Carbon-Doped TiO2 Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment
Abstract
1. Introduction
2. Results
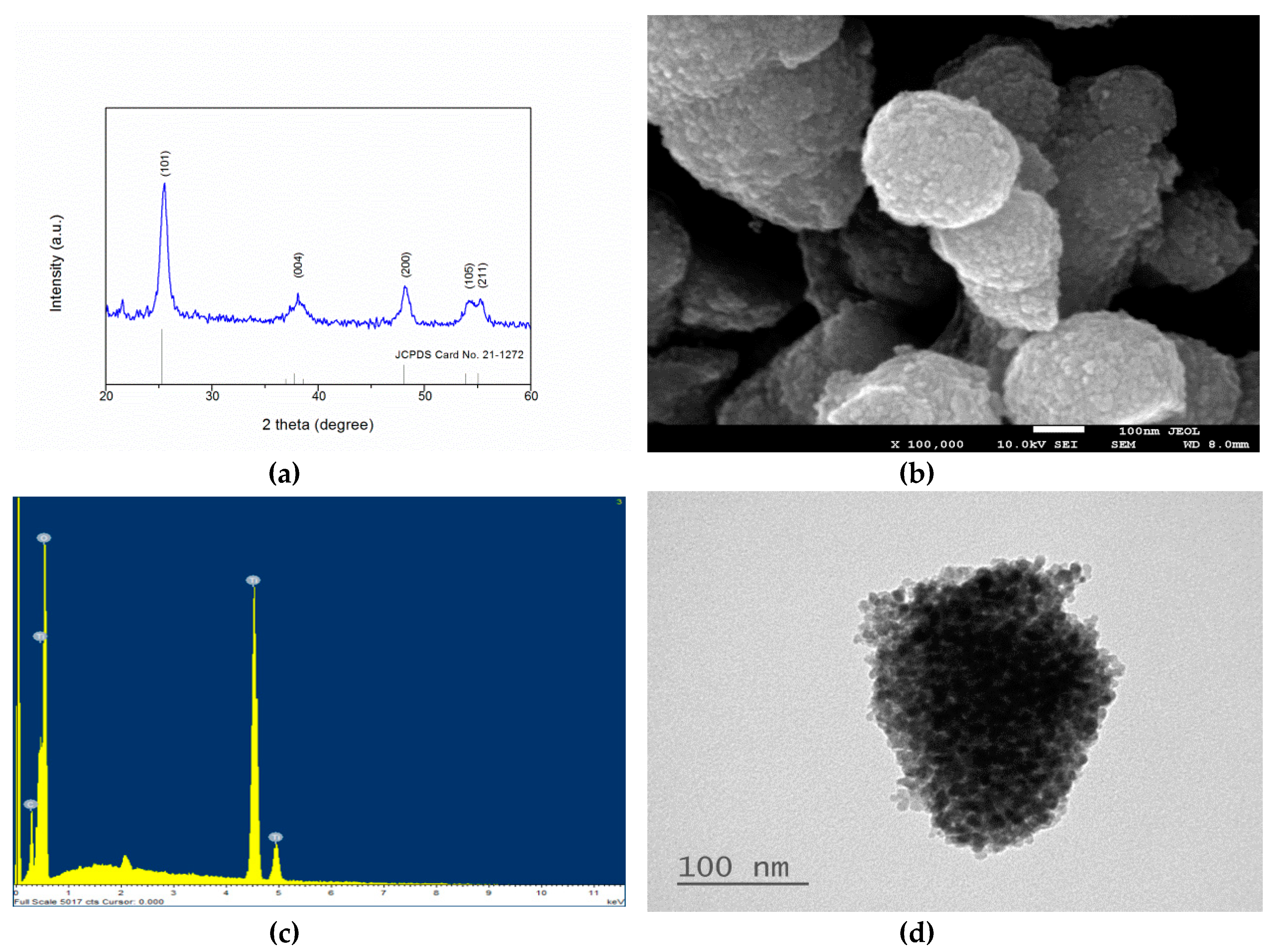
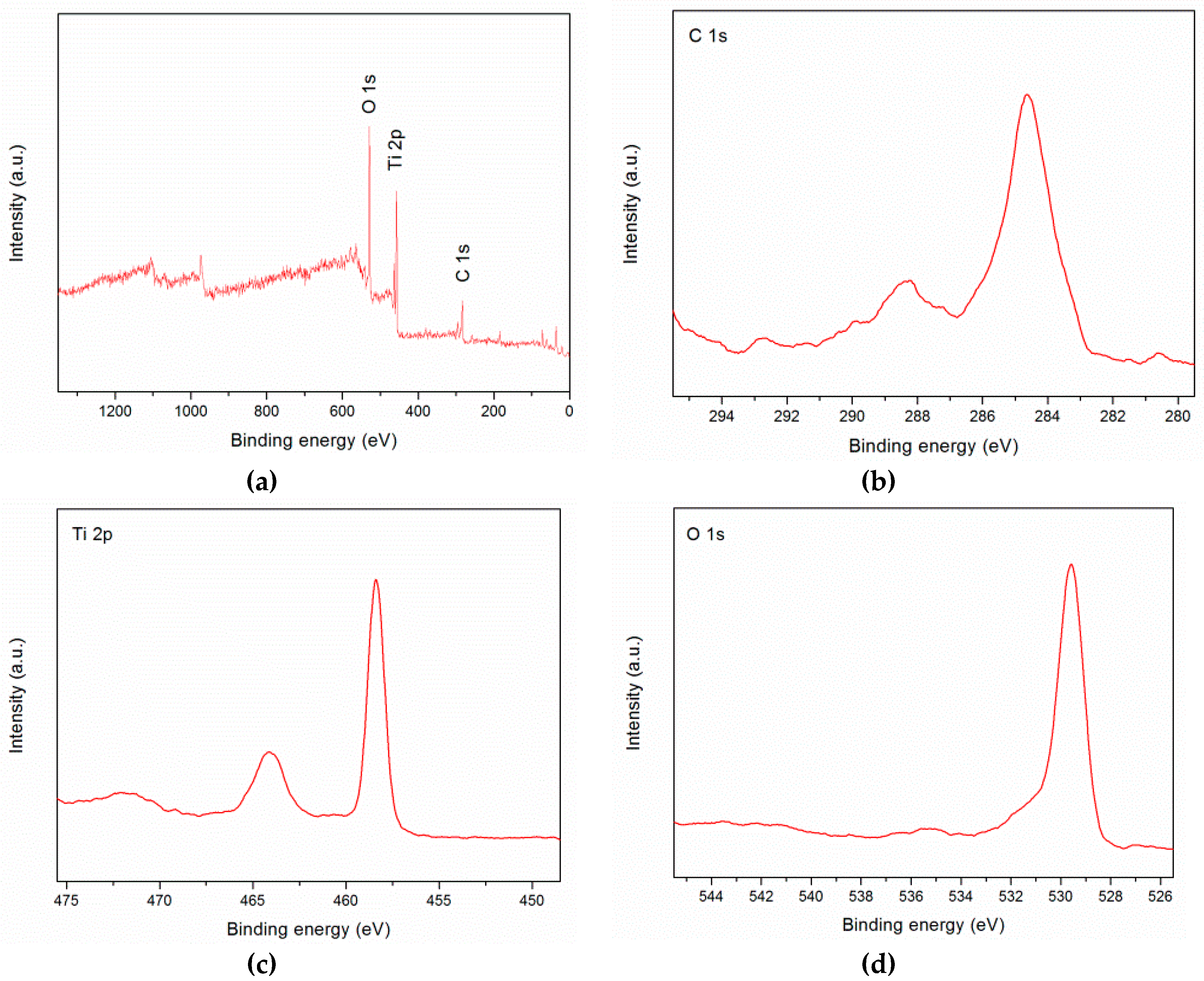
2.1. Material Characterization
2.2. ROS Generation of TiO2:C under X-ray Irradiation
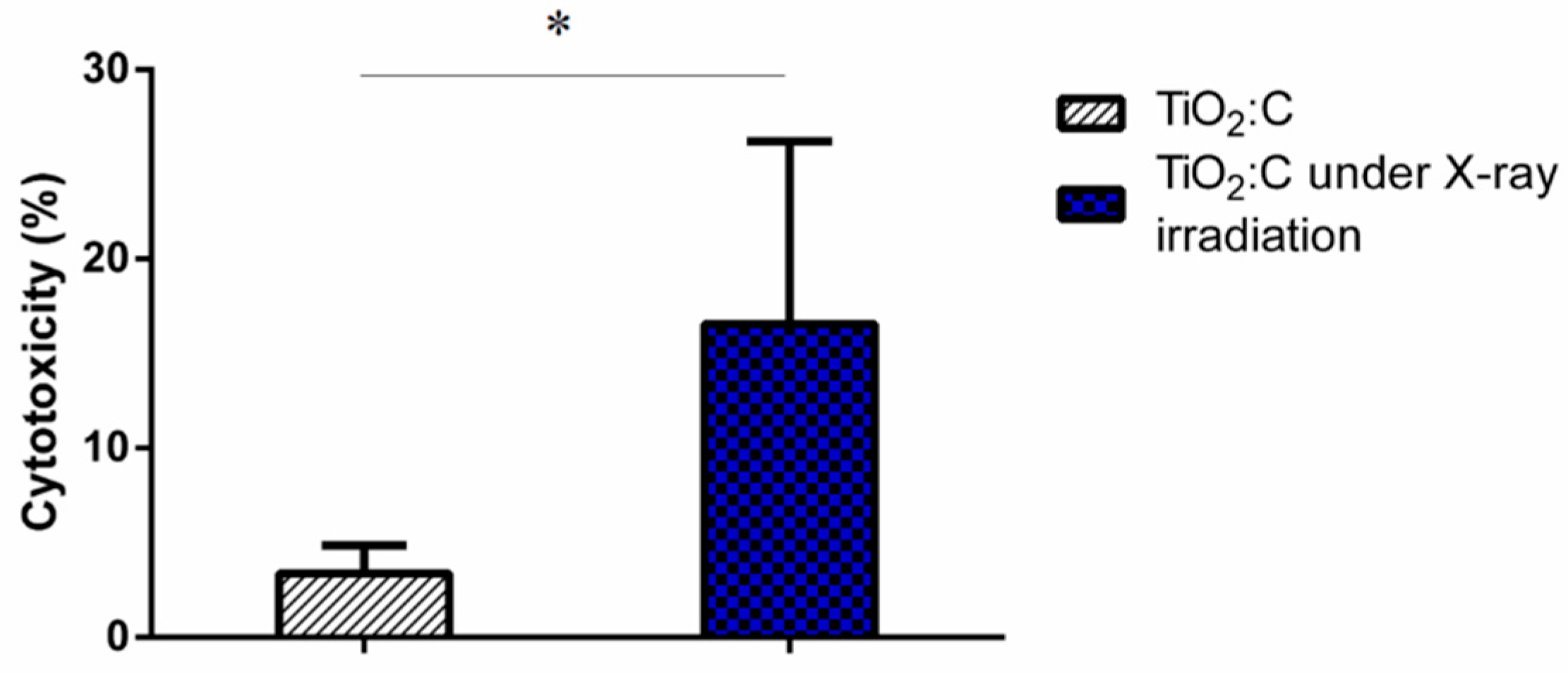
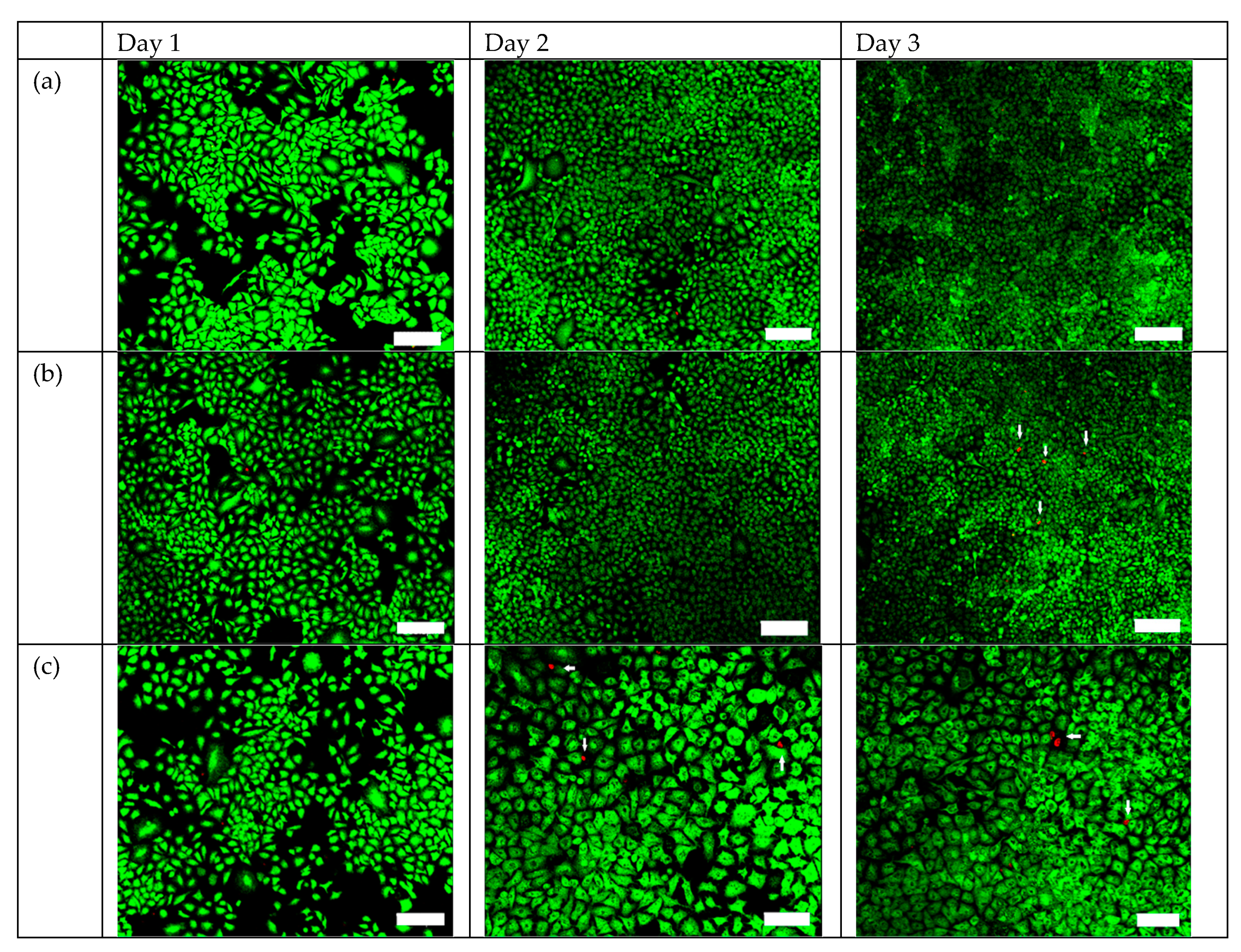
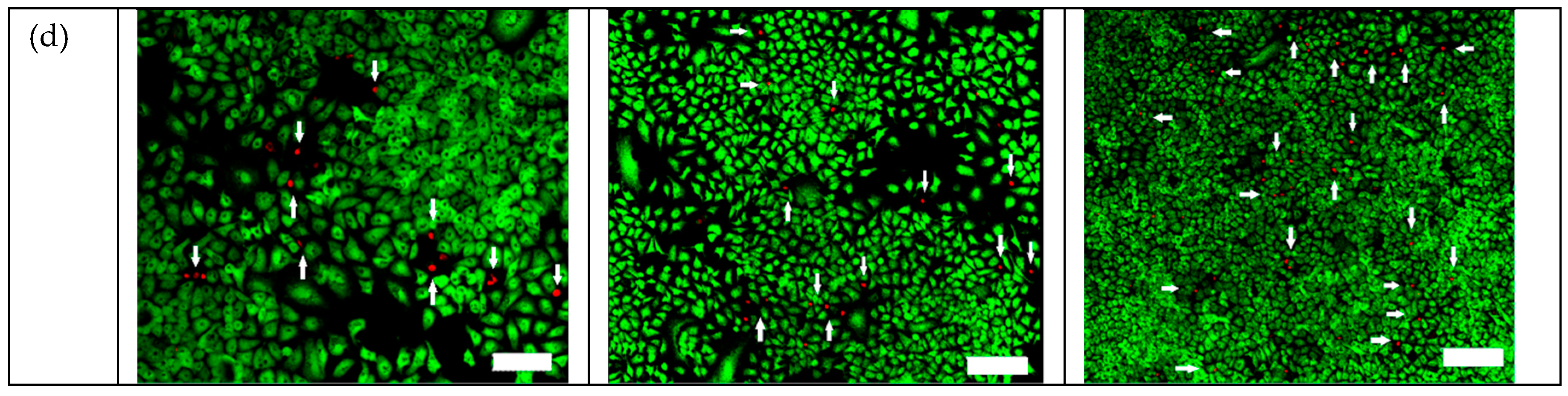
2.3. Efficacy of TiO2:C in Killing A549 Cells under X-ray Irradiation In Vitro
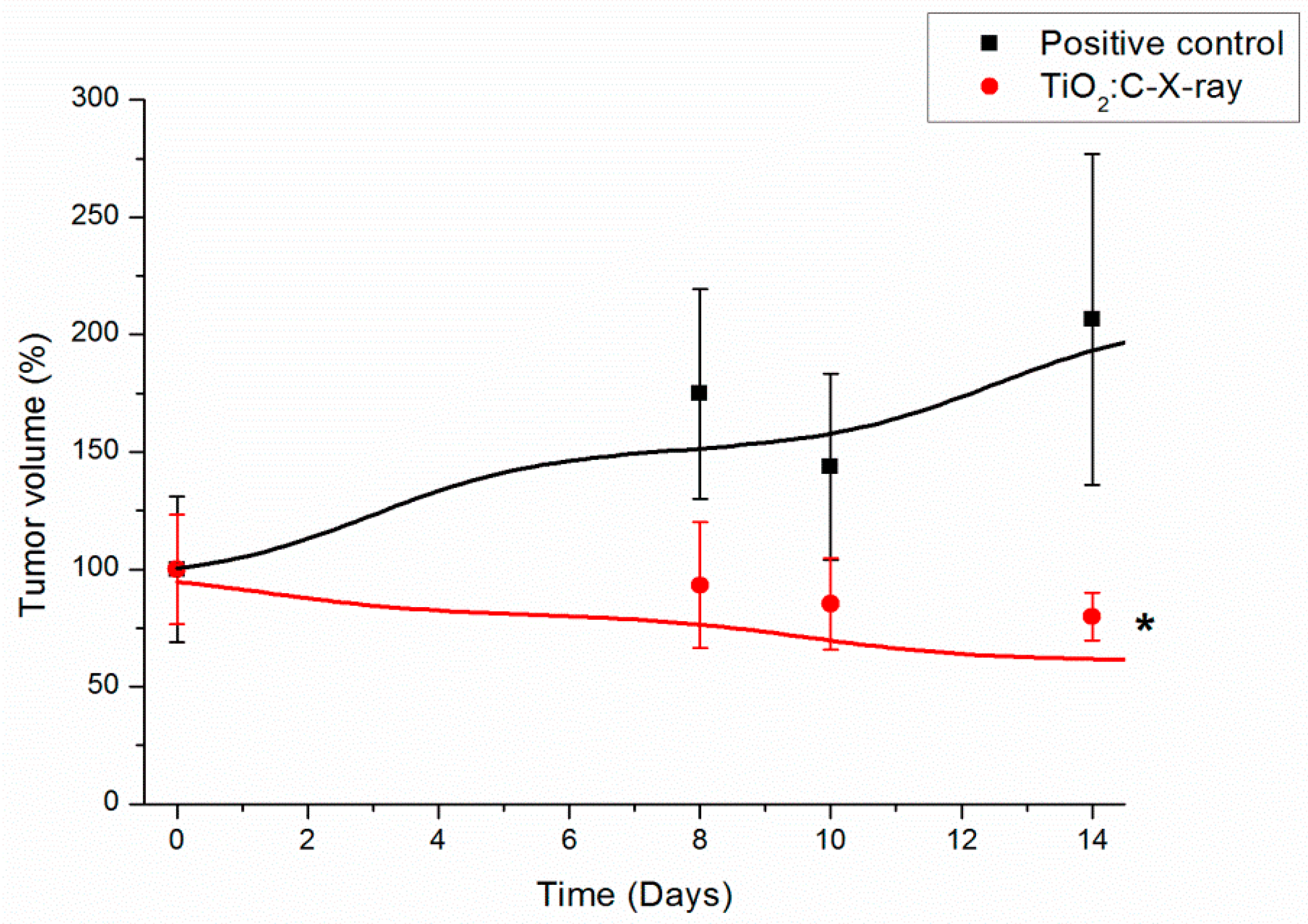
2.4. In Vivo Anti-Tumor Efficacy of TiO2:C under X-ray Irradiation (TiO2:C-X-ray)
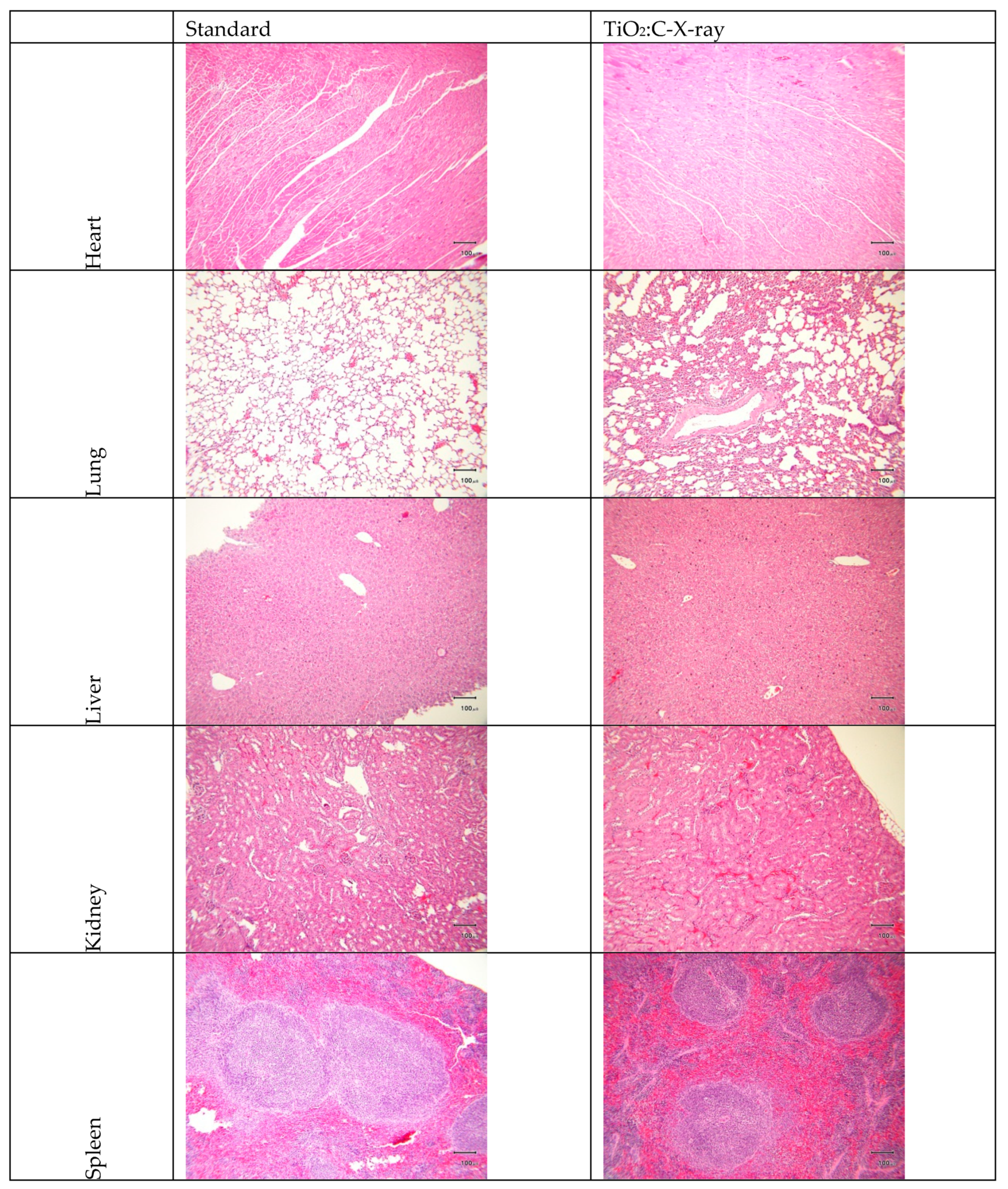
2.5. Blood/Serum Analysis and Hematoxylin and Eosin (H-and-E) Examination
3. Discussion
4. Materials and Methods
4.1. Synthesis of TiO2:C Particles
4.2. Material Characterization
4.3. Degradation of MB for ROS Detection
4.4. LDH Assay: In Vitro Testing of the Synthesized TiO2:C in X-ray-Induced PDT
4.5. Live/Dead Staining: In Vitro Testing of the Synthesized TiO2:C in X-ray-Induced PDT
4.6. In Vivo Evaluation of the PDT Effect of the Synthesized TiO2:C under X-ray Irradiation
4.7. In Vivo Safety Testing of the Synthesized TiO2:C under X-ray Irradiation
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PDT | photodynamic therapy |
| ROS | reactive oxygen species |
| A549 cells | adenocarcinomic human alveolar basal epithelial cells |
| DNA | deoxyribonucleic acid |
| 5 ALA | 5-Aminolevulinic Acid |
| Caco-2 | heterogeneous human epithelial colorectal adenocarcinoma cell line |
| MB | methylene blue |
| A549 | adenocarcinoma human alveolar basal epithelial cell line |
| LDH | lactate dehydrogenase |
| TIP | titanium (IV) isopropoxide |
| PBS | phosphate buffered saline |
| H-and-E | hematoxylin and eosin |
| EDTA | ethylenediaminetetraacetic acid |
| BUN | blood urine nitrogen |
| CREA | creatinine |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| ALB | albumin |
| Ca | calcium |
| IP | inorganic phosphate |
| MPTP | mitochondrial permeability transition pore |
References
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic therapy (PDT): A short review on cellular mechanisms and cancer research applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar]
- Hong, I.S.; Greenberg, M.M. DNA Interstrand Cross-Link Formation Initiated by Reaction between Singlet Oxygen and a Modified Nucleotide. J. Am. Chem. Soc. 2005, 127, 10510–10511. [Google Scholar] [CrossRef] [PubMed]
- Hartl, B.A.; Hirschberg, H.; Marcu, L.; Cherry, S.R. Characterizing low fluence thresholds for in vitro photodynamic therapy. Biomed. Opt. Express 2015, 6, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shuhendler, A.J.; Ye, D.; Xu, J.J.; Chen, H.Y. Two-photon excitation nanoparticles for photodynamic therapy. Chem Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [PubMed]
- Chen, T.-C.; Huang, L.; Liu, C.-C.; Chao, P.-J.; Lin, F.-H. Luminol as the light source for in situ photodynamic therapy. Process. Biochem. 2012, 47, 1903–1908. [Google Scholar] [CrossRef]
- Klein, S.; Dell’Arciprete, M.L.; Wegmann, M.; Distel, L.V.; Neuhuber, W.; Gonzalez, M.C.; Kryschi, C. Oxidized silicon nanoparticles for radiosensitization of cancer and tissue cells. Biochem. Biophys. Res. Commun. 2013, 434, 217–222. [Google Scholar] [CrossRef]
- Kaščáková, S.; Giuliani, A.; Lacerda, S.; Pallier, A.; Mercère, P.; Tóth, É.; Réfrégiers, M. X-ray-induced radiophotodynamic therapy (RPDT) using lanthanide micelles: Beyond depth limitations. Nano Res. 2015, 8, 2373–2379. [Google Scholar] [CrossRef]
- Yang, C.-C.; Wang, W.-Y.; Lin, F.-H.; Hou, C.-H. Rare-Earth-Doped Calcium Carbonate Exposed to X-ray Irradiation to Induce Reactive Oxygen Species for Tumor Treatment. Int. J. Mol. Sci. 2019, 20, 1148. [Google Scholar] [CrossRef]
- Clement, S.; Deng, W.; Camilleri, E.; Wilson, B.C.; Goldys, E.M. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: Determination of singlet oxygen quantum yield. Sci. Rep. 2016, 6, 19954. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-Ray Induced Photodynamic Therapy: A Combination of Radiotherapy and Photodynamic Therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Hamada, N.; Fujimichi, Y. Classification of radiation effects for dose limitation purposes: History, current situation and future prospects. J. Radiat. Res. 2014, 55, 629–640. [Google Scholar] [CrossRef]
- Yang, C.-C.; Sun, Y.-J.; Chung, P.-H.; Chen, W.-Y.; Swieszkowski, W.; Tian, W.; Lin, F.-H. Development of Ce-doped TiO2 activated by X-ray irradiation for alternative cancer treatment. Ceram. Int. 2017, 43, 12675–12683. [Google Scholar] [CrossRef]
- Odling, G.; Robertson, N. Why is Anatase a Better Photocatalyst than Rutile? The Importance of Free Hydroxyl Radicals. ChemSusChem 2015, 8, 1838–1840. [Google Scholar] [CrossRef]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Chen, J.; Cheng, L.; Chang, J.; Sheng, W.; Hu, C.; Cao, S. C-doped hollow TiO2 spheres: In situ synthesis, controlled shell thickness, and superior visible-light photocatalytic activity. Appl. Catal. B Environ. 2015, 165, 715–722. [Google Scholar] [CrossRef]
- Singh, J.; Chang, Y.-Y.; Koduru, J.R.; Yang, J.-K. Potential degradation of methylene blue (MB) by nano-metallic particles: A kinetic study and possible mechanism of MB degradation. Environ. Eng. Res. 2018, 23, 1–9. [Google Scholar] [CrossRef]
- Vadivel, D.; Malaichamy, I. Pyrolytic formation and photoactivity of reactive oxygen species in a SiO (2)/carbon nanocomposite from kraft lignin. F1000Research 2018, 7, 1574. [Google Scholar] [CrossRef] [PubMed]
- Tretter, L.; Horvath, G.; Hölgyesi, A.; Essek, F.; Adam-Vizi, V. Enhanced hydrogen peroxide generation accompanies the beneficial bioenergetic effects of methylene blue in isolated brain mitochondria. Free Radic. Biol. Med. 2014, 77, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-M.; Choi, J.Y.; Wang, K.; Zhang, H.; Tariq, Z.; Wu, D.; Ko, E.; LaDana, C.; Sesaki, H.; Cao, K. Methylene blue alleviates nuclear and mitochondrial abnormalities in progeria. Aging Cell 2016, 15, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Juzenas, P.; Chen, W.; Sun, Y.P.; Coelho, M.A.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [PubMed]
- Townley, H.E.; Kim, J.; Dobson, P.J. In vivo demonstration of enhanced radiotherapy using rare earth doped titania nanoparticles. Nanoscale 2012, 4, 5043–5050. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R.; Bekah, D.; Nadeau, J.L. Gold nanoparticles and their alternatives for radiation therapy enhancement. Front. Chem. 2014, 2, 86. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef]
- Chen, M.H.; Hanagata, N.; Ikoma, T.; Huang, J.Y.; Li, K.Y.; Lin, C.P.; Lin, F.H. Hafnium-doped hydroxyapatite nanoparticles with ionizing radiation for lung cancer treatment. Acta Biomater. 2016, 37, 165–173. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar]
- Mroz, P.; Yaroslavsky, A.; Kharkwal, G.B.; Hamblin, M.R. Cell death pathways in photodynamic therapy of cancer. Cancers (Basel) 2011, 3, 2516–2539. [Google Scholar] [CrossRef]
- Yu, H.; Liu, N.; Wang, H.; Shang, Q.; Jiang, P.; Zhang, Y. Different responses of tumor and normal cells to low-dose radiation. Contemp. Oncol. (Pozn) 2013, 17, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012, 2012, 137289. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxidative Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- De Marchi, E.; Bonora, M.; Giorgi, C.; Pinton, P. The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium. 2014, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kwong, J.Q.; Molkentin, J.D. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. 2015, 21, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Bratton, S.B.; Salvesen, G.S. Regulation of the Apaf-1-caspase-9 apoptosome. J. Cell Sci. 2010, 123, 3209–3214. [Google Scholar] [CrossRef]
- Liu, S.W.; Weng, C.S.; Wang, W.J.; Liu, Y.H.; Wu, V.C.; Wang, M.C. BIOCOMPATIBILITY EVALUATION OF DRUG RELEASING ABSORBABLE VASCULAR STENTS. Biomed. Eng. Appl. Basis Commun. 2018, 30, 1850035. [Google Scholar] [CrossRef]

| Parameter | Unit | Standard | TiO2:C-X-ray |
|---|---|---|---|
| BUN | mg/dL | 39.15 ± 12.16 | 30.81 |
| CREA | mg/dL | <0.2 (0.1) | <0.2 (0.1) |
| ALT(SGPT) | U/L | 33.5 ± 2.12 | 30.00 |
| AST(SGOT) | U/L | 80 ± 13.22 | 71.50 |
| ALB | g/L | 28.3 ± 3.89 | 25.55 |
| Ca | mmol/L | 2.23 ± 0.11 | 2.29 |
| IP | mg/dL | 6.29 ± 2.22 | 6.70 |
| Parameter | Unit | Standard | TiO2:C-X-ray |
|---|---|---|---|
| RBC | M/μL | 8.64 ± 0.48 | 8.46 |
| HGB | g/dL | 13.5 ± 0.69 | 13.20 |
| HCT | % | 41.3 ± 2.23 | 41.50 |
| MCV | fL | 45.65 ± 3.04 | 47.53 |
| MCH | pg | 14.31 ± 1.86 | 15.55 |
| MCHC | g/dL | 31.34 ± 1.9 | 31.72 |
| WBC | K/μL | 2.81 ± 1.49 | 2.30 |
| NEUT | K/μL | 2.14 ± 0.32 | 1.55 |
| LYMPH | K/μL | 1.62 ± 0.44 | 1.20 |
| MONO | K/μL | 0.13 ± 0.02 | 0.24 |
| PLT | K/μL | 767 ± 208.75 | 780.00 |
| MPV | fL | 6.1 ± 0.43 | 6.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-C.; Tsai, M.-H.; Li, K.-Y.; Hou, C.-H.; Lin, F.-H. Carbon-Doped TiO2 Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment. Int. J. Mol. Sci. 2019, 20, 2072. https://doi.org/10.3390/ijms20092072
Yang C-C, Tsai M-H, Li K-Y, Hou C-H, Lin F-H. Carbon-Doped TiO2 Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment. International Journal of Molecular Sciences. 2019; 20(9):2072. https://doi.org/10.3390/ijms20092072
Chicago/Turabian StyleYang, Chun-Chen, Min-Hsiung Tsai, Keng-Yuan Li, Chun-Han Hou, and Feng-Huei Lin. 2019. "Carbon-Doped TiO2 Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment" International Journal of Molecular Sciences 20, no. 9: 2072. https://doi.org/10.3390/ijms20092072
APA StyleYang, C.-C., Tsai, M.-H., Li, K.-Y., Hou, C.-H., & Lin, F.-H. (2019). Carbon-Doped TiO2 Activated by X-Ray Irradiation for the Generation of Reactive Oxygen Species to Enhance Photodynamic Therapy in Tumor Treatment. International Journal of Molecular Sciences, 20(9), 2072. https://doi.org/10.3390/ijms20092072






