Defining the Genetic Basis of Plant–Endophytic Bacteria Interactions
Abstract
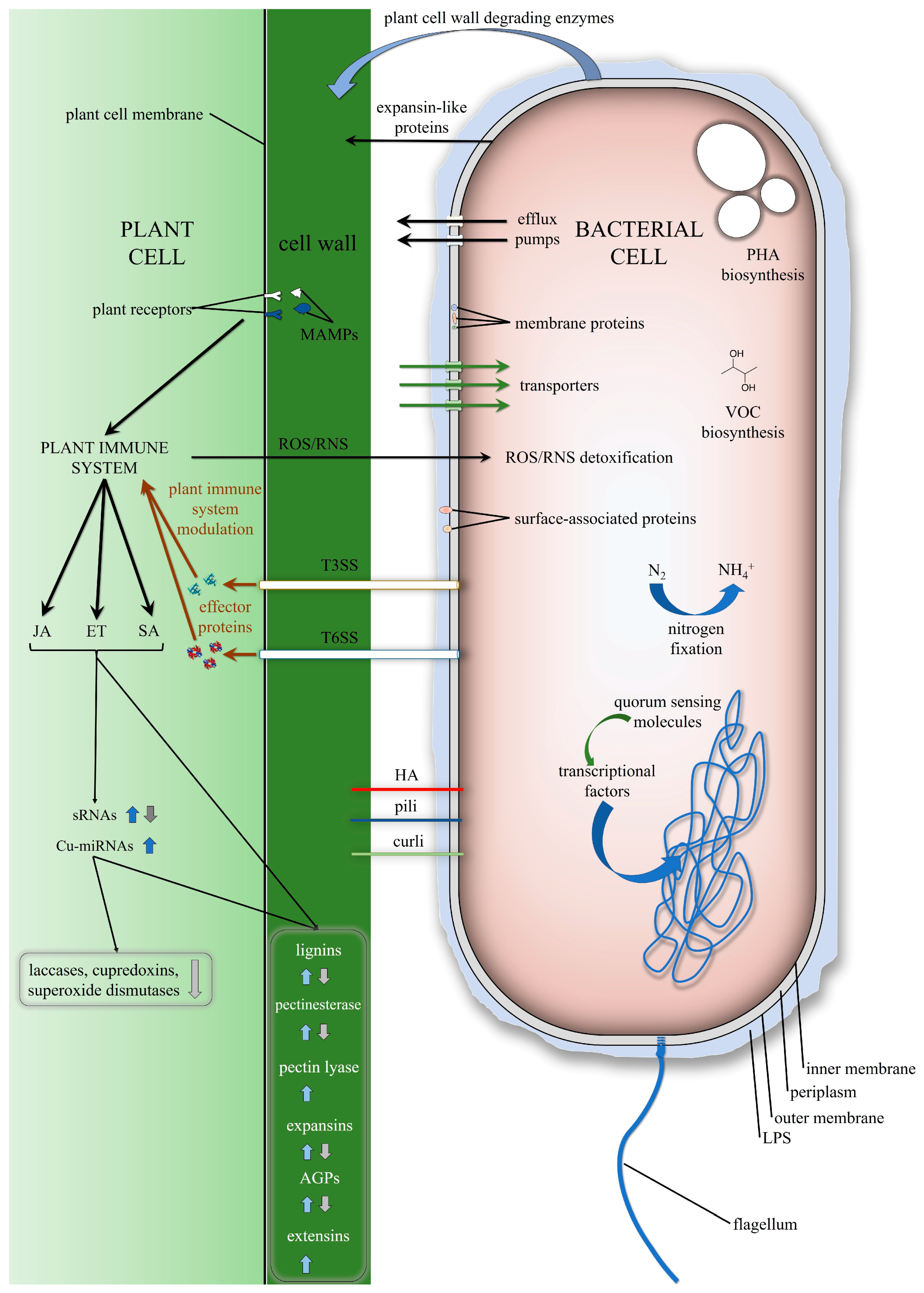
1. Introduction
2. Genetic Features of the Endophytic Bacteria That Are Involved in Interactions with Plants
2.1. Chemotaxis, Motility
2.2. Adhesion, Biofilm Production
2.3. Lipopolysaccharide, Membrane Proteins
2.4. Plant Cell Wall Modifications
2.5. Substrate Utilisation, Transport
2.6. Stress Protection
2.7. Bacterial Secretion Systems
2.8. Transcriptional Regulators, Sensor Proteins
3. Plant Genetic Features that are Involved in Interactions with Endophytic Bacteria
3.1. Plant Receptors
3.2. Plant Hormone-Signalling Pathways
3.3. Small RNAs
3.4. Cell Growth, Expansion
3.5. Lignin Biosynthesis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.; Schenk, P.M. Inner plant values: Diversity, colonization and benefits from endophytic bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef] [PubMed]
- Bécard, G. How Plants Communicate with Their Biotic Environment; Academic Press: New York, NY, USA, 2017; Volume 82. [Google Scholar]
- Compant, S.; Reiter, B.; Sessitsch, A.; Nowak, J.; Clément, C.; Barka, E.A. Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl. Environ. Microbiol. 2005, 71, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.; Joubert, P.; Doty, S. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Chen, L.; Xin, H.L.; Zheng, C.J.; Rahman, K.; Han, T.; Qin, L.P. A friendly relationship between endophytic fungi and medicinal plants: A systematic review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Khan, A.L.; Bilal, S.; Asaf, S.; Lee, I.-J. What is there in seeds? Vertically transmitted endophytic resources for sustainable improvement in plant growth. Front. Plant Sci. 2018, 9, 24. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Compant, S.; Campisano, A.; Doring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Gagne-Bourque, F.; Mayer, B.F.; Charron, J.B.; Vali, H.; Bertrand, A.; Jabaji, S. Accelerated growth rate and increased drought stress resilience of the model grass Brachypodium distachyon colonized by Bacillus subtilis B26. PLoS ONE 2015, 10, e0130456. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Khan, Q.M.; Sessitsch, A. Endophytic bacteria: Prospects and applications for the phytoremediation of organic pollutants. Chemosphere 2014, 117, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Bell, T.H.; Joly, S.; Pitre, F.E.; Yergeau, E. Increasing phytoremediation efficiency and reliability using novel omics approaches. Trends Biotechnol. 2014, 32, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.; Sherrier, J. Plant microbe interactions; Academic Press: New York, NY, USA, 2015; Volume 75. [Google Scholar]
- Schikora, A. Plant-microbe interactions in the rhizosphere; Caister Academic Press: Norfolk, UK, 2018. [Google Scholar]
- Kaul, S.; Sharma, T.; M, K.D. “Omics” tools for better understanding the plant-endophyte interactions. Front. Plant Sci. 2016, 7, 955. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Gonzalez, I.S.; Mittelviefhaus, M.; Clingenpeel, S.; Paredes, S.H.; Miao, J.; Wang, K.; Devescovi, G.; Stillman, K.; Monteiro, F. Genomic features of bacterial adaptation to plants. Nat. Genet. 2018, 50, 138. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Hardoim, C.C.P. Genomic features of mutualistic plant bacteria. In Endophytes: Biology and Biotechnology; Springer: New York, NY, USA, 2017; pp. 99–125. [Google Scholar]
- Scharf, B.E.; Hynes, M.F.; Alexandre, G.M. Chemotaxis signaling systems in model beneficial plant-bacteria associations. Plant Mol. Biol. 2016, 90, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Balsanelli, E.; Tadra-Sfeir, M.Z.; Faoro, H.; Pankievicz, V.C.; de Baura, V.A.; Pedrosa, F.O.; de Souza, E.M.; Dixon, R.; Monteiro, R.A. Molecular adaptations of Herbaspirillum seropedicae during colonization of the maize rhizosphere. Environ. Microbiol. 2016, 18, 2343–2356. [Google Scholar] [CrossRef] [PubMed]
- Greer-Phillips, S.E.; Stephens, B.B.; Alexandre, G. An energy taxis transducer promotes root colonization by Azospirillum brasilense. J. Bacteriol. 2004, 186, 6595–6604. [Google Scholar] [CrossRef]
- Buschart, A.; Sachs, S.; Chen, X.; Herglotz, J.; Krause, A.; Reinhold-Hurek, B. Flagella mediate endophytic competence rather than act as MAMPS in rice–Azoarcus sp. strain BH72 interactions. Mol. Plant Microbe Interact. 2012, 25, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Croes, C.L.; Moens, S.; van Bastelaere, E.; Vanderleyden, J.; Michiels, K.W. The polar flagellum mediates Azospirillum brasilense adsorption to wheat roots. Microbiology 1993, 139, 2261–2269. [Google Scholar] [CrossRef]
- Shidore, T.; Dinse, T.; Ohrlein, J.; Becker, A.; Reinhold-Hurek, B. Transcriptomic analysis of responses to exudates reveal genes required for rhizosphere competence of the endophyte Azoarcus sp. strain BH72. Environ. Microbiol. 2012, 14, 2775–2787. [Google Scholar] [CrossRef]
- Böhm, M.; Hurek, T.; Reinhold-Hurek, B. Twitching motility is essential for endophytic rice colonization by the N2-fixing endophyte Azoarcus sp. strain BH72. Mol. Plant Microbe Interact. 2007, 20, 526–533. [Google Scholar] [CrossRef]
- Pankievicz, V.C.; Camilios-Neto, D.; Bonato, P.; Balsanelli, E.; Tadra-Sfeir, M.Z.; Faoro, H.; Chubatsu, L.S.; Donatti, L.; Wajnberg, G.; Passetti, F.; et al. RNA-seq transcriptional profiling of Herbaspirillum seropedicae colonizing wheat (Triticum aestivum) roots. Plant Mol. Biol. 2016, 90, 589–603. [Google Scholar] [CrossRef]
- Cole, B.J.; Feltcher, M.E.; Waters, R.J.; Wetmore, K.M.; Mucyn, T.S.; Ryan, E.M.; Wang, G.; Ul-Hasan, S.; McDonald, M.; Yoshikuni, Y.; et al. Genome-wide identification of bacterial plant colonization genes. PLoS Biol. 2017, 15, e2002860. [Google Scholar] [CrossRef]
- Zur, J.; Wojcieszynska, D.; Guzik, U. Metabolic responses of bacterial cells to immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef] [PubMed]
- Meneses, C.H.; Rouws, L.F.; Simões-Araújo, J.L.; Vidal, M.S.; Baldani, J.I. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte Gluconacetobacter diazotrophicus. Mol. Plant Microbe Interact. 2011, 24, 1448–1458. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Wilkinson, A.; Krehenbrink, M.; Russo, D.M.; Zorreguieta, A.; Downie, J.A. Glucomannan-mediated attachment of Rhizobium leguminosarum to pea root hairs is required for competitive nodule infection. J. Bacteriol. 2008, 190, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Barak, J.D.; Gorski, L.; Naraghi-Arani, P.; Charkowski, A.O. Salmonella enterica virulence genes are required for bacterial attachment to plant tissue. Appl. Environ. Microbiol. 2005, 71, 5685–5691. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Balsanelli, E.; Tuleski, T.; Faoro, H.; Cruz, L.M.; Wassem, R.; de Baura, V.A.; Tadra-Sfeir, M.Z.; Weiss, V.; DaRocha, W.D.; et al. Genomic comparison of the endophyte Herbaspirillum seropedicae SmR1 and the phytopathogen Herbaspirillum rubrisubalbicans M1 by suppressive subtractive hybridization and partial genome sequencing. FEMS Microbiol. Ecol. 2012, 80, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Barak, J.D.; Jahn, C.E.; Gibson, D.L.; Charkowski, A.O. The role of cellulose and O-antigen capsule in the colonization of plants by Salmonella enterica. Mol. Plant Microbe Interact. 2007, 20, 1083–1091. [Google Scholar] [CrossRef]
- Ainelo, H.; Lahesaare, A.; Teppo, A.; Kivisaar, M.; Teras, R. The promoter region of lapA and its transcriptional regulation by Fis in Pseudomonas putida. PLoS ONE 2017, 12, e0185482. [Google Scholar] [CrossRef]
- Martinez-Gil, M.; Yousef-Coronado, F.; Espinosa-Urgel, M. LapF, the second largest Pseudomonas putida protein, contributes to plant root colonization and determines biofilm architecture. Mol. Microbiol. 2010, 77, 549–561. [Google Scholar] [CrossRef]
- Barnhart, M.M.; Chapman, M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef]
- Taghavi, S.; van der Lelie, D.; Hoffman, A.; Zhang, Y.B.; Walla, M.D.; Vangronsveld, J.; Newman, L.; Monchy, S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. 638. PLoS Genet. 2010, 6, e1000943. [Google Scholar] [CrossRef]
- Mitter, B.; Petric, A.; Shin, M.W.; Chain, P.S.; Hauberg-Lotte, L.; Reinhold-Hurek, B.; Nowak, J.; Sessitsch, A. Comparative genome analysis of Burkholderia phytofirmans PsJN reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front. Plant Sci. 2013, 4, 120. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, F.O.; Monteiro, R.A.; Wassem, R.; Cruz, L.M.; Ayub, R.A.; Colauto, N.B.; Fernandez, M.A.; Fungaro, M.H.; Grisard, E.C.; Hungria, M.; et al. Genome of Herbaspirillum seropedicae strain SmR1, a specialized diazotrophic endophyte of tropical grasses. PLoS Genet. 2011, 7, e1002064. [Google Scholar] [CrossRef] [PubMed]
- Steimle, A.; Autenrieth, I.B.; Frick, J.S. Structure and function: Lipid A modifications in commensals and pathogens. Int. J. Med. Microbiol. 2016, 306, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Camilios-Neto, D.; Bonato, P.; Wassem, R.; Tadra-Sfeir, M.Z.; Brusamarello-Santos, L.C.; Valdameri, G.; Donatti, L.; Faoro, H.; Weiss, V.A.; Chubatsu, L.S. Dual RNA-seq transcriptional analysis of wheat roots colonized by Azospirillum brasilense reveals up-regulation of nutrient acquisition and cell cycle genes. BMC Genom. 2014, 15, 378. [Google Scholar] [CrossRef]
- Balsanelli, E.; Serrato, R.V.; de Baura, V.A.; Sassaki, G.; Yates, M.G.; Rigo, L.U.; Pedrosa, F.O.; de Souza, E.M.; Monteiro, R.A. Herbaspirillum seropedicae rfbB and rfbC genes are required for maize colonization. Environ. Microbiol. 2010, 12, 2233–2244. [Google Scholar] [CrossRef]
- Jofré, E.; Lagares, A.; Mori, G. Disruption of dTDP-rhamnose biosynthesis modifies lipopolysaccharide core, exopolysaccharide production, and root colonization in Azospirillum brasilense. FEMS Microbiol. Lett. 2004, 231, 267–275. [Google Scholar] [CrossRef]
- Balsanelli, E.; Tuleski, T.R.; de Baura, V.A.; Yates, M.G.; Chubatsu, L.S.; Pedrosa Fde, O.; de Souza, E.M.; Monteiro, R.A. Maize root lectins mediate the interaction with Herbaspirillum seropedicae via N-acetyl glucosamine residues of lipopolysaccharides. PLoS ONE 2013, 8, e77001. [Google Scholar] [CrossRef] [PubMed]
- De Weert, S.; Vermeiren, H.; Mulders, I.H.; Kuiper, I.; Hendrickx, N.; Bloemberg, G.V.; Vanderleyden, J.; De Mot, R.; Lugtenberg, B.J. Flagella-driven chemotaxis towards exudate components is an important trait for tomato root colonization by Pseudomonas fluorescens. Mol. Plant Microbe Interact. 2002, 15, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Romling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Tadra-Sfeir, M.Z.; Souza, E.M.; Faoro, H.; Muller-Santos, M.; Baura, V.A.; Tuleski, T.R.; Rigo, L.U.; Yates, M.G.; Wassem, R.; Pedrosa, F.O.; et al. Naringenin regulates expression of genes involved in cell wall synthesis in Herbaspirillum seropedicae. Appl. Environ. Microbiol. 2011, 77, 2180–2183. [Google Scholar] [CrossRef]
- Crespo, M.C.A.; Valverde, C. A single mutation in the oprF mRNA leader confers strict translational control by the Gac/Rsm system in Pseudomonas fluorescens CHA0. Curr. Microbiol. 2009, 58, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Reinhold-Hurek, B.; Maes, T.; Gemmer, S.; Van Montagu, M.; Hurek, T. An endoglucanase is involved in infection of rice roots by the not-cellulose-metabolizing endophyte Azoarcus sp. strain BH72. Mol. Plant Microbe Interact. 2006, 19, 181–188. [Google Scholar] [CrossRef]
- Fan, X.; Yang, R.; Qiu, S.; Cai, X.; Zou, H.; Hu, F. The endo-beta-1,4-glucanase of Bacillus amyloliquefaciens is required for optimum endophytic colonization of plants. J. Microbiol. Biotechnol. 2016, 26, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Kerff, F.; Amoroso, A.; Herman, R.; Sauvage, E.; Petrella, S.; Filée, P.; Charlier, P.; Joris, B.; Tabuchi, A.; Nikolaidis, N. Crystal structure and activity of Bacillus subtilis YoaJ (EXLX1), a bacterial expansin that promotes root colonization. Proc. Natl. Acad. Sci. USA 2008, 105, 16876–16881. [Google Scholar] [CrossRef] [PubMed]
- Kost, T.; Stopnisek, N.; Agnoli, K.; Eberl, L.; Weisskopf, L. Oxalotrophy, a widespread trait of plant-associated Burkholderia species, is involved in successful root colonization of lupin and maize by Burkholderia phytofirmans. Front. Microbiol. 2014, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.B.; Ampomah, O.Y.; Darrah, R.; Peters, N.K.; Bhuvaneswari, T. Role of trehalose transport and utilization in Sinorhizobium meliloti-alfalfa interactions. Mol. Plant Microbe Interact. 2005, 18, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Sy, A.; Timmers, A.C.; Knief, C.; Vorholt, J.A. Methylotrophic metabolism is advantageous for Methylobacterium extorquens during colonization of Medicago truncatula under competitive conditions. Appl. Environ. Microbiol. 2005, 71, 7245–7252. [Google Scholar] [CrossRef]
- Krause, A.; Bischoff, B.; Miché, L.; Battistoni, F.; Reinhold-Hurek, B. Exploring the function of alcohol dehydrogenases during the endophytic life of Azoarcus sp. strain BH72. Mol. Plant Microbe Interact. 2011, 24, 1325–1332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alqueres, S.; Meneses, C.; Rouws, L.; Rothballer, M.; Baldani, I.; Schmid, M.; Hartmann, A. The bacterial superoxide dismutase and glutathione reductase are crucial for endophytic colonization of rice roots by Gluconacetobacter diazotrophicus PAL5. Mol. Plant Microbe Interact. 2013, 26, 937–945. [Google Scholar] [CrossRef]
- Garcia-Leon, G.; Hernandez, A.; Hernando-Amado, S.; Alavi, P.; Berg, G.; Martinez, J.L. A function of SmeDEF, the major quinolone resistance determinant of Stenotrophomonas maltophilia, is the colonization of plant roots. Appl. Environ. Microbiol. 2014, 80, 4559–4565. [Google Scholar] [CrossRef]
- Yi, H.-S.; Ahn, Y.-R.; Song, G.C.; Ghim, S.-Y.; Lee, S.; Lee, G.; Ryu, C.-M. Impact of a bacterial volatile 2, 3-butanediol on Bacillus subtilis rhizosphere robustness. Front. Microbiol. 2016, 7, 993. [Google Scholar] [CrossRef] [PubMed]
- Press, C.; Loper, J.; Kloepper, J. Role of iron in rhizobacteria-mediated induced systemic resistance of cucumber. Phytopathology 2001, 91, 593–598. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Balsanelli, E.; Faoro, H.; Cruz, L.M.; Wassem, R.; de Baura, V.A.; Weiss, V.; Yates, M.G.; Madeira, H.M.; Pereira-Ferrari, L. The type III secretion system is necessary for the development of a pathogenic and endophytic interaction between Herbaspirillum rubrisubalbicans and Poaceae. BMC Microbiol. 2012, 12, 98. [Google Scholar] [CrossRef] [PubMed]
- Alavi, P.; Muller, H.; Cardinale, M.; Zachow, C.; Sanchez, M.B.; Martinez, J.L.; Berg, G. The DSF quorum sensing system controls the positive influence of Stenotrophomonas maltophilia on plants. PLoS ONE 2013, 8, e67103. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.L.; Zhang, L.Q. Quorum-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie Van Leeuwenhoek 2006, 89, 267–280. [Google Scholar] [CrossRef]
- Zuniga, A.; Poupin, M.J.; Donoso, R.; Ledger, T.; Guiliani, N.; Gutierrez, R.A.; Gonzalez, B. Quorum sensing and indole-3-acetic acid degradation play a role in colonization and plant growth promotion of Arabidopsis thaliana by Burkholderia phytofirmans PsJN. Mol. Plant Microbe Interact. 2013, 26, 546–553. [Google Scholar] [CrossRef]
- Dos Santos, M.F.; Muniz de Padua, V.L.; de Matos Nogueira, E.; Hemerly, A.S.; Domont, G.B. Proteome of Gluconacetobacter diazotrophicus co-cultivated with sugarcane plantlets. J. Proteomics 2010, 73, 917–931. [Google Scholar] [CrossRef]
- Yi, Y.; de Jong, A.; Frenzel, E.; Kuipers, O.P. Comparative transcriptomics of Bacillus mycoides strains in response to potato-root exudates reveals different genetic adaptation of endophytic and soil isolates. Front. Microbiol. 2017, 8, 1487. [Google Scholar] [CrossRef]
- Wallace, J.G.; May, G. Endophytes: The other maize genome. In The Maize Genome; Springer: New York, NY, USA, 2018; pp. 213–246. [Google Scholar]
- Ali, S.; Duan, J.; Charles, T.C.; Glick, B.R. A bioinformatics approach to the determination of genes involved in endophytic behavior in Burkholderia spp. J. Theor. Biol. 2014, 343, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Martin-Moldes, Z.; Zamarro, M.T.; Del Cerro, C.; Valencia, A.; Gomez, M.J.; Arcas, A.; Udaondo, Z.; Garcia, J.L.; Nogales, J.; Carmona, M.; et al. Whole-genome analysis of Azoarcus sp. strain CIB provides genetic insights to its different lifestyles and predicts novel metabolic features. Syst. Appl. Microbiol. 2015, 38, 462–471. [Google Scholar] [CrossRef]
- Badri, D.V.; Weir, T.L.; van der Lelie, D.; Vivanco, J.M. Rhizosphere chemical dialogues: Plant-microbe interactions. Curr. Opin. Biotechnol. 2009, 20, 642–650. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef]
- Ampomah, O.Y.; Avetisyan, A.; Hansen, E.; Svenson, J.; Huser, T.; Jensen, J.B.; Bhuvaneswari, T. The thuEFGKAB operon of rhizobia and Agrobacterium tumefaciens code for transport of trehalose, maltitol and isomers of sucrose and their assimilation through the formation of their 3-ketoderivatives. J. Bacteriol. 2013, 195, 3797–3807. [Google Scholar] [CrossRef] [PubMed]
- Batista, M.B.; Teixeira, C.S.; Sfeir, M.Z.T.; Alves, L.P.S.; Valdameri, G.; Pedrosa, F.O.; Sassaki, G.L.; Steffens, M.B.R.; de Souza, E.M.; Dixon, R.; et al. PHB biosynthesis counteracts redox stress in Herbaspirillum seropedicae. Front. Microbiol. 2018, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Fibach-Paldi, S.; Burdman, S.; Okon, Y. Key physiological properties contributing to rhizosphere adaptation and plant growth promotion abilities of Azospirillum brasilense. FEMS Microbiol. Lett. 2012, 326, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.P.S.; do Amaral, F.P.; Kim, D.; Bom, M.T.; Gavidia, M.P.; Teixeira, C.S.; Holthman, F.; Pedrosa, F.O.; Maltempi de Souza, E.; Chubatsu, L.S.; et al. Importance of poly-3-hydroxybutyrate (PHB) metabolism to the ability of Herbaspirillum seropedicae to promote plant growth. Appl. Environ. Microbiol. 2019, 85, e02586-18. [Google Scholar] [CrossRef]
- Alberton, D.; Muller-Santos, M.; Brusamarello-Santos, L.C.; Valdameri, G.; Cordeiro, F.A.; Yates, M.G.; de Oliveira Pedrosa, F.; de Souza, E.M. Comparative proteomics analysis of the rice roots colonized by Herbaspirillum seropedicae strain SmR1 reveals induction of the methionine recycling in the plant host. J. Proteome Res. 2013, 12, 4757–4768. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda Mdel, C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Sheibani-Tezerji, R.; Rattei, T.; Sessitsch, A.; Trognitz, F.; Mitter, B. Transcriptome profiling of the endophyte Burkholderia phytofirmans PsJN indicates sensing of the plant environment and drought stress. mBio 2015, 6, e00621-15. [Google Scholar] [CrossRef]
- Tadra-Sfeir, M.Z.; Faoro, H.; Camilios-Neto, D.; Brusamarello-Santos, L.; Balsanelli, E.; Weiss, V.; Baura, V.A.; Wassem, R.; Cruz, L.M.; De Oliveira Pedrosa, F.b.; et al. Genome wide transcriptional profiling of Herbaspirillum seropedicae SmR1 grown in the presence of naringenin. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Coutinho, B.G.; Licastro, D.; Mendonca-Previato, L.; Camara, M.; Venturi, V. Plant-influenced gene expression in the rice endophyte Burkholderia kururiensis M130. Mol. Plant Microbe Interact. 2015, 28, 10–21. [Google Scholar] [CrossRef]
- Drogue, B.; Sanguin, H.; Chamam, A.; Mozar, M.; Llauro, C.; Panaud, O.; Prigent-Combaret, C.; Picault, N.; Wisniewski-Dye, F. Plant root transcriptome profiling reveals a strain-dependent response during Azospirillum-rice cooperation. Front. Plant Sci. 2014, 5, 607. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.; Santa Brígida, A.B.; Mota Filho, J.P.; de Carvalho, T.G.; Rojas, C.A.; Vaneechoutte, D.; Van Bel, M.; Farrinelli, L.; Ferreira, P.C.; Vandepoele, K. Drought tolerance conferred to sugarcane by association with Gluconacetobacter diazotrophicus: A transcriptomic view of hormone pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef]
- Straub, D.; Yang, H.; Liu, Y.; Tsap, T.; Ludewig, U. Root ethylene signalling is involved in Miscanthus sinensis growth promotion by the bacterial endophyte Herbaspirillum frisingense GSF30(T). J. Exp. Bot. 2013, 64, 4603–4615. [Google Scholar] [CrossRef]
- Irizarry, I.; White, J.F. Bacillus amyloliquefaciens alters gene expression, ROS production and lignin synthesis in cotton seedling roots. J. Appl. Microbiol. 2018, 124, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Brusamarello-Santos, L.C.; Gilard, F.; Brule, L.; Quillere, I.; Gourion, B.; Ratet, P.; Maltempi de Souza, E.; Lea, P.J.; Hirel, B. Metabolic profiling of two maize (Zea mays L.) inbred lines inoculated with the nitrogen fixing plant-interacting bacteria Herbaspirillum seropedicae and Azospirillum brasilense. PLoS ONE 2017, 12, e0174576. [Google Scholar] [CrossRef] [PubMed]
- Scherling, C.; Ulrich, K.; Ewald, D.; Weckwerth, W. A metabolic signature of the beneficial interaction of the endophyte Paenibacillus sp. isolate and in vitro–grown poplar plants revealed by metabolomics. Mol. Plant Microbe Interact. 2009, 22, 1032–1037. [Google Scholar] [CrossRef]
- Lòpez-Fernàndez, S.; Compant, S.; Vrhovsek, U.; Bianchedi, P.L.; Sessitsch, A.; Pertot, I.; Campisano, A. Grapevine colonization by endophytic bacteria shifts secondary metabolism and suggests activation of defense pathways. Plant Soil 2015, 405, 155–175. [Google Scholar] [CrossRef]
- Van Bastelaere, E.; De Mot, R.; Michiels, K.; Vanderleyden, J. Differential gene expression in Azospirillum spp. by plant root exudates: Analysis of protein profiles by two-dimensional polyacrylamide gel electrophoresis. FEMS Microbiol. Lett. 1993, 112, 335–342. [Google Scholar] [CrossRef]
- Miché, L.; Battistoni, F.; Gemmer, S.; Belghazi, M.; Reinhold-Hurek, B. Upregulation of jasmonate-inducible defense proteins and differential colonization of roots of Oryza sativa cultivars with the endophyte Azoarcus sp. Mol. Plant Microbe Interact. 2006, 19, 502–511. [Google Scholar] [CrossRef]
- Green, E.R.; Mecsas, J. Bacterial secretion systems: An overview. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef]
- Thomas, N.A.; Deng, W.; Puente, J.L.; Frey, E.A.; Yip, C.K.; Strynadka, N.C.; Finlay, B.B. CesT is a multi-effector chaperone and recruitment factor required for the efficient type III secretion of both LEE- and non-LEE-encoded effectors of enteropathogenic Escherichia coli. Mol. Microbiol. 2005, 57, 1762–1779. [Google Scholar] [CrossRef]
- Valentini, M.; Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: Lessons from Pseudomonas aeruginosa and other bacteria. J. Biol. Chem. 2016, 291, 12547–12555. [Google Scholar] [CrossRef]
- Chaudhry, V.; Patil, P.B. Genomic investigation reveals evolution and lifestyle adaptation of endophytic Staphylococcus epidermidis. Sci. Rep. 2016, 6, 19263. [Google Scholar] [CrossRef]
- Chaudhry, V.; Sharma, S.; Bansal, K.; Patil, P.B. Glimpse into the genomes of rice endophytic bacteria: Diversity and distribution of Firmicutes. Front. Microbiol. 2016, 7, 2115. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Morgenstern, B.; Voss, B.; Hess, W.R.; Reva, O.; et al. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef]
- Loper, J.E.; Hassan, K.A.; Mavrodi, D.V.; Davis, E.W.; Lim, C.K.; Shaffer, B.T.; Elbourne, L.D.; Stockwell, V.O.; Hartney, S.L.; Breakwell, K.; et al. Comparative genomics of plant-associated Pseudomonas spp.: Insights into diversity and inheritance of traits involved in multitrophic interactions. PLoS Genet. 2012, 8, e1002784. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Monchy, S.; Taghavi, S.; Zhu, W.; Ramos, J.; van der Lelie, D. Comparative genomics and functional analysis of niche-specific adaptation in Pseudomonas putida. FEMS Microbiol. Rev. 2011, 35, 299–323. [Google Scholar] [CrossRef] [PubMed]
- Taghavi, S.; Garafola, C.; Monchy, S.; Newman, L.; Hoffman, A.; Weyens, N.; Barac, T.; Vangronsveld, J.; van der Lelie, D. Genome survey and characterization of endophytic bacteria exhibiting a beneficial effect on growth and development of poplar trees. Appl. Environ. Microbiol. 2009, 75, 748–757. [Google Scholar] [CrossRef]
- Wisniewski-Dye, F.; Lozano, L.; Acosta-Cruz, E.; Borland, S.; Drogue, B.; Prigent-Combaret, C.; Rouy, Z.; Barbe, V.; Herrera, A.M.; Gonzalez, V.; et al. Genome sequence of Azospirillum brasilense CBG497 and comparative analyses of Azospirillum core and accessory genomes provide insight into niche adaptation. Genes (Basel) 2012, 3, 576–602. [Google Scholar] [CrossRef]
- Straub, D.; Rothballer, M.; Hartmann, A.; Ludewig, U. The genome of the endophytic bacterium H. frisingense GSF30(T) identifies diverse strategies in the Herbaspirillum genus to interact with plants. Front. Microbiol. 2013, 4, 168. [Google Scholar] [CrossRef] [PubMed]
- Sheibani-Tezerji, R.; Naveed, M.; Jehl, M.A.; Sessitsch, A.; Rattei, T.; Mitter, B. The genomes of closely related Pantoea ananatis maize seed endophytes having different effects on the host plant differ in secretion system genes and mobile genetic elements. Front. Microbiol. 2015, 6, 440. [Google Scholar] [CrossRef]
- Lopez-Fernandez, S.; Sonego, P.; Moretto, M.; Pancher, M.; Engelen, K.; Pertot, I.; Campisano, A. Whole-genome comparative analysis of virulence genes unveils similarities and differences between endophytes and other symbiotic bacteria. Front. Microbiol. 2015, 6, 419. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Patz, S.; Becker, Y.; Berger, B.; Drungowski, M.; Bunk, B.; Overmann, J.; Spröer, C.; Reetz, J.; Tchuisseu Tchakounte, G.V.; et al. Comparative genomics reveal a flagellar system, a type VI secretion system and plant growth-promoting gene clusters unique to the endophytic bacterium Kosakonia radicincitans. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Antonielli, L.; Sessitsch, A.; Compant, S.; Trognitz, F. Comparative genome analysis of the vineyard weed endophyte Pseudomonas viridiflava CDRTc14 showing selective herbicidal activity. Sci. Rep. 2017, 7, 17336. [Google Scholar] [CrossRef] [PubMed]
- Faoro, H.; Rene Menegazzo, R.; Battistoni, F.; Gyaneshwar, P.; do Amaral, F.P.; Taule, C.; Rausch, S.; Goncalves Galvao, P.; de Los Santos, C.; Mitra, S.; et al. The oil-contaminated soil diazotroph Azoarcus olearius DQS-4(T) is genetically and phenotypically similar to the model grass endophyte Azoarcus sp. BH72. Environ. Microbiol. Rep. 2017, 9, 223–238. [Google Scholar] [CrossRef]
- Carvalho, T.L.; Ballesteros, H.G.; Thiebaut, F.; Ferreira, P.C.; Hemerly, A.S. Nice to meet you: Genetic, epigenetic and metabolic controls of plant perception of beneficial associative and endophytic diazotrophic bacteria in non-leguminous plants. Plant Mol. Biol. 2016, 90, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, F.; Vargas, C.; Schwarcz, K.; Cavalcante, J.; Nogueira, E.M.; Baldani, J.I.; Ferreira, P.C.; Hemerly, A.S. SHR5: A novel plant receptor kinase involved in plant-N2-fixing endophytic bacteria association. J. Exp. Bot. 2006, 57, 559–569. [Google Scholar] [CrossRef]
- Trda, L.; Fernandez, O.; Boutrot, F.; Heloir, M.C.; Kelloniemi, J.; Daire, X.; Adrian, M.; Clement, C.; Zipfel, C.; Dorey, S. The grapevine flagellin receptor VvFLS2 differentially recognizes flagellin-derived epitopes from the endophytic growth-promoting bacterium Burkholderia phytofirmans and plant pathogenic bacteria. New Phytol. 2014, 201, 1371–1384. [Google Scholar] [CrossRef]
- Jelenska, J.; Davern, S.M.; Standaert, R.F.; Mirzadeh, S.; Greenberg, J.T. Flagellin peptide flg22 gains access to long-distance trafficking in Arabidopsis via its receptor, FLS2. J. Exp. Bot. 2017, 68, 1769–1783. [Google Scholar] [CrossRef] [PubMed]
- Lebeis, S.L.; Paredes, S.H.; Lundberg, D.S.; Breakfield, N.; Gehring, J.; McDonald, M.; Malfatti, S.; Del Rio, T.G.; Jones, C.D.; Tringe, S.G. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 2015, 349, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Iniguez, A.L.; Dong, Y.; Carter, H.D.; Ahmer, B.M.; Stone, J.M.; Triplett, E.W. Regulation of enteric endophytic bacterial colonization by plant defenses. Mol. Plant Microbe Interact. 2005, 18, 169–178. [Google Scholar] [CrossRef]
- Cavalcante, J.J.; Vargas, C.; Nogueira, E.M.; Vinagre, F.; Schwarcz, K.; Baldani, J.I.; Ferreira, P.C.; Hemerly, A.S. Members of the ethylene signalling pathway are regulated in sugarcane during the association with nitrogen-fixing endophytic bacteria. J. Exp. Bot. 2007, 58, 673–686. [Google Scholar] [CrossRef]
- Long, H.H.; Sonntag, D.G.; Schmidt, D.D.; Baldwin, I.T. The structure of the culturable root bacterial endophyte community of Nicotiana attenuata is organized by soil composition and host plant ethylene production and perception. New Phytol. 2010, 185, 554–567. [Google Scholar] [CrossRef] [PubMed]
- De Souza, A.R.; De Souza, S.; De Oliveira, M.; Ferraz, T.; Figueiredo, F.; Da Silva, N.; Rangel, P.; Panisset, C.; Olivares, F.; Campostrini, E. Endophytic colonization of Arabidopsis thaliana by Gluconacetobacter diazotrophicus and its effect on plant growth promotion, plant physiology, and activation of plant defense. Plant Soil 2016, 399, 257–270. [Google Scholar] [CrossRef]
- Kniskern, J.M.; Traw, M.B.; Bergelson, J. Salicylic acid and jasmonic acid signaling defense pathways reduce natural bacterial diversity on Arabidopsis thaliana. Mol. Plant Microbe Interact. 2007, 20, 1512–1522. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, N.; Kumar, A.; Munjal, V.; Nadakkakath, A.V.; Eapen, S.J. Pseudomonas putida BP25 alters root phenotype and triggers salicylic acid signaling as a feedback loop in regulating endophytic colonization in Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 2016, 93, 99–111. [Google Scholar] [CrossRef]
- Robertson-Albertyn, S.; Alegria Terrazas, R.; Balbirnie, K.; Blank, M.; Janiak, A.; Szarejko, I.; Chmielewska, B.; Karcz, J.; Morris, J.; Hedley, P.E.; et al. Root hair mutations displace the barley rhizosphere microbiota. Front. Plant Sci. 2017, 8, 1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Carvalhais, L.C.; Schenk, P.M.; Dennis, P.G. Effects of jasmonic acid signalling on the wheat microbiome differ between body sites. Sci. Rep. 2017, 7, 41766. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, L.C.; Dennis, P.G.; Badri, D.V.; Tyson, G.W.; Vivanco, J.M.; Schenk, P.M. Activation of the jasmonic acid plant defence pathway alters the composition of rhizosphere bacterial communities. PLoS ONE 2013, 8, e56457. [Google Scholar] [CrossRef] [PubMed]
- Kwak, M.J.; Kong, H.G.; Choi, K.; Kwon, S.K.; Song, J.Y.; Lee, J.; Lee, P.A.; Choi, S.Y.; Seo, M.; Lee, H.J.; et al. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018, 36, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, Q.; Zhang, J.; Chen, Y.; Lin, X.; Sun, C.; Wang, W.; Liu, H.; Zhang, B. Migration of endophytic diazotroph Azorhizobium caulinodans ORS571 inside wheat (Triticum aestivum L) and its effect on microRNAs. Funct. Integr. Genom. 2017, 17, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Rojas, C.A.; Grativol, C.; Motta, M.R.; Vieira, T.; Regulski, M.; Martienssen, R.A.; Farinelli, L.; Hemerly, A.S.; Ferreira, P.C. Genome-wide identification of microRNA and siRNA responsive to endophytic beneficial diazotrophic bacteria in maize. BMC Genom. 2014, 15, 766. [Google Scholar] [CrossRef]
- Pilon, M. The copper microRNAs. New Phytol. 2017, 213, 1030–1035. [Google Scholar] [CrossRef]
- Johnson, K.L.; Cassin, A.M.; Lonsdale, A.; Bacic, A.; Doblin, M.S.; Schultz, C.J. A motif and amino acid bias bioinformatics pipeline to identify hydroxyproline-rich glycoproteins. Plant Physiol. 2017, 174, 294. [Google Scholar] [CrossRef] [PubMed]
- Castilleux, R.; Plancot, B.; Ropitaux, M.; Carreras, A.; Leprince, J.; Boulogne, I.; Follet-Gueye, M.-L.; Popper, Z.A.; Driouich, A.; Vicré, M. Cell wall extensins in root–microbe interactions and root secretions. J. Exp. Bot. 2018, 69, 4235–4247. [Google Scholar] [CrossRef] [PubMed]
- Nguema-Ona, E.; Vicre-Gibouin, M.; Cannesan, M.A.; Driouich, A. Arabinogalactan proteins in root-microbe interactions. Trends Plant Sci. 2013, 18, 440–449. [Google Scholar] [CrossRef]
- Xie, S.S.; Wu, H.J.; Zang, H.Y.; Wu, L.M.; Zhu, Q.Q.; Gao, X.W. Plant growth promotion by spermidine-producing Bacillus subtilis OKB105. Mol. Plant Microbe Interact. 2014, 27, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Niu, E.; Shang, X.; Cheng, C.; Bao, J.; Zeng, Y.; Cai, C.; Du, X.; Guo, W. Comprehensive analysis of the COBRA-Like (COBL) gene family in Gossypium identifies two COBLs potentially associated with fiber quality. PLoS ONE 2015, 10, e0145725. [Google Scholar] [CrossRef]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Strain(s) | Gene (Accession Number) | Encoded Feature/Characteristic | Plant(s) | Major Findings | References |
|---|---|---|---|---|---|
| Chemotaxis, Motility | |||||
| H. seropedicae SmR1 | (Hsero_3720) | methyl-accepting chemotaxis transducer transmembrane protein | Zea mays | decreased chemotaxis towards the plant and attachment to the roots | [17] |
| P. fluorescens OE28.3, SBW25, F113 and WCS365 | cheA | chemotaxis protein | Solanum lycopersicum | ten- to 1000-fold decrease in the ability to colonise the root tip | [42] |
| A. brasilense Sp7 | tlp1 | transducer-like protein 1, chemoreceptor-like protein | Triticum aestivum | significant reduction in root colonisation | [18] |
| A. brasilense Sp7 | mot3 | bacterial flagellar motility | T. aestivum | significant decrease in adsorption capacity to roots | [20] |
| Azoarcus sp. BH72 | fliC3 (azo2704) | major structural protein flagellin | Oryza sativa subsp. indica cv. IR36 | significant reduction in root colonisation | [19] |
| Azoarcus sp. BH72 | pilX (azo2916) | type IV fimbrial biogenesis protein PilX | O. sativa ssp. japonica cv. Nipponbare | reduced root colonisation | [21] |
| Azoarcus sp. BH72 | pilA (azo3355) | major structural component of the pilus structure | O. sativa subsp. indica cv. IR36 | strong reduction in endophytic and surface colonisation | [22] |
| Azoarcus sp. BH72 | pilT (azo3468) | type IV pilus retraction protein | O. sativa subsp. indica cv. IR36 | strong reduction in endophytic colonisation, 50% reduction in surface colonisation | [22] |
| Adhesion, biofilm formation | |||||
| Pseudomonas putida KT2440 | lapA | surface adhesion protein | Z. mays | impaired root colonisation | [32] |
| Salmonella enterica serovar Enteritidis | agpB | curli subunit | Medicago sativa | reduced attachment capacity to the roots | [28] |
| G. diazotrophicus PAL5 | gumD (YP_001602791) | polysaccharide biosynthesis glycosyltransferase, exopolysaccharide biosynthesis | O. sativa | defective rice root surface attachment, reduced root surface and endophytic colonisation | [26] |
| R. leguminosarum biovar viciae 3841 | gmsA (CAK07156.1) | glucomannan production protein | Pisum sativum | defective attachment and biofilm formation on the root hairs | [27] |
| R. leguminosarum biovar viciae 3841 | pssA (CAK09242.1) | CDP-diacylglycerol-serine O-phosphatidyltransferase, acidic exopolysaccharide biosynthesis | P. sativum | defective attachment to the root hairs | [27] |
| S. enterica | yihO | glucuronide transporter, O-antigen capsule assembly and translocation, colonic acid biosynthesis | M. sativa | reduced ability to attach and colonise sprouts | [30] |
| R. leguminosarum biovar viciae 3841 | celA (CAK07141.1); bcsA according to the nomenclature by Romling and Galperin [43] | cellulose synthase catalytic subunit [UDP-forming], cellulose biosynthesis | P. sativum | deficient biofilm formation on the roots | [27] |
| S. enterica | bcsA | cellulose synthase catalytic subunit [UDP-forming], bacterial cellulose synthesis | M. sativa | reduced ability to attach and colonise sprouts | [30] |
| H. rubrisubalbicans M1 | wssD (Hrubri_1119); bcsZ according to the nomenclature by Romling and Galperin [43] | beta-1,4-glucanase (cellulase) (EC 3.2.1.4), part of the cellulose biosynthesis operon | Z. mays | decrease root surface attachment and endophytic colonisation | [29] |
| Lipopolysaccharide, membrane proteins | |||||
| H. seropedicae SmR1 | rfbB (Hsero_4410) | dTDP-D-glucose 4,6-dehydratase, biosynthesis of rhamnose | Z. mays | 100-fold lower attachment to the root surface, decreased efficiency in endophytic colonisation | [39] |
| H. seropedicae SmR1 | rfbC (Hsero_4411) | dTDP-4-keto-6-deoxy-D-glucose 3,5-epimeras, biosynthesis of rhamnose | Z. mays | 100-fold lower attachment to the root surface, decreased efficiency in endophytic colonisation | [39] |
| A. brasilense | rfbD (AMK58_RS28935) | dTDP-4-dehydrorhamnose reductase, biosynthesis of rhamnose | Z. mays cv. ‘Funk’s Tronador G422T’ | impaired attachment to the roots and decreased root colonisation | [40] |
| H. seropedicae SmR1 | waaL (Hsero_3570) | O-antigen ligase, catalyse a key step for LPS biosynthesis | Z. mays | severely impaired in colonisation | [41] |
| H. seropedicae SmR1 | ampG (YP_003773531.1) | muropeptide permease of the major facilitator superfamily, recycling of the cell wall peptidoglycan | Z. mays | 10-fold decrease in endophytic population in roots one and three days after inoculation | [44] |
| P. fluorescens CHA0 | oprF (EF592174) | outer membrane porin F | Cucumis sativus var. Poinset 76 and S. lycopersicum Platense | decrease in adsorption capacity to roots | [45] |
| Plant cell wall modifications | |||||
| Azoarcus sp. BH72 | eglA (azo2236) | beta-1,4-glucanase (cellulase) (EC 3.2.1.4) | O. sativa subsp. indica cv. IR36 | decrease of endophytic colonisation | [46] |
| Bacillus amyloliquefaciens TB2 | eglS | endo-beta-1,4-glucanase | Brassica rapa subsp. pekinensis and chinensis | decrease of endophytic colonisation | [47] |
| B. subtilis 168 | yoaJ | expansin, causes loosening and extension of plant cell walls by disrupting the non-covalent bonding between the cellulose microfibrils and matrix glucans | Z. mays | significant decrease in ability to colonise roots | [48] |
| Substrate utilisation, transport | |||||
| B. phytofirmans PsJN | oxc | oxalate decarboxylase | Lupinus albus L., cv. Amiga, Z. mays subsp. mays, cv. Birko | impaired early colonisation | [49] |
| R. meliloti 1021 | thuA | trehalose utilisation-related protein | M. sativa | impaired colonisation of roots | [50] |
| R. meliloti 1021 | thuB | trehalose utilisation-related protein | M. sativa | impaired colonisation of roots | [50] |
| H. seropedicae SmR1 | phbC1 (Hsero_2999) | polyhydroxybutyrate (PHB) synthase subunit C | Z. mays | eight-fold fewer planktonic and epiphytic cells during the early stages of root colonisation | [17] |
| M. extorquens AM1 | mxaF (MexAM1_META1p4538) | methanol dehydrogenase subunit alpha, oxidise methanol to formaldehyde (together with MxaI) | M. truncatula | decreased colonisation abilities and persistence in different parts of the plant | [51] |
| M. extorquens AM1 | mptG (MexAM1_META1p1760) | beta-ribofuranosyl aminobenzene 5’-phosphate synthase, involved in tetrahydromethanopterin biosynthesis | M. truncatula | decreased colonisation abilities and persistence in different parts of the plant, increased sensitivity to formaldehyde and methanol | [51] |
| Azoarcus sp. BH72 | exaA2 (azo2972) | quino(hemo)protein alcohol dehydrogenase, pyrroloquinoline quinone (PQQ)-dependent (EC 1.1.2.8) | O. sativa subsp. indica cv. IR36 | decrease in root colonisation | [52] |
| Azoarcus sp. BH72 | exaA3 (azo2975) | quino(hemo)protein alcohol dehydrogenase, PQQ-dependent (EC 1.1.2.8) | O. sativa subsp. indica cv. IR36 | decrease in root colonisation | [52] |
| Stress protection | |||||
| G. diazotrophicus PAL5 | sodB (GDI_2168) | superoxide dismutase [Mn/Fe] (EC 1.15.1.1) | O. sativa subsp. indica cv. IR42 | decrease in number of tightly root-associated bacterial and endophytic colonisation | [53] |
| G. diazotrophicus PAL5 | gor (GDI_2216) | glutathione reductase (EC 1.8.1.7) | O. sativa subsp. indica cv. IR42 | decrease in number of tightly root-associated bacterial and endophytic colonisation | [53] |
| Stenotrophomonas maltophilia D457 | smeE (SMD_3657) | RND efflux system, inner membrane transporter, part of the SmeDEF efflux pump involved in quinolone resistance | Brassica napus cv. Californium | impaired colonisation of roots | [54] |
| H. seropedicae SmR1 | (Hsero_4782) | ABC-type multidrug transporter | Z. mays | 20-fold lower endophytic and epiphytic populations three days after inoculation | [17] |
| Paraburkholderia kururiensis M130 | (2553408008) | outer membrane efflux transporter, nodT family | O. sativa var. BALDO | four- to six-fold decrease ability to colonise roots | [14] |
| B. subtilis 168 | pta, als | 2,3-butanediol biosynthesis | Capsicum annuum | decreased ability to persist in the rhizosphere | [55] |
| S. marcescens 90-166 | entA (AAY84_01970) | 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase, enterobactin biosynthesis | Cucumis sativus | significant decrease in root population | [56] |
| Bacterial secretion systems | |||||
| H. rubrisubalbicans M1 | hrcN (Hrubri_2444) | T3SS ATPase | O. sativa | 100-fold decrease in endophytic population in roots nine days after inoculation | [57] |
| H. rubrisubalbicans M1 | hrpE (Hrubri_2433) | T3SS structural apparatus | O. sativa | 100-fold decrease in endophytic population in roots nine days after inoculation | [57] |
| Azoarcus sp. BH72 | ppkA (azo3888) | T6SS serine/threonine protein kinase | O. sativa ssp. japonica cv. Nipponbare | increased colonisation capacity | [21] |
| P. kururiensis M130 | cesT (2553406074) | Tir chaperone protein | O. sativa var. BALDO | four-six-fold decrease in the ability to colonise roots | [14] |
| Transcriptional regulators, sensor proteins | |||||
| S. maltophilia R551-3 | rpfF (Smal_1830) | enoyl-CoA hydratase, synthesis of quorum sensing molecule - diffusible signal factor (DSF) | B. napus cv. Californium | decreased colonisation efficiency and plant growth promotion | [58] |
| P. fluorescens 2P24 | pcoI | acyl-homoserine-lactone synthase, quorum sensing molecules biosynthesis | T. aestivum | deficiency in colonisation of rhizosphere | [59] |
| B. phytofirmans PsJN | bpI.1 (Bphyt_0126) | AHL synthase of chromosome 1 QS system | A. thaliana Col-0 | decreased root colonisation | [60] |
| B. phytofirmans PsJN | bpI.2 (Bphyt_4275) | AHL synthase of chromosome 2 QS system | A. thaliana Col-0 | decreased root colonisation | [60] |
| Azoarcus sp. BH72 | (azo1544) | GGDEF/EAL/PAC/PAS-domain-containing protein | O. sativa ssp. japonica cv. Nipponbare | reduced root colonisation | [21] |
| Azoarcus sp. BH72 | (azo2408) | GGDEF domain-containing protein | O. sativa ssp. japonica cv. Nipponbare | decreased root colonisation | [21] |
| S. enterica serovar Newport | rpoS | stationary-phase sigma factor, regulating biofilm formation, agfD and other adhesins | M. sativa | decreased colonisation of sprouts | [28] |
| Type of Analysis | Strain(s) | Plant(s) | Major Findings | References |
|---|---|---|---|---|
| Transcriptomic analysis of Azoarcus sp. BH72 in response to the root exudates | Azoarcus sp. BH72 | O. sativa cv. Nipponbare | changes in the expression of 176 genes, primarily those encoding for hypothetical proteins, which are the proteins that are involved in the metabolic processes, especially producing and conserving energy, amino acid transport and metabolism | [21] |
| Transcriptomic analysis of H. seropedicae SmR1 of bacteria attached to the roots of T. aestivum cv. CD104 | H. seropedicae SmR1 | T. aestivum cv. CD104 | upregulation of the genes related to the type IV pili, PHB biosynthesis, nitrogen fixation, stress tolerance, adhesion and utilisation of the plant-derived compounds | [23] |
| Transcriptomic analysis of B. mycoides EC18 and B. mycoides SB8 in response to the root exudates | B. mycoides EC18 (endophyte) and B. mycoides SB8 (soil bacteria) | Solanum tuberosum cv. Seresta | more pronounced response of the endophytic strain to root exudates, upregulation of the genes related to amino acid metabolism, membrane proteins, transcriptional regulators, stress-related genes and plant cell wall-degrading enzymes in the endophytic strain | [62] |
| Transcriptomic analysis of B. phytofirmans PsJN colonising potato plant | B. phytofirmans PsJN | S. tuberosum cv. Bionta | expression of the genes related to regulating transcription, general metabolism (sugars, amino acids, lipids and nucleotides), secretion systems, energy production and cellular homeostasis | [74] |
| Transcriptomic analysis of H. seropedicae SmR1 in response to the naringenin | H. seropedicae SmR1 | - | changes in the expression profile of the genes related to the bacterial cell wall, repression of the genes related to the chemotaxis, flagella biosynthesis, downregulation of the genes related to amino acid and sugar transport, upregulation of the multidrug transport efflux encoding genes | [75] |
| Transcriptomic analysis of P. kururiensis M130 in response to the plant extract | P. kururiensis M130 | O. sativa var. BALDO | diversified expression of the genes related to the cellular processes, metabolism, secretion and transport; diversified expression of potential antisense RNAs (asRNAs) and sense mRNA that can possibly be affected by asRNAs | [76] |
| Transcriptomic analysis of A. brasilense FP2 and T. aestivum (dual RNA sequencing) | A. brasilense FP2 | T. aestivum cv. CD-104 | bacterial response: high expression of the surface layer protein (sbpA), ABC sugar transporters (gguA and gguB), monosaccharide transporters, calcium-binding proteins, superoxide dismutase and proteins related to polysaccharides, LPS biosynthesis, transport of exopolysaccharides, PHAs biosynthesis and nitrogen fixation; plant response: differential expression of 776 of the expressed sequence tag that are involved in transport, biological regulation, defence mechanisms, production of phytohormones and phytochemicals | [38] |
| Transcriptomic analysis of O. sativa roots in response to inoculation with bacteria | A. lipoferum 4B (isolated from Cigalon), Azospirillum sp. B510 (isolated from Nipponbare) | O. sativa japonica cv. Cigalon and Nipponbare | diversified expression of 7384 genes of rice after inoculation with bacteria, primarily those involved in primary metabolism, transport, regulating transcription and protein fate, 34 genes similarly regulated by both strains, among them the pathogenesis-related gene (PR-10); B510 strain leads to the repression of a larger number of the genes that are related to stress and plant defence than the 4B strain | [77] |
| Transcriptomic analysis of Saccharum officinarum inoculated with bacteria and uninoculated specimens that were subjected to water depletion for three days | G. diazotrophicus PAL5 | S. officinarum cv. SP70-1143 | higher level of colonisation by the endophytic strain of water-deficit roots; higher tolerance to drought stress of the inoculated plants; diversified expression of drought-related genes in the inoculated plants | [78] |
| Transcriptomic and proteomic analyses of Miscanthus sinensis three hours and three weeks after inoculation | H. frisingense GSF30T | M. sinensis | prominent upregulation of the genes involved in jasmonate signalling and biosynthesis in the early (3 h) response to the inoculation; upregulation of the ethylene receptor and downregulation of the ethylene response factor after three weeks | [79] |
| Transcriptomic analysis of cotton roots ten days after inoculation with bacteria | B. amyloliquefaciens pb1 | Gossypium sp. | upregulation of the genes related to nitrate assimilation, cell growth, transport, hormones, transcription factors and antioxidants | [80] |
| Metabolomic analysis of Z. mays roots and leaves in response to inoculation with bacteria | H. seropedicae SmR1 and SmR54, A. brasilense FP2 and FP10 | Z. mays FV252 and FV2 | maize genotype-specific pattern of accumulation of metabolites, primarily changes in the mannitol, trehalose and isocitrate concentrations | [81] |
| Metabolomic analysis of poplar in response to Paenibacillus sp. P22 | Paenibacillus sp. P22 | Populus alba × (P. davidiana + P. simonii) × P. tomentosa] | increased concentration of asparagine, urea and threitol; depletion of sugars and organic acids of the tricarboxylic acid cycle in the plants that had been inoculated with bacteria | [82] |
| Metabolomic analysis of Vitis vinifera roots and stems in response to Enterobacter ludwigii EnVs6 | E. ludwigii EnVs6 | V. vinifera cv. Pinot noir clone I-SMA 185 | increased concentration of vanillic acid and decreased concentration of catechin, esculin, arbutin, astringin, pallidol, ampelopsin, D-quadrangularin and isohopeaphenol in the plants that had been inoculated by bacteria; effect of plant colonisation more prominent in the stems than in roots | [83] |
| Proteomic analysis of G. diazotrophicus PAL5 in response to plantlets | G. diazotrophicus PAL5 | S. officinarum cv. SP70-1143 | differential expression of 38 proteins that are associated with carbohydrates and the energy metabolism, folding, sorting, degradation processes, transcription and translation; in bacterial cells responding to the plantlets, a higher expression of the transcription elongation factor (GreA), a 60 kDa chaperonin (GroEL) and outer membrane lipoprotein (Omp16) | [61] |
| Proteomic analysis of Azospirillum spp. in response to plant root exudates | A. brasilense Sp7, Sp245, Sp246; A. lipoferum Sp59b, SpBrl7, DN64, SF50 | T. aestivum cv. Fidel | induction of the acidic 40-kDa protein in response to root exudates | [84] |
| Proteomic analysis of rice roots colonised by H. seropedicae SmR1 | H. seropedicae SmR1 | O. sativa ssp. japonica cv. Nipponbare | bacterial response: higher level of dinitrogenase reductase NifH and glutamine synthetase GlnA; plant response: upregulation of S-adenosylmethionine synthetase, methylthioribose kinase and acireductone dioxygenase 1; stimulation of the phytosiderophores biosynthesis | [72] |
| Proteomic analysis of rice roots colonised by Azoarcus sp. BH72 and in response to ethylene and JA treatment | Azoarcus sp. BH72 | O. sativa subsp. indica cv. IR36 and IR42 | plant defence responses involving JA may contribute to restriction of endophytic colonisation in grasses; endophytic colonisation and JA induce the expression of the pathogenesis-related proteins and salt-stress induced proteins | [85] |
| Strain(s) | Major Findings | References |
|---|---|---|
| 92 strains, including four endophytes: Staphylococcus epidermidis SE4.7, SE4.8, SE4.6 and SE2.9 | identification of a distinct sub-lineage of S. epidermidis isolated from surface-sterilised rice seeds, which is different from the majority of human isolates; identification of the five genomic regions that are associated with rice S. epidermidis endophytes, i.e., encoding for methionine sulfoxide reductase (msrA), haloacid dehydrogenase (HAD), 4-hydroxythreonine-4-phosphate dehydrogenase (pdxA), repair protein (radC) | [89] |
| 21 rice seed endophytes belonging to the phylum Firmicutes | identification of numerous genes that are related to the production of auxin and siderophores, tolerance of oxidative, heat and osmotic stresses in the endophytic strains | [90] |
| 40 endophytes, 42 nodule-forming symbionts, 42 rhizosphere bacteria, 29 plant pathogens, 49 soil bacteria | enrichment of the endophyte-related genes: response regulator proteins CheBR and CheC, flagellum biosynthesis, motility mechanisms, transcriptional regulation of nitrogen assimilation (nifA), reduction of nitric oxide (norR), regulation of carbon storage (sdiA), beta-lactamase resistance (ampR), pyrimidine metabolism (pyrR), thiamine metabolism (tenA), glutathione peroxidase (btuE), glutathione S-transferase (gst), catalase (katE), nitric oxide reductase (norR), ATP-binding cassette (ABC), major facilitator superfamily (MFS), phosphotransferase system (PTS), solute carrier family (SLC), type IV conjugal DNA-protein transfer secretion system and nitrogenase (nifH) | [7] |
| nine endophytes: B. phytofirmans PsJN, Burkholderia spp. JK006, A. lipoferum 4B, E. cloacae ENHKU01, Klebsiella pneumoniae 342, P. putida W619, Enterobacter spp. 638, Azoarcus spp. BH72, Serratia proteamaculans 568 | identification of the gene sets that are putatively responsible for endophytic behaviour, transporters: major facilitator superfamily (MFS) and ATP-binding cassette (ABC) transporter; secretion systems: type VI secretion system; transcriptional regulator: AraC, FrmR, AsnC, LrgB, LysR, DeoR, WrbA and two components of the winged helix transcriptional regulator proteins; plant polymer degradation: cellulases, hemicellulases, endoglucanases; detoxification: glutathione S-transferase, dehydrogenases, synthases, hydratases | [64] |
| three strains: Stenotrophomonas rhizophila DSM 14405T (endophyte), S. maltophilia R551-3 (endophyte), S. maltophilia K279 (human pathogen) | presence in the genome of S. rhizophila DSM 14405T of unique genes related to the endophytic lifestyle: osmotic stress protection: cbg1, xynB, ggpS, ycaD, algJ and type VI secretion system | [58] |
| six strains: B. amyloliquefaciens FZB42 (plant-associated bacteria), B. subtilis 168, Bacillus licheniformis, Bacillus clausii, Bacillus halodurans, Bacillus cereus | presence of 214 unique genes in the genome of B. amyloliquefaciens FZB42, among them, three encoding proteins with a collagen-related GXT structural motif, which are putatively involved in surface adhesion or biofilm formation | [91] |
| 10 Pseudomonas spp. strains | diversified presence of type II, III, IV, VI secretion systems | [92] |
| four strains: P. putida KT2440 (rhizospheric bacteria), P. putida W619 (endophyte), P. putida F1 (aromatic hydrocarbon-degrading strain), P. putida GB-1 (manganese-oxidising strain) | presence of the ndvB (encoding beta-(1,2)-glucan) and two genes that are putatively involved in adhesion that encode for the autotransporter proteins (secretion type V) with a pectin/lyase/pertactin domain genes in the W619 strain that is absent in KT2440 strain | [93] |
| four strains: Enterobacter sp. 638, S. maltophilia R551-3, P. putida W619, S. proteamaculans 568 | adaptation to utilise a broad spectrum of plant-derived compounds as a carbon source | [94] |
| five endophytic strains: Azoarcus sp. CIB, “Aromatoleum aromaticum” EbN1, Azoarcus sp. BH72; Azoarcus sp. KH32C; Azoarcus toluclasticus MF63 | no unique gene cluster in Azoarcus sp. CIB exclusive for an endophytic lifestyle; presence of the genes that are related to the motility, adhesion, adaptation to the plant defence responses, type II, IV and VI secretion systems in Azoarcus spp. | [65] |
| four strains: A. brasilense CBG457 (endophyte), A. brasilense Sp245 (rhizospheric bacteria), A. lipoferum 4B (endophyte), Azospirillum sp. B510 (endophyte) | niche-specific presence of the genes that are related to EPS and LPS biosynthesis, adhesion, catabolic properties and plant cell wall degrading enzymes | [95] |
| B. phytofirmans PsJN and 8 other endophytic strains | presence in the genome of B. phytofirmans PsJN of numerous genes that are connected with the degradation of complex organic compounds and detoxification, cell surface signalling, secretion systems and quorum sensing system | [35] |
| H. frisingense GSF30T and 14 other strains | lack of a type III secretion system in H. frisingense GSF30T that is present in some related Herbaspirillum grass endophytes; differences in respiration, carbon, nitrogen and cell wall metabolism among Herbaspirillum isolates partially correlate with their different habitats | [96] |
| seven Pantoea ananatis strains | genomic differences in the genes encoding the secretion systems, putative effectors and transposase/integrases/phage-related genes | [97] |
| seven grapevine endophytic bacteria and 12 reference strains | lifestyle of pathogens or endophytes might be the outcome of complex, multifactorial interactions | [98] |
| Kosakonia radicincitans DSM 16,656 and 31 other strains | presence in the genome of K. radicincitans DSM 16656T of two flagellar systems and three type VI secretion systems, which may help them to avoid pattern-triggered immunity in plants | [99] |
| Pseudomonas viridiflava CDRTc14 (endophyte) and ten other Pseudomonas spp. strains | presence of the genes that are related to plant-bacteria interactions: type IV pili, motility, chemotaxis, transporters, type I, II, IV, V, VI and VII secretion systems, stress response, quorum sensing and quorum quenching | [100] |
| Azoarcus olearius DQS-4 (isolated from soil, rice endophyte) and Azoarcus sp. BH72 (endophyte) | presence of similar genes in A. olearius DQS-4 and Azoarcus sp. BH72 that are related to plant-bacteria interactions | [101] |
| 3837 bacterial genomes including 484 newly sequenced bacteria that had been isolated from the roots of Brassicaceae, poplar and maize | identification of 64 plant-associated protein domains that potentially mimic plant domains, enrichment in the genomes of plant-associated bacteria of the genes encoding the proteins that are related to the carbohydrate metabolism and lower number of mobile elements; in Acidovorax the presence of the characteristic gene families Jekyll and Hyde for non-pathogens and pathogens, respectively | [14] |
| 108 bacterial endophytes, 56 plant bacterial pathogens, 96 rhizosphere bacteria | enrichment of the genes encoding for nitrogenase, involved in the uptake of urea cycle, transport, secretion systems and transcriptional regulation | [15] |
| Plant | Mutant Name | Phenotype Characteristics | Bacterial Strain | Major Findings | References |
|---|---|---|---|---|---|
| M. truncatula | sickle (skl) | ethylene-insensitive (sickle) | K. pneumoniae 342 | higher level of skl plant colonisation by bacteria | [107] |
| Nicotiana attenuata | ir-aco1 | deficient in ethylene biosynthesis | - | lower bacterial diversity | [109] |
| N. attenuata | 35S-etr1 | deficient in ethylene perception | - | lower bacterial diversity | [109] |
| A. thaliana | fad3/7/8 | three fatty acid desaturase genes (FAD3, FAD7 and FAD8) that are necessary to produce wild-type levels of JA | - | greater epiphytic bacterial diversity | [111] |
| A. thaliana | nahG | SA degradation, defective in defence by expressing the bacterial salicylate hydroxylase gene, nahG | K. pneumoniae 342, S. enterica serovar Typhimurium 14,028 | no changes in colonisation by the 342 strain, a higher level of nahG plant colonisation by the 14,028 strain | [107] |
| A. thaliana | nahG | SA degradation, defective in defence by expressing the bacterial salicylate hydroxylase gene, nahG | G. diazotrophicus PAL5 | higher level of colonisation of the roots and leaves of NahG plants by bacteria | [110] |
| A. thaliana | npr1 | regulates the DNA binding ability of transcription factors that are involved in plant defence, disrupts the SA-mediated and SA-independent defence responses | - | reduced endophytic bacterial community diversity | [111] |
| A. thaliana | npr1 | regulates the DNA binding ability of the transcription factors that are involved in plant defence, disrupts the SA-mediated and SA-independent defence responses | K. pneumoniae 342, S. enterica serovar Typhimurium 14,028 | higher level of colonisation npr1 plants by the 342 and 14,028 strains | [107] |
| A. thaliana | sid2 (also known as eds16) | deficient in the accumulation of SA | - | reduced endophytic bacterial community diversity | [111] |
| Hordeum vulgare var. Karat | rhl1.a | completely hairless mutant that exhibits a disturbed pattern of root epidermis cells with indistinguishable trichoblasts | - | reduced complexity community | [113] |
| H. vulgare var. Dema | rhp1.b | develops only to the primordium stage and their tip growth is arrested after bulge forms | - | reduced complexity community | [113] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinski, A.; Betekhtin, A.; Hupert-Kocurek, K.; Mur, L.A.J.; Hasterok, R. Defining the Genetic Basis of Plant–Endophytic Bacteria Interactions. Int. J. Mol. Sci. 2019, 20, 1947. https://doi.org/10.3390/ijms20081947
Pinski A, Betekhtin A, Hupert-Kocurek K, Mur LAJ, Hasterok R. Defining the Genetic Basis of Plant–Endophytic Bacteria Interactions. International Journal of Molecular Sciences. 2019; 20(8):1947. https://doi.org/10.3390/ijms20081947
Chicago/Turabian StylePinski, Artur, Alexander Betekhtin, Katarzyna Hupert-Kocurek, Luis A. J. Mur, and Robert Hasterok. 2019. "Defining the Genetic Basis of Plant–Endophytic Bacteria Interactions" International Journal of Molecular Sciences 20, no. 8: 1947. https://doi.org/10.3390/ijms20081947
APA StylePinski, A., Betekhtin, A., Hupert-Kocurek, K., Mur, L. A. J., & Hasterok, R. (2019). Defining the Genetic Basis of Plant–Endophytic Bacteria Interactions. International Journal of Molecular Sciences, 20(8), 1947. https://doi.org/10.3390/ijms20081947









