Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link?
Abstract
1. Introduction
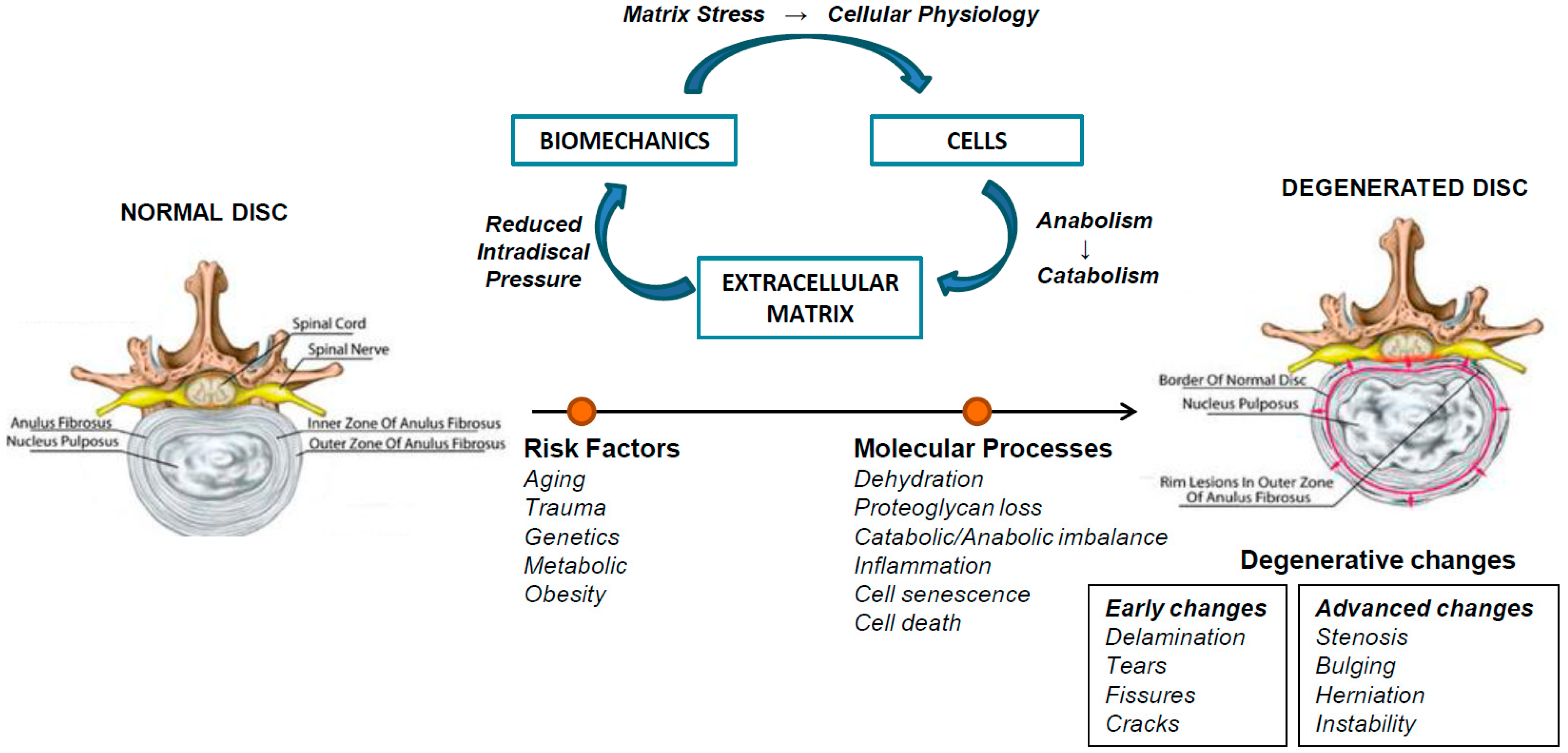
2. Intervertebral Disc Degeneration (IVDD)
3. Inflammation in IVDD
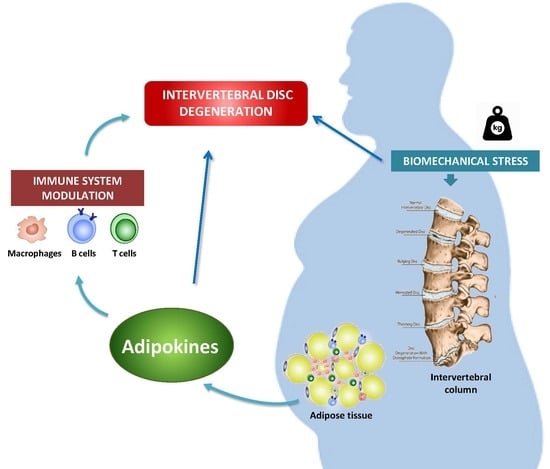
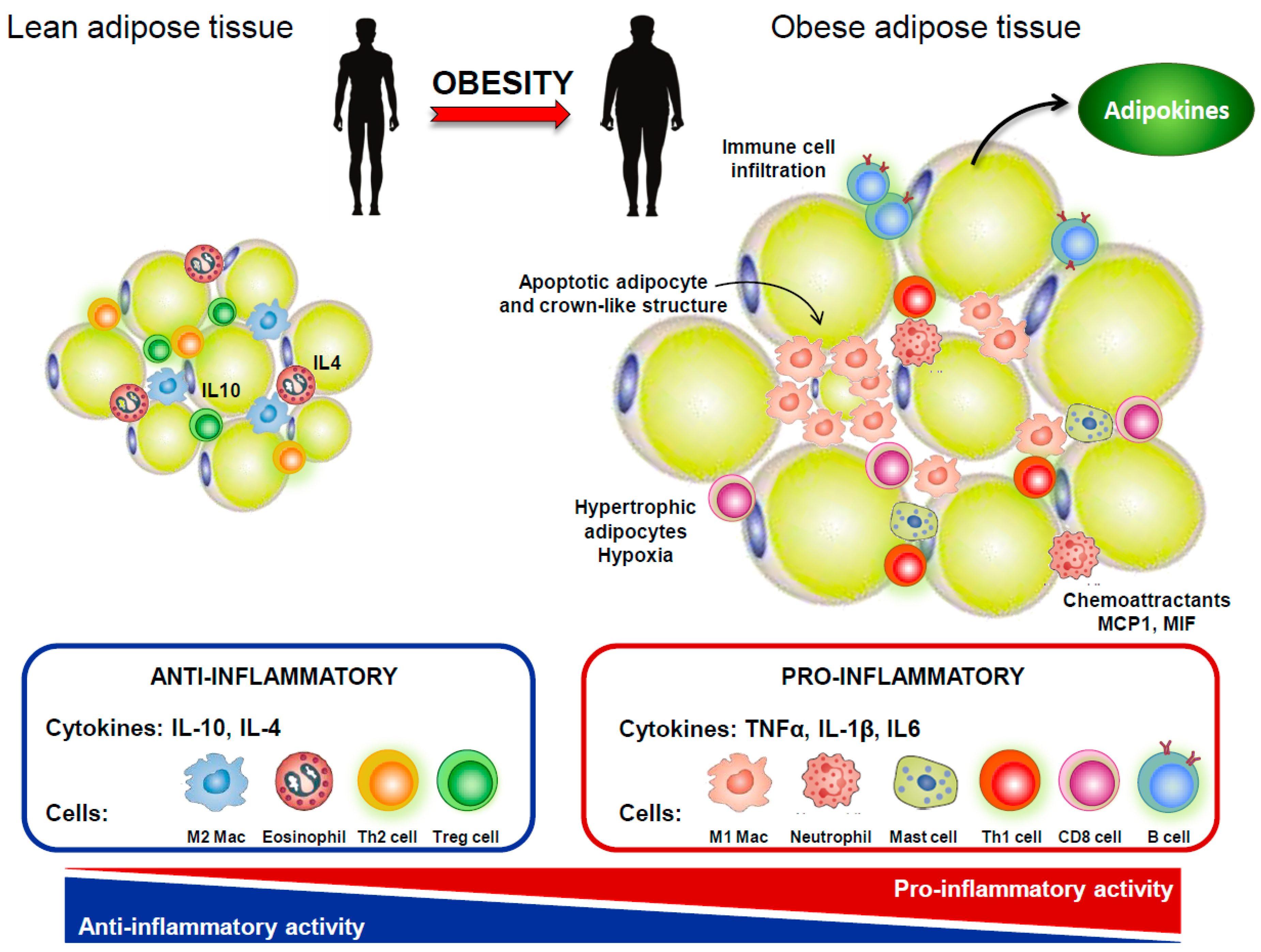
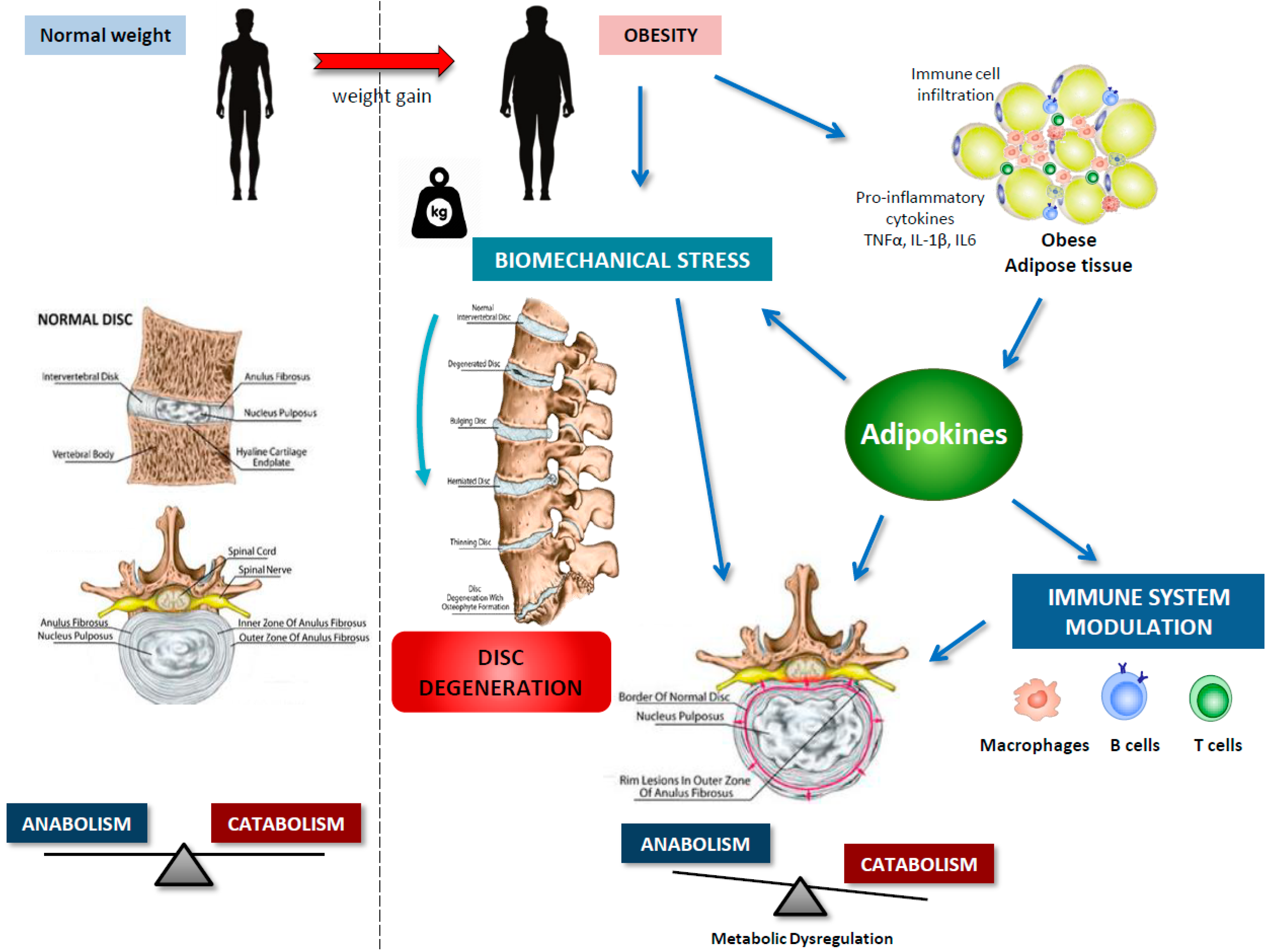
4. IVDD and Obesity
5. Adipokines in IVDD
5.1. Leptin
5.2. Adiponectin
5.3. Resistin
5.4. Progranulin
5.5. Visfatin
5.6. Lipocalin-2
5.7. Ghrelin
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IVDD | Intervertebral disc (IVD) degeneration |
| OA | Osteoarthritis |
| RA | Rheumatoid arthritis |
| IL | Interleukin |
| TNF | Tumor necrosis factor |
| NP | Nucleus pulposus |
| AF | Annulus fibrosus |
| CEP | Cartilaginous end plate |
| ECM | Extracellular matrix |
| MMP | Matrix metalloprotease |
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| NOS | Nitric oxide (NO) synthase |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription |
| ERK | Extracellular-signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen Activated Protein Kinase |
| PI3K | Phosphoinositide 3-kinase |
| LEPR | Leptin receptor |
| PGRN | Progranulin |
| TNFR | TNF-α receptor |
| LCN2 | Lipocalin-2 |
References
- Vergroesen, P.-P.; Kingma, I.; Emanuel, K.S.; Hoogendoorn, R.J.; Welting, T.J.; Van Royen, B.J.; Van Dieën, J.H. Mechanics and biology in intervertebral disc degeneration: A vicious circle. Osteoarthr. Cartil. 2015, 23, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Sampara, P.; Banala, R.R.; Vemuri, S.K.; Av, G.R.; Gpv, S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: A review. Gene Ther. 2018, 25, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Kadow, T.; Sowa, G.; Vo, N.; Kang, J.D. Molecular Basis of Intervertebral Disc Degeneration and Herniations: What Are the Important Translational Questions? Clin. Orthop. Relat. Res. 2015, 473, 1903–1912. [Google Scholar] [CrossRef] [PubMed]
- Samartzis, D.; Karppinen, J.; Chan, D.; Luk, K.D.K.; Cheung, K.M.C. The association of lumbar intervertebral disc degeneration on magnetic resonance imaging with body mass index in overweight and obese adults: A population-based study. Arthritis Rheum. 2012, 64, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Potter, V.J. Inflammation and macrophage modulation in adipose tissues. Cell. Microbiol. 2014, 16, 1484–1492. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaimi, E.A.; Shehzad, A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. Eur. J. Med. Res. 2013, 18, 12. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Pérez, T.; Pino, J.; López, V.; Franco, E.; Alonso, A.; Gonzalez-Gay, M.A.; Mera, A.; Lago, F.; Gómez, R.; et al. Biomechanics, obesity, and osteoarthritis. The role of adipokines: When the levee breaks. J. Orthop. Res. 2017, 36, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Pino, J.; Gonzalez-Gay, M.A.; Mera, A.; Lago, F.; Gómez, R.; Mobasheri, A.; Gualillo, O. Adipokines and inflammation: Is it a question of weight? Br. J. Pharmacol. 2018, 175, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Johnson, Z.I.; Schoepflin, Z.R.; Choi, H.; Shapiro, I.M.; Risbud, M.V. Disc in flames: Roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur. Cells Mater. 2015, 30, 104–117. [Google Scholar] [CrossRef]
- Gruber, H.E.; Ingram, J.A.; Hoelscher, G.L.; Hanley, E.N. Leptin expression by annulus cells in the human intervertebral disc. Spine J. 2007, 7, 437–443. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Liu, D.; Li, H.; Jiang, L.S.; Dai, L.Y. Expression of leptin and its functional receptor on disc cells: Contribution to cell proliferation. Spine (Phila. Pa. 1976) 2008, 33, 858–864. [Google Scholar] [CrossRef]
- Daniels, J.; Binch, A.A.L.; Le Maitre, C.L. Inhibiting IL-1 signaling pathways to inhibit catabolic processes in disc degeneration. J. Orthop. Res. 2017, 35, 74–85. [Google Scholar] [CrossRef]
- De Geer, C.M. Cytokine Involvement in Biological Inflammation Related to Degenerative Disorders of the Intervertebral Disk: A Narrative Review. J. Chiropr. Med. 2018, 17, 54–62. [Google Scholar] [CrossRef]
- Kubaszewski, Ł.; Zioła-Frankowska, A.; Frankowski, M.; Rogala, P.; Gasik, Z.; Kaczmarczyk, J.; Nowakowski, A.; Dabrowski, M.; Labedz, W.; Miękisiak, G.; et al. Comparison of trace element concentration in bone and intervertebral disc tissue by atomic absorption spectrometry techniques. J. Orthop. Surg. Res. 2014, 9, 99. [Google Scholar] [CrossRef]
- Martirosyan, N.L.; Patel, A.A.; Carotenuto, A.; Kalani, M.Y.S.; Belykh, E.; Walker, C.T.; Preul, M.C.; Theodore, N. Genetic Alterations in Intervertebral Disc Disease. Front. Surg. 2016, 3. [Google Scholar] [CrossRef]
- Le Maitre, C.; Freemont, A.J.; Hoyland, J.; Luoma, K.; Riihimaki, H.; Luukkonen, R.; Raininko, R.; Viikari-Juntura, E.; Lamminen, A.; Freemont, A.; et al. The role of interleukin-1 in the pathogenesis of human Intervertebral disc degeneration. Arthritis Res. Ther. 2005, 7, R732. [Google Scholar] [CrossRef]
- Phillips, K.L.E.; Jordan-Mahy, N.; Nicklin, M.J.H.; Le Maitre, C.L. Interleukin-1 receptor antagonist deficient mice provide insights into pathogenesis of human intervertebral disc degeneration. Ann. Rheum. Dis. 2013, 72, 1860–1867. [Google Scholar] [CrossRef]
- Hoyland, J.A.; Le Maitre, C.; Freemont, A.J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008, 47, 809–814. [Google Scholar] [CrossRef]
- Sun, Z.; Yin, Z.; Liu, C.; Liang, H.; Jiang, M.; Tian, J. IL-1β promotes ADAMTS enzyme-mediated aggrecan degradation through NF-κB in human intervertebral disc. J. Orthop. Surg. Res. 2015, 10. [Google Scholar] [CrossRef]
- Hu, B.; Shi, C.; Xu, C.; Cao, P.; Tian, Y.; Zhang, Y.; Deng, L.; Chen, H.; Yuan, W. Heme oxygenase-1 attenuates IL-1β induced alteration of anabolic and catabolic activities in intervertebral disc degeneration. Sci. Rep. 2016, 6, 21190. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Hlavna, M.; Tahmasseb, J.A.; Zvick, J.; Kunz, D.; Ito, K.; Ferguson, S.J.; Wuertz-Kozak, K. An inflammatory nucleus pulposus tissue culture model to test molecular regenerative therapies: Validation with epigallocatechin 3-gallate. Int. J. Mol. Sci. 2016, 17, 1640. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, Y.; Phillips, K.L.E.; Chiverton, N.; Haddock, G.; Bunning, R.A.; Cross, A.K.; Shapiro, I.M.; Le Maitre, C.L.; Risbud, M.V. Tumor necrosis factor α- And interleukin-1β-dependent induction of CCL3 expression by nucleus pulposus cells promotes macrophage migration through CCR1. Arthritis Rheum. 2013, 65, 832–842. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Yao, J.; Ji, G.; Qian, L.; Wang, J.; Zhang, G.; Tian, J.; Nie, Y.; Zhang, Y.E.; et al. Obesity: Pathophysiology and intervention. Nutrients 2014, 6, 5153–5183. [Google Scholar] [CrossRef] [PubMed]
- Liuke, M.; Solovieva, S.; Lamminen, A.; Luoma, K.; Leino-Arjas, P.; Luukkonen, R.; Riihimäki, H. Disc degeneration of the lumbar spine in relation to overweight. Int. J. Obes. 2005, 29, 903–908. [Google Scholar] [CrossRef]
- Dowdell, J.; Erwin, M.; Choma, T.; Vaccaro, A.; Iatridis, J.; Cho, S.K. Intervertebral disk degeneration and repair. Clin. Neurosurg. 2017, 80, S46–S54. [Google Scholar] [CrossRef] [PubMed]
- Dario, A.B.; Ferreira, M.L.; Refshauge, K.M.; Lima, T.S.; Ordoñana, J.R.; Ferreira, P.H. The relationship between obesity, low back pain, and lumbar disc degeneration when genetics and the environment are considered: A systematic review of twin studies. Spine J. 2015, 15, 1106–1117. [Google Scholar] [CrossRef]
- Videman, T.; Gibbons, L.E.; Kaprio, J.; Battié, M.C. Challenging the cumulative injury model: Positive effects of greater body mass on disc degeneration. Spine J. 2010, 10, 26–31. [Google Scholar] [CrossRef]
- Sharma, A. The Role of Adipokines in Intervertebral Disc Degeneration. Med. Sci. 2018, 6, 34. [Google Scholar] [CrossRef]
- Segar, A.H.; Fairbank, J.C.T.; Urban, J. Leptin and the intervertebral disc: A biochemical link exists between obesity, intervertebral disc degeneration and low back pain—an in vitro study in a bovine model. Eur. Spine J. 2019, 28, 214–223. [Google Scholar] [CrossRef]
- Huh, J.Y.; Park, Y.J.; Ham, M.; Kim, J.B. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Mol Cells 2014, 37, 365–371. [Google Scholar] [CrossRef]
- Vrselja, Z.; Curic, G. Vertebral marrow adipose tissue adipokines as a possible cause of intervertebral disc inflammation. Jt. Bone Spine 2018, 85, 143–146. [Google Scholar] [CrossRef]
- Francisco, V.; Pino, J.; Campos-Cabaleiro, V.; Ruiz-Fernández, C.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Gualillo, O. Obesity, fat mass and immune system: Role for leptin. Front. Physiol. 2018, 9, 640. [Google Scholar] [CrossRef]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2014, 7, 207–222. [Google Scholar] [CrossRef]
- Koerner, J.D.; Markova, D.Z.; Yadla, S.; Mendelis, J.; Hilibrand, A.; Vaccaro, A.R.; Risbud, M.V.; Albert, T.J.; Anderson, D.G.; Kepler, C.K. Differential Gene Expression in Anterior and Posterior Annulus Fibrosus. Spine (Phila. Pa. 1976) 2014, 39, 1917–1923. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Wu, W.K.K.; Yu, X.; Liang, J.; Qiu, G.; Liu, J. Leptin Induces Cyclin D1 Expression and Proliferation of Human Nucleus Pulposus Cells via JAK/STAT, PI3K/Akt and MEK/ERK Pathways. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Li, Z.; Shen, J.; Wu, W.K.K.; Yu, X.; Liang, J.; Qiu, G.; Liu, J. The role of leptin on the organization and expression of cytoskeleton elements in nucleus pulposus cells. J. Orthop. Res. 2013, 31, 847–857. [Google Scholar] [CrossRef]
- Li, Z.; Liang, J.; Wu, W.K.K.; Yu, X.; Yu, J.; Weng, X.; Shen, J. Leptin activates RhoA/ROCK pathway to induce cytoskeleton remodeling in nucleus pulposus cells. Int. J. Mol. Sci. 2014, 15, 1176–1188. [Google Scholar] [CrossRef]
- Miao, D.; Zhang, L. Leptin modulates the expression of catabolic genes in rat nucleus pulposus cells through the mitogen-activated protein kinase and Janus kinase 2/signal transducer and activator of transcription 3 pathways. Mol. Med. Rep. 2015, 12, 1761–1768. [Google Scholar] [CrossRef]
- Li, Z.; Yu, X.; Liang, J.; Ka, W.; Wu, K.; Yu, J.; Shen, J.; Li, Z.; Liang, Y.X.; Wkk, W.; et al. Leptin Downregulates Aggrecan through the p38-ADAMST Pathway in Human Nucleus Pulposus Cells. PLoS ONE 2014, 9, e109595. [Google Scholar] [CrossRef]
- Han, Y.C.; Ma, B.; Guo, S.; Yang, M.; Li, L.J.; Wang, S.J.; Tan, J. Leptin regulates disc cartilage endplate degeneration and ossification through activation of the MAPK-ERK signalling pathway in vivo and in vitro. J. Cell. Mol. Med. 2018, 22, 2098–2109. [Google Scholar] [CrossRef]
- Ding, W.; Zhao, C.; Cao, L.; Zhang, K.; Sun, W.; Xie, Y.; Li, H.; Zhao, J. Leptin induces terminal differentiation of rat annulus fibrosus cells via activation of MAPK signaling. Anat. Rec. 2013, 296, 1806–1812. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Tian, J.-W.; Wang, Y.-H. Leptin-induced inflammation by activating IL-6 expression contributes to the fibrosis and hypertrophy of ligamentum flavum in lumbar spinal canal stenosis. Biosci. Rep. 2018, 38, 20171214. [Google Scholar] [CrossRef]
- Sun, Y.; Xun, K.; Wang, C.; Zhao, H.; Bi, H.; Chen, X.; Wang, Y. Adiponectin, an unlocking adipocytokine. Cardiovasc. Ther. 2009, 27, 59–75. [Google Scholar] [CrossRef]
- Liu, M.; Liu, F. Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Khabour, O.F.; Abu-Rumeh, L.; Al-Jarrah, M.; Jamous, M.; Alhashimi, F. Association of adiponectin protein and ADIPOQ gene variants with lumbar disc degeneration. Exp. Ther. Med. 2014, 8, 1340–1344. [Google Scholar] [CrossRef]
- Yuan, B.; Huang, L.; Yan, M.; Zhang, S.; Zhang, Y.; Jin, B.; Ma, Y.; Luo, Z. Adiponectin Downregulates TNF-α Expression in Degenerated Intervertebral Discs. Spine (Phila. Pa. 1976) 2018, 43, E381–E389. [Google Scholar] [CrossRef]
- Terashima, Y.; Kakutani, K.; Yurube, T.; Takada, T.; Maeno, K.; Hirata, H.; Miyazaki, S.; Ito, M.; Kakiuchi, Y.; Takeoka, Y.; et al. Expression of adiponectin receptors in human and rat intervertebral disc cells and changes in receptor expression during disc degeneration using a rat tail temporary static compression model. J. Orthop. Surg. Res. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Tarkowski, A.; Bjersing, J.; Shestakov, A.; Bokarewa, M.I. Resistin competes with lipopolysaccharide for binding to toll-like receptor 4. J. Cell. Mol. Med. 2010, 14, 1419–1431. [Google Scholar] [CrossRef]
- De Boer, T.N.; van Spil, W.E.; Huisman, A.M.; Polak, A.A.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; Mastbergen, S.C. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr. Cartil. 2012, 20, 846–853. [Google Scholar] [CrossRef]
- Presle, N.; Pottie, P.; Dumond, H.; Guillaume, C.; Lapicque, F.; Pallu, S.; Mainard, D.; Netter, P.; Terlain, B. Differential distribution of adipokines between serum and synovial fluid in patients with osteoarthritis. Contribution of joint tissues to their articular production. Osteoarthr. Cartil. 2006, 14, 690–695. [Google Scholar] [CrossRef]
- Fang, W.Q.; Zhang, Q.; Peng, Y.B.; Chen, M.; Lin, X.P.; Wu, J.H.; Cai, C.H.; Mei, Y.F.; Jin, H. Resistin level is positively correlated with thrombotic complications in Southern Chinese metabolic syndrome patients. J. Endocrinol. Invest. 2011, 34, e36–e42. [Google Scholar] [CrossRef]
- Tarkowski, A.; Bokarewa, M.; Nagaev, I.; Dahlberg, L.; Smith, U. Proinflammatory Properties Resistin, an Adipokine with Potent. J. Immunol. Ref. 2005, 174, 5789–5795. [Google Scholar]
- Su, C.-M.; Hsu, C.-J.; Tsai, C.-H.; Huang, C.-Y.; Wang, S.-W.; Tang, C.-H. Resistin Promotes Angiogenesis in Endothelial Progenitor Cells Through Inhibition of MicroRNA206: Potential Implications for Rheumatoid Arthritis. Stem Cells 2015, 33, 2243–2255. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Pan, H.; Yang, H.; Li, X.; Zhang, K.; Wang, H.; Zheng, Z.; Liu, H.; Wang, J. Resistin promotes CCL4 expression through toll-like receptor-4 and activation of the p38-MAPK and NF-κB signaling pathways: Implications for intervertebral disc degeneration. Osteoarthr. Cartil. 2017, 25, 341–350. [Google Scholar] [CrossRef]
- Liu, C.; Yang, H.; Gao, F.; Li, X.; An, Y.; Wang, J.; Jin, A. Resistin promotes intervertebral disc degeneration by upregulation of ADAMTS-5 through p38 MAPK signaling pathway. Spine (Phila. Pa. 1976) 2016, 41, 1414–1420. [Google Scholar] [CrossRef]
- Rajan, N.E.; Bloom, O.; Maidhof, R.; Stetson, N.; Sherry, B.; Levine, M.; Chahine, N.O. Toll-Like Receptor 4 (TLR4) expression and stimulation in a model of intervertebral disc inflammation and degeneration. Spine (Phila. Pa. 1976) 2013, 38, 1343–1351. [Google Scholar] [CrossRef]
- Wei, J.; Hettinghouse, A.; Liu, C. The role of progranulin in arthritis. Ann. N. Y. Acad. Sci. 2016, 1383, 5–20. [Google Scholar] [CrossRef]
- Jian, J.; Li, G.; Hettinghouse, A.; Liu, C. Progranulin: A key player in autoimmune diseases. Cytokine 2018, 101, 48–55. [Google Scholar] [CrossRef]
- Wang, S.; Wei, J.; Fan, Y.; Ding, H.; Tian, H.; Zhou, X.; Cheng, L. Progranulin Is Positively Associated with Intervertebral Disc Degeneration by Interaction with IL-10 and IL-17 Through TNF Pathways. Inflammation 2018, 41, 1852–1863. [Google Scholar] [CrossRef]
- Naphade, S.B.; Kigerl, K.A.; Jakeman, L.B.; Kostyk, S.K.; Popovich, P.G.; Kuret, J. Progranulin expression is upregulated after spinal contusion in mice. Acta Neuropathol. 2010, 119, 123–133. [Google Scholar] [CrossRef]
- Zhao, Y.P.; Tian, Q.Y.; Liu, B.; Cuellar, J.; Richbourgh, B.; Jia, T.H.; Liu, C.J. Progranulin Knockout Accelerates Intervertebral Disc Degeneration in Aging Mice. Sci. Rep. 2015, 5, 9102. [Google Scholar] [CrossRef]
- Ding, H.; Wei, J.; Zhao, Y.; Liu, Y.; Liu, L.; Cheng, L. Progranulin derived engineered protein Atsttrin suppresses TNF-α-mediated inflammation in intervertebral disc degenerative disease. Oncotarget 2017, 8, 109692–109702. [Google Scholar] [CrossRef]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; McNiece, I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol. Cell. Biol. 1994, 14, 1431–1437. [Google Scholar] [CrossRef]
- Shi, C.; Wu, H.; Du, D.; Im, H.J.; Zhang, Y.; Hu, B.; Chen, H.; Wang, X.; Liu, Y.; Cao, P.; et al. Nicotinamide Phosphoribosyltransferase Inhibitor APO866 Prevents IL-1β-Induced Human Nucleus Pulposus Cell Degeneration via Autophagy. Cell. Physiol. Biochem. 2018, 49, 2463–2482. [Google Scholar] [CrossRef]
- Kao, T.-H.; Peng, Y.-J.; Salter, D.M.; Lee, H.-S.; Kao, T.-H.; Lee, H.-S.; Peng, Y.-J.; Salter, D.M. Nerve growth factor increases MMP9 activity in annulus fibrosus cells by upregulating lipocalin 2 expression. Eur. Spine J. 2015, 24, 1959–1968. [Google Scholar] [CrossRef]
- Kao, T.-H.; Peng, Y.-J.; Tsou, H.-K.; Salter, D.M.; Lee, H.-S. Nerve growth factor promotes expression of novel genes in intervertebral disc cells that regulate tissue degradation. J. Neurosurg. Spine 2014, 21, 653–661. [Google Scholar] [CrossRef]
- Lorenzi, T.; Meli, R.; Marzioni, D.; Morroni, M.; Baragli, A.; Castellucci, M.; Gualillo, O.; Muccioli, G. Ghrelin: A metabolic signal affecting the reproductive system. Cytokine Growth Factor Rev. 2009, 20, 137–152. [Google Scholar] [CrossRef]
- Colldén, G.; Tschöp, M.H.; Müller, T.D. Molecular Sciences Therapeutic Potential of Targeting the Ghrelin Pathway. Int. J. Mol. Sci. 2017, 18, 798. [Google Scholar] [CrossRef]
- Caminos, J.E.; Gualillo, O.; Lago, F.; Otero, M.; Blanco, M.; Gallego, R.; Garcia-Caballero, T.; Goldring, M.B.; Casanueva, F.F.; Gomez-Reino, J.J.; et al. The endogenous growth hormone secretagogue (ghrelin) is synthesized and secreted by chondrocytes. Endocrinology 2005, 146, 1285–1292. [Google Scholar] [CrossRef]
- Pereira, J.A.D.S.; da Silva, F.C.; de Moraes-Vieira, P.M.M. The Impact of Ghrelin in Metabolic Diseases: An Immune Perspective. J. Diabetes Res. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Li, W.; Wu, X.; Qu, R.; Wang, W.; Chen, X.; Cheng, L. Ghrelin protects against nucleus pulposus degeneration through inhibition of NF-κB signaling pathway and activation of Akt signaling pathway. Oncotarget 2017, 8, 91887–91901. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Fernández, C.; Francisco, V.; Pino, J.; Mera, A.; González-Gay, M.A.; Gómez, R.; Lago, F.; Gualillo, O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? Int. J. Mol. Sci. 2019, 20, 2030. https://doi.org/10.3390/ijms20082030
Ruiz-Fernández C, Francisco V, Pino J, Mera A, González-Gay MA, Gómez R, Lago F, Gualillo O. Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? International Journal of Molecular Sciences. 2019; 20(8):2030. https://doi.org/10.3390/ijms20082030
Chicago/Turabian StyleRuiz-Fernández, Clara, Vera Francisco, Jesus Pino, Antonio Mera, Miguel Angel González-Gay, Rodolfo Gómez, Francisca Lago, and Oreste Gualillo. 2019. "Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link?" International Journal of Molecular Sciences 20, no. 8: 2030. https://doi.org/10.3390/ijms20082030
APA StyleRuiz-Fernández, C., Francisco, V., Pino, J., Mera, A., González-Gay, M. A., Gómez, R., Lago, F., & Gualillo, O. (2019). Molecular Relationships among Obesity, Inflammation and Intervertebral Disc Degeneration: Are Adipokines the Common Link? International Journal of Molecular Sciences, 20(8), 2030. https://doi.org/10.3390/ijms20082030