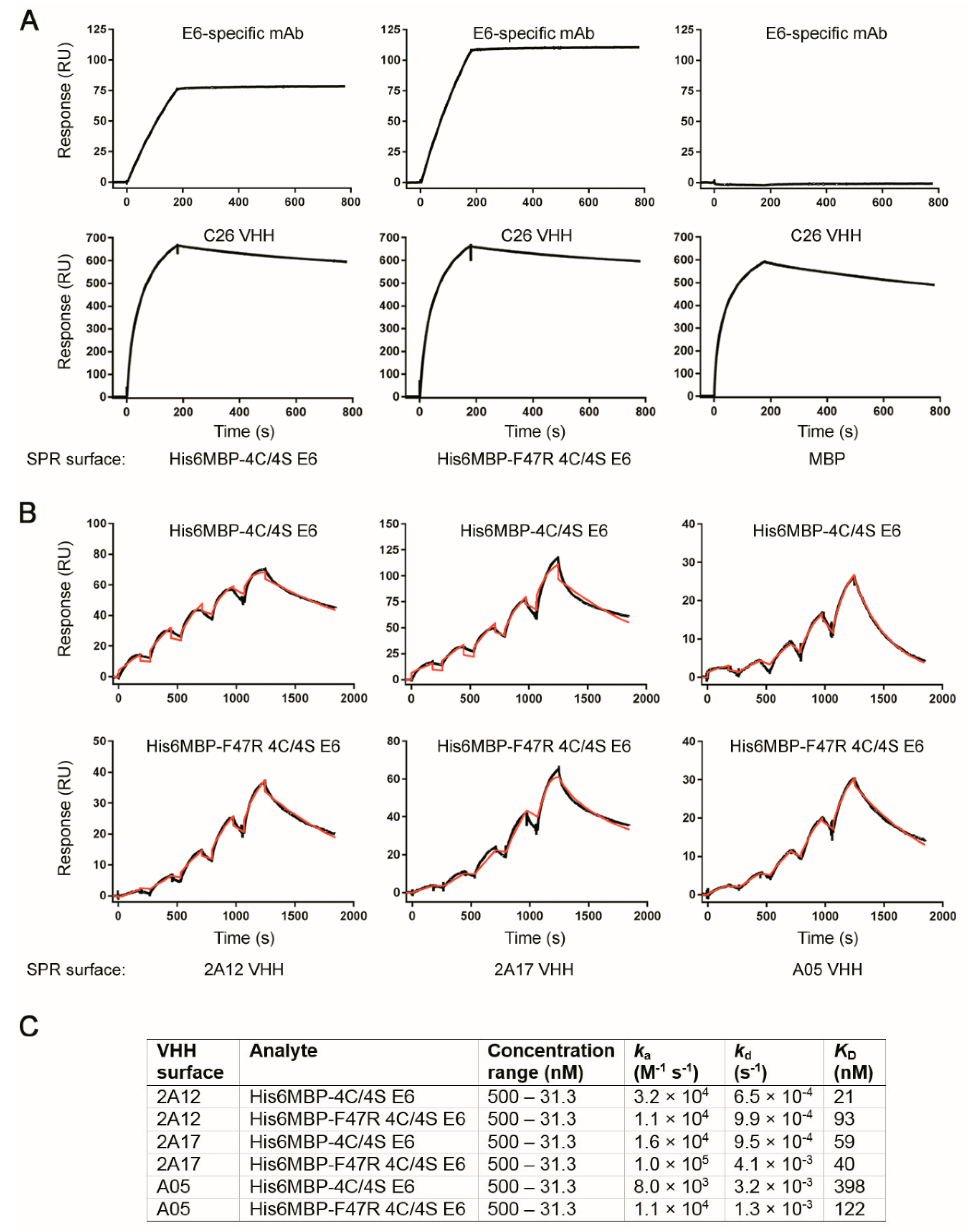
2.6. Surface Plasmon Resonance Analyses Demonstrated Several VHHs Bound Recombinant E6 with Nanomolar Affinity
3. Discussion
4. Materials and Methods
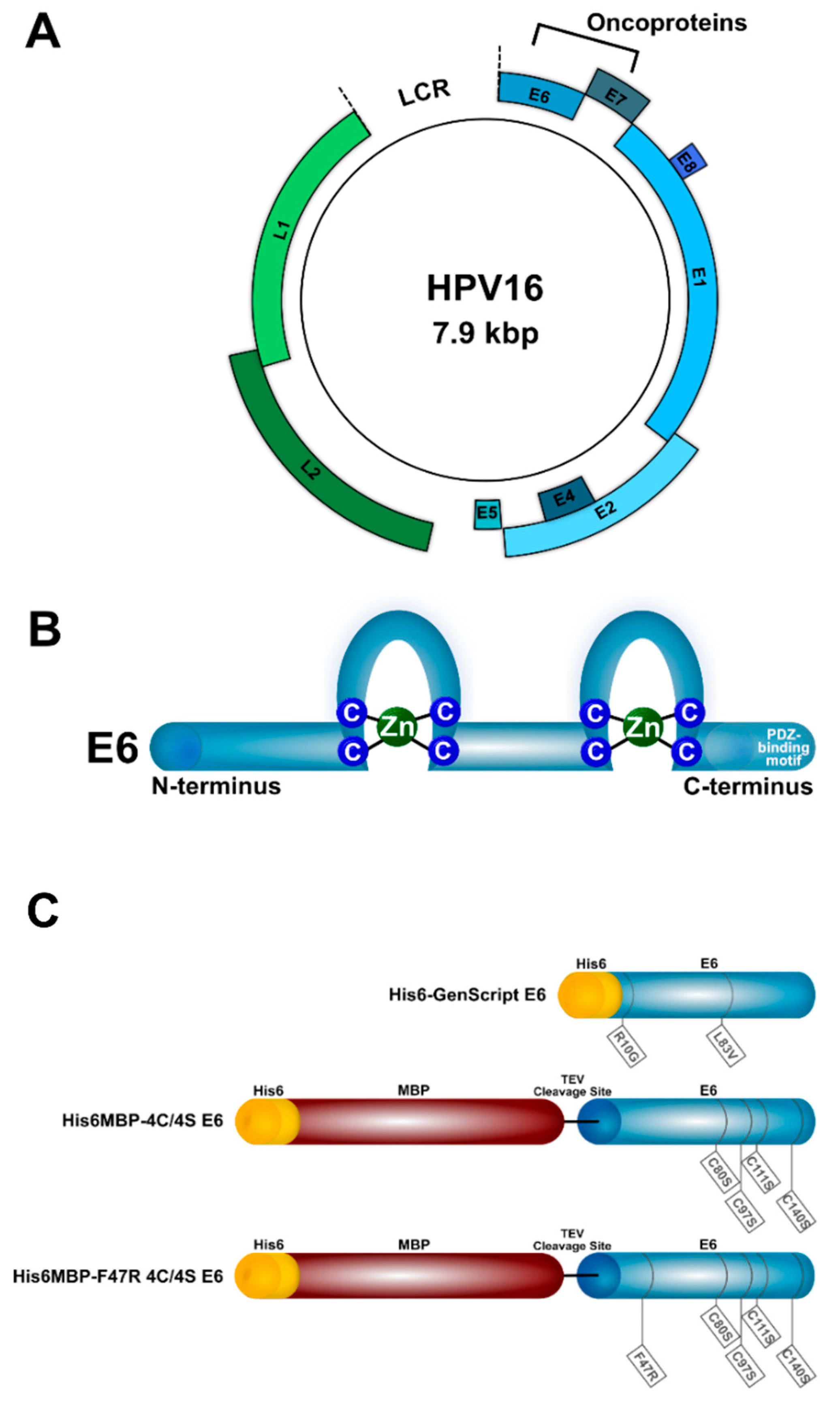
4.1. Recombinant Proteins
4.2. VHH Phage Display Library Construction, Selection, and Screening
4.3. VHH Expression and Purification
4.4. ELISA and Dot/Western Blot of Purified VHHs
4.5. Size Exclusion Chromatography and Surface Plasmon Resonance Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Zur Hausen, H. The search for infectious causes of human cancers: Where and why. Virology 2009, 392, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hoppe-Seyler, K.; Bossler, F.; Braun, J.A.; Herrmann, A.L.; Hoppe-Seyler, F. The HPV E6/E7 oncogenes: Key factors for viral carcinogenesis and therapeutic targets. Trends Microbiol. 2018, 26, 158–168. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- World Health Organization. Fact Sheet: Human Papillomavirus (HPV) and Cervical Cancer. Available online: http://www.who.int/mediacentre/factsheets/fs380/en/ (accessed on 22 February 2018).
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens—Part B: Biological agents. Lancet Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Crow, J.M. HPV: The global burden. Nature 2012, 488, S2–S3. [Google Scholar] [CrossRef]
- Mehanna, H.; Beech, T.; Nicholson, T.; El-Hariry, I.; McConkey, C.; Paleri, V.; Roberts, S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer—Systematic review and meta-analysis of trends by time and region. Head Neck 2013, 35, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, A.R.; Nyitray, A.G.; Kreimer, A.R.; Pierce Campbell, C.M.; Goodman, M.T.; Sudenga, S.L.; Monsonego, J.; Franceschi, S. E UROGIN 2014 roadmap: Differences in human papillomavirus infection natural history, transmission and human papillomavirus-related cancer incidence by gender and anatomic site of infection. Int. J. Cancer 2015, 136, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Society; National Cancer Institute of Canada Advisory Committee on Records and Registries. Canadian Cancer Statistics; Canadian Cancer Society: Toronto, ON, Canada, 2016. [Google Scholar]
- Centers for Disease Control and Prevention, Division of Cancer Prevention and Control. How Many Cancers Are Linked with HPV Each Year? Available online: https://www.cdc.gov/cancer/hpv/statistics/cases.htm#5 (accessed on 1 February 2018).
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–3. [Google Scholar] [CrossRef]
- Klingelhutz, A.J.; Roman, A. Cellular transformation by human papillomaviruses: Lessons learned by comparing high-and low-risk viruses. Virology 2012, 424, 77–98. [Google Scholar] [CrossRef] [PubMed]
- Vande Pol, S.B.; Klingelhutz, A.J. Papillomavirus E6 oncoproteins. Virology 2013, 445, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Zapien, D.; Ruiz, F.X.; Poirson, J.; Mitschler, A.; Ramirez, J.; Forster, A.; Cousido-Siah, A.; Masson, M.; Pol, S.V.; Podjarny, A. Structure of the E6/E6AP/p53 complex required for HPV-mediated degradation of p53. Nature 2016, 529, 541–545. [Google Scholar] [CrossRef]
- Dreer, M.; van de Poel, S.; Stubenrauch, F. Control of viral replication and transcription by the papillomavirus E8^E2 protein. Virus Res. 2017, 231, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Harden, M.E.; Munger, K. Human papillomavirus molecular biology. Mutat. Res. Rev. Mutat. Res. 2017, 772, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gibb, C.M.; Jackson, R.; Mohammed, S.; Fiaidhi, J.; Zehbe, I. Pathogen–Host Analysis Tool (PHAT): An integrative platform to analyze next-generation sequencing data. Bioinformatics 2018, bty1003. [Google Scholar] [CrossRef]
- Stone, D.; Niyonzima, N.; Jerome, K.R. Genome editing and the next generation of antiviral therapy. Hum. Genet. 2016, 135, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Togtema, M.; Pichardo, S.; Jackson, R.; Lambert, P.F.; Curiel, L.; Zehbe, I. Sonoporation delivery of monoclonal antibodies against human papillomavirus 16 E6 restores p53 expression in transformed cervical keratinocytes. PLoS ONE 2012, 7, e50730. [Google Scholar] [CrossRef] [PubMed]
- Togtema, M.; Jackson, R.; Grochowski, J.; Villa, P.L.; Mellerup, M.; Chattopadhyaya, J.; Zehbe, I. Synthetic siRNA targeting human papillomavirus 16 E6: A perspective on in vitro nanotherapeutic approaches. Nanomedicine 2018, 13, 455–474. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Heng, B.C. Intracellular antibodies (intrabodies) versus RNA interference for therapeutic applications. Ann. Clin. Lab. Sci. 2005, 35, 227–229. [Google Scholar] [PubMed]
- Giovane, C.; Trave, G.; Briones, A.; Lutz, Y.; Wasylyk, B.; Weiss, E. Targeting of the N-terminal domain of the human papillomavirus type 16 E6 oncoprotein with monomeric scFvs blocks the E6-mediated degradation of cellular p53. J. Mol. Recognit. 1999, 12, 141–152. [Google Scholar] [CrossRef]
- Masson, M.; Hindelang, C.; Sible, A.P.; Schwalbach, G.; Travé, G.; Weiss, E. Preferential nuclear localization of the human papillomavirus type 16 E6 oncoprotein in cervical carcinoma cells. J. Gen. Virol. 2003, 84, 2099–2104. [Google Scholar] [CrossRef]
- Lagrange, M.; Charbonnier, S.; Orfanoudakis, G.; Robinson, P.; Zanier, K.; Masson, M.; Lutz, Y.; Trave, G.; Weiss, E.; Deryckere, F. Binding of human papillomavirus 16 E6 to p53 and E6AP is impaired by monoclonal antibodies directed against the second zinc-binding domain of E6. J. Gen. Virol. 2005, 86, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Courtête, J.; Sibler, A.P.; Zeder-Lutz, G.; Dalkara, D.; Oulad-Abdelghani, M.; Zuber, G.; Weiss, E. Suppression of cervical carcinoma cell growth by intracytoplasmic codelivery of anti-oncoprotein E6 antibody and small interfering RNA. Mol. Cancer Ther. 2007, 6, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Freund, G.; Sibler, A.P.; Desplancq, D.; Oulad-Abdelghani, M.; Vigneron, M.; Gannon, J.; Van Regenmortel, M.H.; Weiss, E. Targeting endogenous nuclear antigens by electrotransfer of monoclonal antibodies in living cells. MAbs 2013, 5, 518–522. [Google Scholar] [CrossRef]
- Postupalenko, V.; Sibler, A.P.; Desplancq, D.; Nominé, Y.; Spehner, D.; Schultz, P.; Weiss, E.; Zuber, G. Intracellular delivery of functionally active proteins using self-assembling pyridythiourea-polyethylenimine. J. Control. Release 2014, 178, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Sibler, A.P.; Nordhammer, A.; Masson, M.; Martineau, P.; Travé, G.; Weiss, E. Nucleocytoplasmic shuttling of antigen in mammalian cells conferred by a soluble versus insoluble single-chain antibody fragment equipped with import/export signals. Exp. Cell Res. 2003, 286, 276–287. [Google Scholar] [CrossRef]
- Griffin, H.; Elston, R.; Jackson, D.; Ansell, K.; Coleman, M.; Winter, G.; Doorbar, J. Inhibition of papillomavirus protein function in cervical cancer cells by intrabody targeting. J. Mol. Biol. 2006, 355, 360–378. [Google Scholar] [CrossRef]
- Lagrange, M.; Boulade-Ladame, C.; Mailly, L.; Weiss, E.; Orfanoudakis, G.; Deryckere, F. Intracellular scFvs against the viral E6 oncoprotein provoke apoptosis in human papillomavirus-positive cancer cells. Biochem. Biophys. Res. Commun. 2007, 361, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Amici, C.; Visintin, M.; Verachi, F.; Paolini, F.; Percario, Z.; Di Bonito, P.; Mandarino, A.; Affabris, E.; Venuti, A.; Accardi, L. A novel intracellular antibody against the E6 oncoprotein impairs growth of human papillomavirus 16-positive tumor cells in mouse models. Oncotarget 2016, 7, 15539–15553. [Google Scholar] [CrossRef][Green Version]
- Shepard, H.M.; Phillips, G.L.; Thanos, C.D.; Feldmann, M. Developments in therapy with monoclonal antibodies and related proteins. Clin. Med. 2017, 17, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Hamers-Casterman, C.T.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hammers, C.; Songa, E.B.; Bendahman, N.; Hammers, R. Naturally occurring antibodies devoid of light chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Harmsen, M.M.; De Haard, H.J. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [Google Scholar] [CrossRef]
- Steeland, S.; Vandenbroucke, R.E.; Libert, C. Nanobodies as therapeutics: Big opportunities for small antibodies. Drug Discov. Today 2016, 21, 1076–1113. [Google Scholar] [CrossRef] [PubMed]
- Flajnik, M.F.; Deschacht, N.; Muyldermans, S. A case of convergence: Why did a simple alternative to canonical antibodies arise in sharks and camels? PLoS Biol. 2011, 9, e1001120. [Google Scholar] [CrossRef] [PubMed]
- Helma, J.; Cardoso, M.C.; Muyldermans, S.; Leonhardt, H. Nanobodies and recombinant binders in cell biology. J. Cell Biol. 2015, 209, 633–644. [Google Scholar] [CrossRef]
- Lauwereys, M.; Ghahroudi, M.A.; Desmyter, A.; Kinne, J.; Hölzer, W.; De Genst, E.; Wyns, L.; Muyldermans, S. Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J. 1998, 17, 3512–3520. [Google Scholar] [CrossRef]
- Stijlemans, B.; Conrath, K.; Cortez-Retamozo, V.; Van Xong, H.; Wyns, L.; Senter, P.; Revets, H.; De Baetselier, P.; Muyldermans, S.; Magez, S. Efficient targeting of conserved cryptic epitopes of infectious agents by single domain antibodies. African trypanosomes as paradigm. J. Biol. Chem. 2004, 279, 1256–1261. [Google Scholar] [CrossRef]
- De Genst, E.; Silence, K.; Decanniere, K.; Conrath, K.; Loris, R.; Kinne, J.; Muyldermans, S.; Wyns, L. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl. Acad. Sci. USA 2006, 103, 4586–4591. [Google Scholar] [CrossRef]
- Dumoulin, M.; Conrath, K.; Van Meirhaeghe, A.; Meersman, F.; Heremans, K.; Frenken, L.G.; Muyldermans, S.; Wyns, L.; Matagne, A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002, 11, 500–515. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural single-domain antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef] [PubMed]
- Revets, H.; de Baetselier, P.; Muyldermans, S. Nanobodies as novel agents for cancer therapy. Expert Opin. Biol. Ther. 2005, 5, 111–124. [Google Scholar] [CrossRef]
- Böldicke, T. Single domain antibodies for the knockdown of cytosolic and nuclear proteins. Protein Sci. 2017, 26, 925–945. [Google Scholar] [CrossRef]
- Vu, K.B.; Ghahroudi, M.A.; Wyns, L.; Muyldermans, S. Comparison of llama VH sequences from conventional and heavy chain antibodies. Mol. Immunol. 1997, 34, 1121–1131. [Google Scholar] [CrossRef]
- Vincke, C.; Loris, R.; Saerens, D.; Martinez-Rodriguez, S.; Muyldermans, S.; Conrath, K. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J. Biol. Chem. 2009, 284, 3273–3284. [Google Scholar] [CrossRef] [PubMed]
- Van Audenhove, I.; Gettemans, J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. EBioMedicine 2016, 8, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Sanofi Press Release, FDA Approves Cablivi® (Caplacizumab-Yhdp), the First Nanobody®-Based Medicine, for Adults with Acquired Thrombotic Thrombocytopenic Purpura (aTTP). Available online: http://hugin.info/152918/R/2233733/878824.pdf (accessed on 26 April 2019).
- Pattillo, R.A.; Hussa, R.O.; Story, M.T.; Ruckert, A.C.; Shalaby, M.R.; Mattingly, R.F. Tumor antigen and human chorionic gonadotropin in CaSki cells: A new epidermoid cervical cancer cell line. Science 1977, 196, 1456–1458. [Google Scholar] [CrossRef] [PubMed]
- Meissner, J.D. Nucleotide sequences and further characterization of human papillomavirus DNA present in the CaSki, SiHa and HeLa cervical carcinoma cell lines. J. Gen. Virol. 1999, 80, 1725–1733. [Google Scholar] [CrossRef]
- Zehbe, I.; Richard, C.; DeCarlo, C.A.; Shai, A.; Lambert, P.F.; Lichtig, H.; Tommasino, M.; Sherman, L. Human papillomavirus 16 E6 variants differ in their dysregulation of human keratinocyte differentiation and apoptosis. Virology 2009, 383, 69–77. [Google Scholar] [CrossRef][Green Version]
- Zanier, K.; ould M’hamed ould Sidi, A.; Boulade-Ladame, C.; Rybin, V.; Chappelle, A.; Atkinson, A.; Kieffer, B.; Travé, G. Solution structure analysis of the HPV16 E6 oncoprotein reveals a self-association mechanism required for E6-mediated degradation of p53. Structure 2012, 20, 604–617. [Google Scholar] [CrossRef] [PubMed]
- Zanier, K.; Charbonnier, S.; Sidi, A.O.; McEwen, A.G.; Ferrario, M.G.; Poussin-Courmontagne, P.; Cura, V.; Brimer, N.; Babah, K.O.; Ansari, T. Structural basis for hijacking of cellular LxxLL motifs by papillomavirus E6 oncoproteins. Science 2013, 339, 694–698. [Google Scholar] [CrossRef]
- Ramirez, J.; Poirson, J.; Foltz, C.; Chebaro, Y.; Schrapp, M.; Meyer, A.; Bonetta, A.; Forster, A.; Jacob, Y.; Masson, M. Targeting the two oncogenic functional sites of the HPV E6 oncoprotein with a high-affinity bivalent ligand. Angew. Chem. Int. Ed. Engl. 2015, 54, 7958–7962. [Google Scholar] [CrossRef] [PubMed]
- Schulman, F.Y.; Krafft, A.E.; Janczewski, T.; Reupert, R.; Jackson, K.; Garner, M.M. Camelid mucoutaneous fibropapillomas: Clinicopathologic findings and association with papillomavirus. Vet. Pathol. 2003, 40, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Ure, A.E.; Elfadl, A.K.; Khalafalla, A.I.; Gameel, A.A.; Dillner, J.; Forslund, O. Characterization of the complete genomes of Camelus dromedarius papillomavirus types 1 and 2. J. Gen. Virol. 2011, 92, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Boos, W.; Shuman, H. Maltose/maltodextrin system of Escherichia coli: Transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 1998, 62, 204–229. [Google Scholar]
- Hussack, G.; Arbabi-Ghahroudi, M.; MacKenzie, C.R.; Tanha, J. Isolation and characterization of Clostridium difficile toxin-specific single-domain antibodies. Methods Mol. Bio. 2012, 911, 211–239. [Google Scholar] [CrossRef]
- Baral, T.N.; MacKenzie, R.; Arbab-Ghahroudi, M. Single-domain antibodies and their utility. Curr. Protoc. Immunol. 2013, 2–17. [Google Scholar] [CrossRef]
- Hussack, G.; Arbabi-Ghahroudi, M.; van Faassen, H.; Songer, J.G.; Ng, K.K.; MacKenzie, R.; Tanha, J. Neutralization of Clostridium difficile toxin A with single-domain antibodies targeting the cell receptor binding domain. J. Biol. Chem. 2011, 286, 8961–8976. [Google Scholar] [CrossRef]
- Arbabi-Ghahroudi, M.; MacKenzie, R.; Tanha, J. Selection of non-aggregating VH binders from synthetic VH phage-display libraries. Methods Mol. Biol. 2009, 525, 187–216. [Google Scholar] [CrossRef]
- Suarez, I.; Travé, G. Structural insights in multifunctional papillomavirus oncoproteins. Viruses 2018, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Hussack, G.; Keklikian, A.; Alsughayyir, J.; Hanifi-Moghaddam, P.; Arbabi-Ghahroudi, M.; van Faassen, H.; Hou, S.T.; Sad, S.; MacKenzie, R.; Tanha, J. A VL single-domain antibody library shows a high-propensity to yield non-aggregating binders. Protein Eng. Des. Sel. 2012, 25, 313–318. [Google Scholar] [CrossRef][Green Version]
- Arbabi-Ghahroudi, M.; To, R.; Gaudette, N.; Hirama, T.; Ding, W.; MacKenzie, R.; Tanha, J. Aggregation-resistant VHs selected by in vitro evolution tend to have disulfide-bonded loops and acidic isoelectric points. Protein Eng. Des. Sel. 2009, 22, 59–66. [Google Scholar] [CrossRef]
- Movva, N.R.; Nakamura, K.; Inouye, M. Amino acid sequence of the signal peptide of ompA protein, a major outer membrane protein of Escherichia coli. J. Biol. Chem. 1980, 255, 27–29. [Google Scholar] [PubMed]
- De Marco, A. Strategies for successful recombinant expression of disulfide bond-dependent proteins in Escherichia coli. Microb. Cell Fact. 2009, 8, 26. [Google Scholar] [CrossRef]
- Choulier, L.; Orfanoudakis, G.; Robinson, P.; Laune, D.; Khalifa, M.B.; Granier, C.; Weiss, E.; Altschuh, D. Comparative properties of two peptide–antibody interactions as deduced from epitope delineation. J. Immunol. Methods 2002, 259, 77–86. [Google Scholar] [CrossRef]
- Serruys, B.; van Houtte, F.; Verbrugghe, P.; Leroux-Roels, G.; Vanlandschoot, P. Llama-derived single-domain intrabodies inhibit secretion of hepatitis B virions in mice. Hepatology 2009, 49, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Serruys, B.; van Houtte, F.; Farhoudi-Moghadam, A.; Leroux-Roels, G.; Vanlandschoot, P. Production, characterization and in vitro testing of HBcAg-specific VHH intrabodies. J. Gen. Virol. 2010, 91, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Budzinska, M.A.; Shackel, N.A.; Urban, S. HBV DNA integration: Molecular mechanisms and clinical implications. Viruses 2017, 9, 75. [Google Scholar] [CrossRef]
- Thueng-In, K.; Thanongsaksrikul, J.; Srimanote, P.; Bangphoomi, K.; Poungpair, O.; Maneewatch, S.; Choowongkomon, K.; Chaicumpa, W. Cell penetrable humanized-VH/VHH that inhibit RNA dependent RNA polymerase (NS5B) of HCV. PLoS ONE 2012, 7, e49254. [Google Scholar] [CrossRef]
- Phalaphol, A.; Thueng-in, K.; Thanongsaksrikul, J.; Poungpair, O.; Bangphoomi, K.; Sookrung, N.; Srimanote, P.; Chaicumpa, W. Humanized-VH/VHH that inhibit HCV replication by interfering with the virus helicase activity. J. Virol. Methods 2013, 194, 289–299. [Google Scholar] [CrossRef]
- Jittavisutthikul, S.; Thanongsaksrikul, J.; Thueng-In, K.; Chulanetra, M.; Srimanote, P.; Seesuay, W.; Malik, A.; Chaicumpa, W. Humanized-VHH transbodies that inhibit HCV protease and replication. Viruses 2015, 7, 2030–2056. [Google Scholar] [CrossRef]
- Glab-ampai, K.; Malik, A.A.; Chulanetra, M.; Thanongsaksrikul, J.; Thueng-in, K.; Srimanote, P.; Tongtawe, P.; Chaicumpa, W. Inhibition of HCV replication by humanized-single domain transbodies to NS4B. Biochem. Biophys. Res. Commun. 2016, 476, 654–664. [Google Scholar] [CrossRef]
- Tarr, A.W.; Lafaye, P.; Meredith, L.; Damier-Piolle, L.; Urbanowicz, R.A.; Meola, A.; Jestin, J.L.; Brown, R.J.; McKeating, J.A.; Rey, F.A.; et al. An alpaca nanobody inhibits hepatitis C virus entry and cell-to-cell transmission. Hepatology 2013, 58, 932–939. [Google Scholar] [CrossRef]
- Minaeian, S.; Rahbarizadeh, F.; Zarkesh-Esfahani, S.H.; Ahmadvand, D.; Broom, O.J. Neutralization of human papillomavirus by specific nanobodies against major capsid protein L1. J. Microbiol. Biotechnol. 2012, 22, 721–728. [Google Scholar] [CrossRef]
- Minaeian, S.; Rahbarizadeh, F.; Zarkesh Esfahani, S.H.; Ahmadvand, D. Characterization and enzyme-conjugation of a specific anti-L1 nanobody. J. Immunoassay Immunochem. 2012, 33, 422–434. [Google Scholar] [CrossRef]
- Li, S.; Zhang, W.; Jiang, K.; Shan, H.; Shi, M.; Chen, B.; Hua, Z. Nanobody against the E7 oncoprotein of human papillomavirus 16. Mol. Immunol. 2019, 109, 12–19. [Google Scholar] [CrossRef]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.; Wohlkönig, A.; Ruf, A.; Muyldermans, S.; Hol, W.G.; Kobilka, B.K.; Steyaert, J. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef]
- Henry, K.A.; Tanha, J. Performance evaluation of phage-displayed synthetic human single-domain antibody libraries: A retrospective analysis. J. Immunol. Methods 2018, 456, 81–86. [Google Scholar] [CrossRef]
- Tian, G.; Tang, F.; Yang, C.; Zhang, W.; Bergquist, J.; Wang, B.; Mi, J.; Zhang, J. Quantitative dot blot analysis (QDB), a versatile high throughput immunoblot method. Oncotarget 2017, 8, 58553–58562. [Google Scholar] [CrossRef]
- Bieging, K.T.; Mello, S.S.; Attardi, L.D. Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 2014, 14, 359–370. [Google Scholar] [CrossRef]
- Beerheide, W.; Bernard, H.U.; Tan, Y.J.; Ganesan, A.; Rice, W.G.; Ting, A.E. Potential drugs against cervical cancer: Zinc-ejecting inhibitors of the human papillomavirus type 16 E6 oncoprotein. J. Natl. Cancer Inst. 1999, 91, 1211–1220. [Google Scholar] [CrossRef]
- Beerheide, W.; Sim, M.M.; Tan, Y.J.; Bernard, H.U.; Ting, A.E. Inactivation of the human papillomavirus-16 E6 oncoprotein by organic disulfides. Bioorg. Med. Chem. 2000, 8, 2549–2560. [Google Scholar] [CrossRef]
- Baleja, J.D.; Cherry, J.J.; Liu, Z.; Gao, H.; Nicklaus, M.C.; Voigt, J.H.; Chen, J.J.; Androphy, E.J. Identification of inhibitors to papillomavirus type 16 E6 protein based on three-dimensional structures of interacting proteins. Antivir. Res. 2006, 72, 49–59. [Google Scholar] [CrossRef]
- Cherry, J.J.; Rietz, A.; Malinkevich, A.; Liu, Y.; Xie, M.; Bartolowits, M.; Davisson, V.J.; Baleja, J.D.; Androphy, E.J. Structure based identification and characterization of flavonoids that disrupt human papillomavirus-16 E6 function. PLoS ONE 2013, 8, e84506. [Google Scholar] [CrossRef]
- Malecka, K.A.; Fera, D.; Schultz, D.C.; Hodawadekar, S.; Reichman, M.; Donover, P.S.; Murphy, M.E.; Marmorstein, R. Identification and characterization of small molecule human papillomavirus E6 inhibitors. ACS Chem. Biol. 2014, 9, 1603–1612. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Androphy, E.; Chen, J.; Baleja, J.D. Design and characterization of helical peptides that inhibit the E6 protein of papillomavirus. Biochemistry 2004, 43, 7421–7431. [Google Scholar] [CrossRef]
- Grm, H.S.; Weber, M.; Elston, R.; McIntosh, P.; Griffin, H.; Banks, L.; Doorbar, J. Inhibition of E6-induced degradation of its cellular substrates by novel blocking peptides. J. Mol. Biol. 2004, 335, 971–985. [Google Scholar] [CrossRef]
- Dymalla, S.; Scheffner, M.; Weber, E.; Sehr, P.; Lohrey, C.; Hoppe-Seyler, F.; Hoppe-Seyler, K. A novel peptide motif binding to and blocking the intracellular activity of the human papillomavirus E6 oncoprotein. J. Mol. Med. 2009, 87, 321–331. [Google Scholar] [CrossRef]
- Stutz, C.; Reinz, E.; Honegger, A.; Bulkescher, J.; Schweizer, J.; Zanier, K.; Travé, G.; Lohrey, C.; Hoppe-Seyler, K.; Hoppe-Seyler, F. Intracellular analysis of the interaction between the human papillomavirus type 16 E6 oncoprotein and inhibitory peptides. PLoS ONE 2015, 10, e0132339. [Google Scholar] [CrossRef]
- Zanier, K.; Stutz, C.; Kintscher, S.; Reinz, E.; Sehr, P.; Bulkescher, J.; Hoppe-Seyler, K.; Trave, G.; Hoppe-Seyler, F. The E6AP binding pocket of the HPV16 E6 oncoprotein provides a docking site for a small inhibitory peptide unrelated to E6AP, indicating druggability of E6. PLoS ONE 2014, 9, e112514. [Google Scholar] [CrossRef]
- Butz, K.; Denk, C.; Ullmann, A.; Scheffner, M.; Hoppe-Seyler, F. Induction of apoptosis in human papillomavirus positive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc. Natl. Acad. Sci. USA 2000, 97, 6693–6697. [Google Scholar] [CrossRef]
- Karlsson, O.A.; Ramirez, J.; Öberg, D.; Malmqvist, T.; Engström, Å.; Friberg, M.; Chi, C.N.; Widersten, M.; Travé, G.; Nilsson, M.T.; et al. Design of a PDZbody, a bivalent binder of the E6 protein from human papillomavirus. Sci. Rep. 2015, 5, 9382. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, T.A.; Nicol, C.; Cesur, Ö.; Travé, G.; Blair, G.E.; Stonehouse, N.J. An RNA aptamer targets the PDZ-binding motif of the HPV16 E6 oncoprotein. Cancers 2014, 6, 1553–1569. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Fontela, C.; Mandinova, A.; Aaronson, S.A.; Lee, S.W. Emerging roles of p53 and other tumour-suppressor genes in immune regulation. Nat. Rev. Immunol. 2016, 16, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, e301. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M.; O’Leary, M.N.; Chang, J.; Shao, L.; Liu, S.; Alimirah, F.; Koenig, K.; Le, C.; Mitin, N.; Deal, A.M. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 2017, 7, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, S.; Pasetto, A.; Helman, S.R.; Gartner, J.J.; Prickett, T.D.; Howie, B.; Robins, H.S.; Robbins, P.F.; Klebanoff, C.A.; Rosenberg, S.A.; et al. Landscape of immunogenic tumor antigens in successful immunotherapy of virally induced epithelial cancer. Science 2017, 356, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.P.; Sharei, A.; Ding, X.; Sahay, G.; Langer, R.; Jensen, K.F. In vitro and ex vivo strategies for intracellular delivery. Nature 2016, 538, 183–192. [Google Scholar] [CrossRef]
- Phenix, C.P.; Togtema, M.; Pichardo, S.; Zehbe, I.; Curiel, L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J. Pharm. Pharm. Sci. 2014, 17, 136–153. [Google Scholar] [CrossRef]
- Bai, L.; Wei, L.; Wang, J.; Li, X.; He, P. Extended effects of human papillomavirus 16 E6-specific short hairpin RNA on cervical carcinoma cells. Int. J. Gynecol. Cancer 2006, 16, 718–729. [Google Scholar] [CrossRef]
- Gu, W.; Putral, L.; Hengst, K.; Minto, K.; Saunders, N.A.; Leggatt, G.; McMillan, N.A. Inhibition of cervical cancer cell growth in vitro and in vivo with lentiviral-vector delivered short hairpin RNA targeting human papillomavirus E6 and E7 oncogenes. Cancer Gene Ther. 2006, 13, 1023–1032. [Google Scholar] [CrossRef]
- Yamato, K.; Yamada, T.; Kizaki, M.; Ui-Tei, K.; Natori, Y.; Fujino, M.; Nishihara, T.; Ikeda, Y.; Nasu, Y.; Saigo, K.; et al. New highly potent and specific E6 and E7 siRNAs for treatment of HPV16 positive cervical cancer. Cancer Gene Ther. 2008, 15, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Bousarghin, L.; Touze, A.; Gaud, G.; Iochmann, S.; Alvarez, E.; Reverdiau, P.; Gaitan, J.; Jourdan, M.L.; Sizaret, P.Y.; Coursaget, P.L. Inhibition of cervical cancer cell growth by human papillomavirus-like particles packaged with human papillomavirus oncoprotein short hairpin RNAs. Mol. Cancer Ther. 2009, 8, 357–365. [Google Scholar] [CrossRef]
- Tan, S.; Hougardy, B.M.; Meersma, G.J.; Schaap, B.; de Vries, E.G.; van der Zee, A.G.; de Jong, S. Human papilloma virus 16 E6 RNA interference enhances cisplatin and death receptor-mediated apoptosis in human cervical carcinoma cells. Mol. Pharmacol. 2012, 81, 701–709. [Google Scholar] [CrossRef]
- Jackson, R.; Togtema, M.; Zehbe, I. Subcellular localization and quantitation of the human papillomavirus type 16 E6 oncoprotein through immunocytochemistry detection. Virology 2013, 435, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Beghein, E.; Gettemans, J. Nanobody technology: A versatile toolkit for microscopic imaging, protein–protein interaction analysis, and protein function exploration. Front. Immunol. 2017, 8, 771. [Google Scholar] [CrossRef]
- Maier, J.; Traenkle, B.; Rothbauer, U. Real-time analysis of epithelial-mesenchymal transition using fluorescent single-domain antibodies. Sci. Rep. 2015, 5, 13402. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.B.; Traenkle, B.; Koch, P.A.; Emele, F.; Weiss, F.; Poetz, O.; Stehle, T.; Rothbauer, U. Peptides in headlock—A novel high-affinity and versatile peptide-binding nanobody for proteomics and microscopy. Sci. Rep. 2016, 6, 19211. [Google Scholar] [CrossRef] [PubMed]
- Jullien, D.; Vignard, J.; Fedor, Y.; Béry, N.; Olichon, A.; Crozatier, M.; Erard, M.; Cassard, H.; Ducommun, B.; Salles, B.; et al. Chromatibody, a novel non-invasive molecular tool to explore and manipulate chromatin in living cells. J. Cell Sci. 2016, 129, 2673–2683. [Google Scholar] [CrossRef]
- De Bruin, R.C.; Lougheed, S.M.; van der Kruk, L.; Stam, A.G.; Hooijberg, E.; Roovers, R.C.; en Henegouwen, P.M.; Verheul, H.M.; de Gruijl, T.D.; van der Vliet, H.J. Highly specific and potently activating Vγ9Vδ2-T cell specific nanobodies for diagnostic and therapeutic applications. Clin. Immunol. 2016, 169, 128–138. [Google Scholar] [CrossRef]
- Peyrassol, X.; Laeremans, T.; Gouwy, M.; Lahura, V.; Debulpaep, M.; Van Damme, J.; Steyaert, J.; Parmentier, M.; Langer, I. Development by genetic immunization of monovalent antibodies (nanobodies) behaving as antagonists of the human ChemR23 receptor. J. Immunol. 2016, 196, 2893–2901. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togtema, M.; Hussack, G.; Dayer, G.; Teghtmeyer, M.R.; Raphael, S.; Tanha, J.; Zehbe, I. Single-Domain Antibodies Represent Novel Alternatives to Monoclonal Antibodies as Targeting Agents against the Human Papillomavirus 16 E6 Protein. Int. J. Mol. Sci. 2019, 20, 2088. https://doi.org/10.3390/ijms20092088
Togtema M, Hussack G, Dayer G, Teghtmeyer MR, Raphael S, Tanha J, Zehbe I. Single-Domain Antibodies Represent Novel Alternatives to Monoclonal Antibodies as Targeting Agents against the Human Papillomavirus 16 E6 Protein. International Journal of Molecular Sciences. 2019; 20(9):2088. https://doi.org/10.3390/ijms20092088
Chicago/Turabian StyleTogtema, Melissa, Greg Hussack, Guillem Dayer, Megan R. Teghtmeyer, Shalini Raphael, Jamshid Tanha, and Ingeborg Zehbe. 2019. "Single-Domain Antibodies Represent Novel Alternatives to Monoclonal Antibodies as Targeting Agents against the Human Papillomavirus 16 E6 Protein" International Journal of Molecular Sciences 20, no. 9: 2088. https://doi.org/10.3390/ijms20092088
APA StyleTogtema, M., Hussack, G., Dayer, G., Teghtmeyer, M. R., Raphael, S., Tanha, J., & Zehbe, I. (2019). Single-Domain Antibodies Represent Novel Alternatives to Monoclonal Antibodies as Targeting Agents against the Human Papillomavirus 16 E6 Protein. International Journal of Molecular Sciences, 20(9), 2088. https://doi.org/10.3390/ijms20092088






