C-X-C Motif Chemokine Ligand 14 is a Unique Multifunctional Regulator of Tumor Progression
Abstract
1. Introduction
2. Identification of CXCL14 as an Endogenous Tumor Growth Suppressor
Screening for Tumor-Suppressor Candidates
3. CXCL14 Expression as a Marker for Suppression of Tumors by Cetuximab, an Anti-EGF Receptor (EGFR) Monoclonal Antibody
4. Expression of CXCL14 in Carcinoma Cells and Growth of Tumors
5. CXCL14 Produced by Carcinoma-Associated Fibroblasts (CAFs) and Growth of Tumors
6. CXCL14 is a Multifunctional Tumor Suppressor
7. Increased Survival Rates after Injection of Melanoma Cells into CXCL14 Transgenic Mice
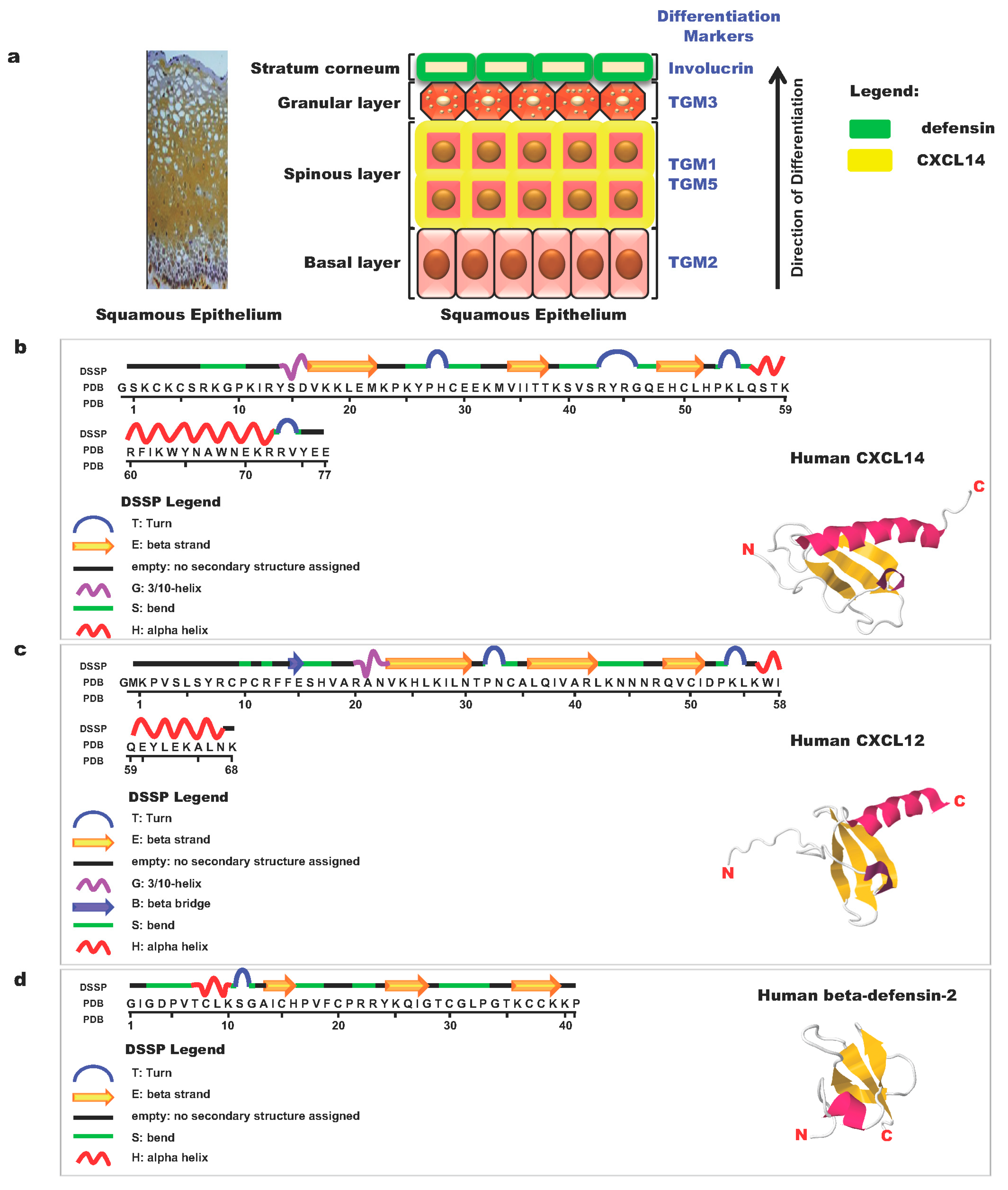
8. Antimicrobial Function
9. Regulation of CXCL14 Expression
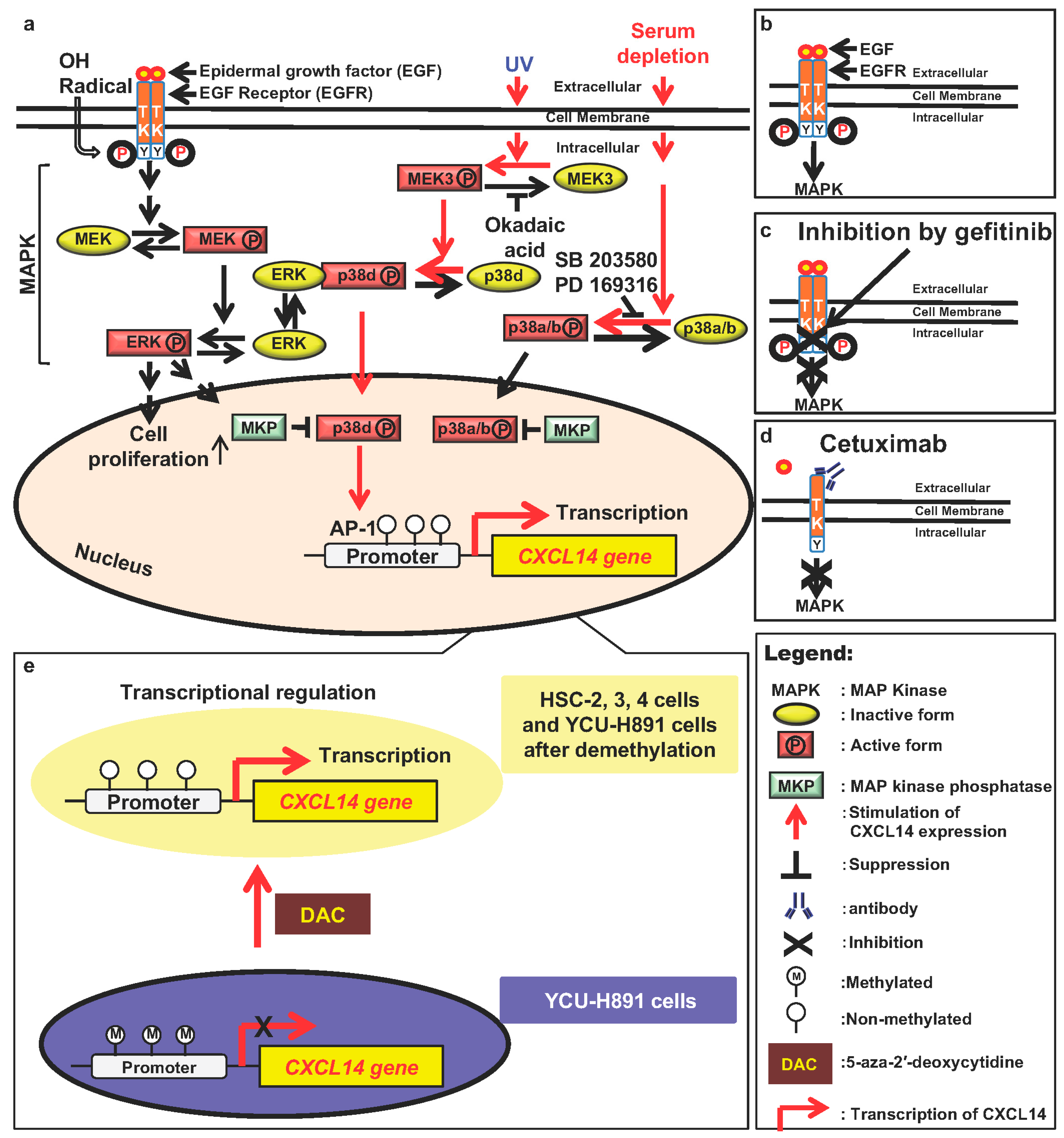
9.1. The Mitogen-Activated Protein Kinase (MAPK)/Extracellular Signal Regulated Kinase (ERK)/p38 Signaling Pathway Regulates CXCL14 Expression
9.2. Transcriptional Regulation of CXCL14
9.3. Epigenetic Regulation of CXCL14 Expression
9.4. Mechanisms of Tumor Suppression by CXCL14
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CXCL14 | C-X-C motif chemokine ligand 14 |
| DAC | 5-Aza-2′-deoxycytidine |
| HNSCC | Head and neck squamous cell carcinoma |
| EGF | Epidermal growth factor |
| MMP-1 | Matrix metalloproteinase-1 |
| TIMP3 | Matrix metalloproteinase 3 |
| IGF | Insulin-like growth factor |
| IGFBP-3 | Insulin-like growth factor binding protein-3 |
| NIH-CXCL14 | CXCL14-secreting NIH-3T3 |
| CAFs | Carcinoma-associated fibroblasts |
| NAFs | Normal-associated fibroblasts |
| MC57 | Mesenchyme-derived fibrosarcoma cells |
| RhoA | Ras-homologous small GTPase |
| ROCK | Rho-associated coiled-coil-containing protein kinase |
| LLC | Lewis lung carcinoma |
| AMP | Antimicrobial peptide |
| TGM | Transglutaminase |
References
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Zlotnik, A.; Yoshie, O.; Nomiyama, H. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome. Biol. 2006, 7, 243. [Google Scholar] [CrossRef] [PubMed]
- Marcuzzi, E.; Angioni, R.; Molon, B.; Calì, B. Chemokines and Chemokine Receptors: Orchestrating tumor metastasization. Int. J. Mol. Sci. 2019, 20, 96. [Google Scholar] [CrossRef]
- Farber, E. The multistep nature of cancer development. Cancer Res. 1984, 44, 4217–4223. [Google Scholar] [PubMed]
- Vogelstein, B.; Kinzler, K.W. The multistep nature of cancer. Trends Genet. 1993, 9, 138–141. [Google Scholar] [CrossRef]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef]
- Ozawa, S.; Kato, Y.; Komori, R.; Maehata, Y.; Kubota, E.; Hata, R. BRAK/CXCL14 expression suppresses tumor growth in vivo in human oral carcinoma cells. Biochem. Biophys. Res. Commun. 2006, 348, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Hromas, R.; Broxmeyer, H.E.; Kim, C.; Nakshatri, H.; Christopherson, K., 2nd; Azam, M.; Hou, Y.H. Cloning of BRAK, a novel divergent CXC chemokine preferentially expressed in normal versus malignant cells. Biochem. Biophys Res. Commun. 1999, 255, 703–706. [Google Scholar] [CrossRef]
- Ozawa, S.; Kato, Y.; Kubota, E.; Hata, R. BRAK/CXCL14 expression in oral carcinoma cells completely suppresses tumor cell xenografts in SCID mouse. Biomed. Res. 2009, 30, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, S.; Kato, Y.; Ito, S.; Komori, R.; Shiiki, N.; Tsukinoki, K.; Ozono, S.; Maehata, Y.; Taguchi, T.; Imagawa-Ishiguro, Y.; et al. Restoration of BRAK/CXCL14 gene expression by gefitinib is associated with antitumor efficacy of the drug in head and neck squamous cell carcinoma. Cancer Sci. 2009, 100, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Ozawa, S.; Ikoma, T.; Yajima, N.; Kiyono, T.; Hata, R. Expression of a chemokine BRAK/CXCL14 in oral floor carcinoma cells reduces the settlement rate of the cells and suppresses their proliferation in vivo. Biomed. Res. 2010, 31, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Frederick, M.J.; Henderson, Y.; Xu, X.; Deavers, M.T.; Sahin, A.A.; Wu, H.; Lewis, D.E.; El-Naggar, A.K.; Clayman, G.L. In vivo expression of the novel CXC chemokine BRAK in normal and cancerous human tissue. Am. J. Pathol. 2000, 156, 1937–1950. [Google Scholar] [CrossRef]
- Hata, R. A new strategy to find targets for anticancer therapy: Chemokine CXCL14/BRAK is a multifunctional tumor suppressor for head and neck squamous cell carcinoma. ISRN Otolaryngol. 2012, 2012, 797619. [Google Scholar] [CrossRef]
- Kondo, T.; Ozawa, S.; Ikoma, T.; Yang, X.Y.; Kanamori, K.; Suzuki, K.; Iwabuchi, H.; Maehata, Y.; Miyamoto, C.; Taguchi, T.; et al. Expression of the chemokine CXCL14 and cetuximab-dependent tumour suppression in head and neck squamous cell carcinoma. Oncogenesis 2016, 5, e240. [Google Scholar] [CrossRef]
- Tessema, M.; Klinge, D.M.; Yingling, C.M.; Do, K.; Van Neste, L.; Belinsky, S.A. Re-expression of CXCL14, a common target for epigenetic silencing in lung cancer, induces tumor necrosis. Oncogene 2010, 29, 5159–5170. [Google Scholar] [CrossRef]
- Cao, B.; Yang, Y.; Pan, Y.; Jia, Y.; Brock, M.V.; Herman, J.G.; Guo, M. Epigenetic silencing of CXCL14 induced colorectal cancer migration and invasion. Discov. Med. 2013, 16, 137–147. [Google Scholar]
- Gu, X.L.; Ou, Z.L.; Lin, F.J.; Yang, X.L.; Luo, J.M.; Shen, Z.Z.; Shao, Z.M. Expression of CXCL14 and its anticancer role in breast cancer. Breast Cancer Res. Treat. 2012, 135, 725–735. [Google Scholar] [CrossRef]
- Wang, W.; Huang, P.; Zhang, L.; Wei, J.; Xie, Q.; Sun, Q.; Zhou, X.; Xie, H.; Zhou, L.; Zheng, S. Antitumor efficacy of C-X-C motif chemokine ligand 14 in hepatocellular carcinoma in vitro and in vivo. Cancer Sci. 2013, 104, 1523–1531. [Google Scholar] [CrossRef]
- Schwarze, S.R.; Luo, J.; Isaacs, W.B.; Jarrard, D.F. Modulation of CXCL14 (BRAK) expression in prostate cancer. Prostate 2005, 64, 67–74. [Google Scholar] [CrossRef]
- Wente, M.N.; Mayer, C.; Gaida, M.M.; Michalski, C.W.; Giese, T.; Bergmann, F.; Giese, N.A.; Buchler, M.W.; Friess, H. CXCL14 expression and potential function in pancreatic cancer. Cancer Lett. 2008, 259, 209–217. [Google Scholar] [CrossRef]
- Zeng, J.; Yang, X.; Cheng, L.; Liu, R.; Lei, Y.; Dong, D.; Li, F.; Lau, Q.C.; Deng, L.; Nice, E.C.; et al. Chemokine CXCL14 is associated with prognosis in patients with colorectal carcinoma after curative resection. J. Transl. Med. 2013, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Oler, G.; Camacho, C.P.; Hojaij, F.C.; Michaluart, P., Jr.; Riggins, G.J.; Cerutti, J.M. Gene expression profiling of papillary thyroid carcinoma identifies transcripts correlated with BRAF mutational status and lymph node metastasis. Clin. Cancer Res. 2008, 14, 4735–4742. [Google Scholar] [CrossRef]
- Bissell, M.J.; Radisky, D. Putting tumours in context. Nat. Rev. Cancer 2001, 1, 46–54. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenviromental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Allinen, M.; Beroukhim, R.; Cai, L.; Brennan, C.; Lahti-Domenici, J.; Huang, H.; Porter, D.; Hu, M.; Chin, L.; Richardson, A.; et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell 2004, 6, 17–32. [Google Scholar] [CrossRef]
- Augsten, M.; Hagglof, C.; Olsson, E.; Stolz, C.; Tsagozis, P.; Levchenko, T.; Frederick, M.J.; Borg, A.; Micke, P.; Egevad, L.; et al. CXCL14 is an autocrine growth factor for fibroblasts and acts as a multi-modal stimulator of prostate tumor growth. Proc. Natl. Acad. Sci. USA 2009, 106, 3414–3419. [Google Scholar] [CrossRef]
- Miyamoto, C.; Maehata, Y.; Ozawa, S.; Ikoma, T.; Kubota, E.; Izukuri, K.; Kato, Y.; Hata, R.; Lee, M.C. Fasudil suppresses fibrosarcoma growth by stimulating secretion of the chemokine CXCL14/BRAK. J. Pharmacol. Sci. 2012, 120, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Shellenberger, T.D.; Wang, M.; Gujrati, M.; Jayakumar, A.; Strieter, R.M.; Burdick, M.D.; Ioannides, C.G.; Efferson, C.L.; El-Naggar, A.K.; Roberts, D.; et al. BRAK/CXCL14 is a potent inhibitor of angiogenesis and a chemotactic factor for immature dendritic cells. Cancer Res. 2004, 64, 8262–8270. [Google Scholar] [CrossRef] [PubMed]
- Izukuri, K.; Suzuki, K.; Yajima, N.; Ozawa, S.; Ito, S.; Kubota, E.; Hata, R. Chemokine CXCL14/BRAK transgenic mice suppress growth of carcinoma cell transplants. [corrected]. Transgenic Res. 2010, 19, 1109–1117. [Google Scholar] [CrossRef]
- Orimo, A.; Gupta, P.B.; Sgroi, D.C.; Arenzana-Seisdedos, F.; Delaunay, T.; Naeem, R.; Carey, V.J.; Richardson, A.L.; Weinberg, R.A. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell 2005, 121, 335–348. [Google Scholar] [CrossRef]
- Saha, A.; Ahn, S.; Blando, J.; Su, F.; Kolonin, M.G.; DiGiovanni, J. Proinflammatory CXCL12-CXCR4/CXCR7 signaling axis drives Myc-induced prostate cancer in obese mice. Cancer Res. 2017, 77, 5158–5168. [Google Scholar] [CrossRef]
- Hara, T.; Tanegashima, K. CXCL14 antagonizes the CXCL12-CXCR4 signaling axis. Biomol. Concepts 2014, 5, 167–173. [Google Scholar] [CrossRef]
- Izukuri, K.; Ito, S.; Nozaki, N.; Yajima, N.; Iwamiya, M.; Kawahara, S.; Suzuki, B.; Kubota, E.; Hata, R.I. Determination of serum BRAK/CXCL14 levels in healthy volunteers. Lab. Med. 2010, 41, 478–482. [Google Scholar] [CrossRef]
- Collins, P.J.; McCully, M.L.; Martinez-Munoz, L.; Santiago, C.; Wheeldon, J.; Caucheteux, S.; Thelen, S.; Cecchinato, V.; Laufer, J.M.; Purvanov, V.; et al. Epithelial chemokine CXCL14 synergizes with CXCL12 via allosteric modulation of CXCR4. FASEB J. 2017, 31, 3084–3097. [Google Scholar] [CrossRef]
- He, Z.; You, C.; Zhao, D. Long non-coding RNA UCA1/miR-182/PFKB2 axis modulates glioblastoma-associated sgtromal cells-medited glycolysis and invasion of glioma cells. Bioche. Biophys. Res. Commun. 2018, 500, 569–576. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.A.; Miyamoto, C.; Mehata, Y.; Suzukio, A.; Maeda, T. Acidic extracellula microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef]
- Maeda, T.; Suzuki, A.; Koga, K.; Miyamoto, C.; Maehata, Y.; Ozawa, S.; Hata, R.; Nagashima, Y.; Nabeshima, K.; Miyazaki, K.; Kato, Y. TRPM5 mediates acidic extracellular pH signaling and TRPM5 inhibition reduces spontaneous metastasis in mouse B16-BL6 melanoma cells. Oncotarget 2017, 8, 78312–78326. [Google Scholar] [CrossRef]
- Wang, Y.; Weng, X.; Wang, L.; Hao, M.; Li, Y.; Hou, L.; Liang, Y.; Wu, T.; Yao, M.; Lin, G.; et al. HIC1 deletion promotes breast cancer progression by activating tumor cell/fibroblast crosstalk. J. Clin. Investig. 2018, 128, 5235–5250. [Google Scholar] [CrossRef]
- Hata, R.; Izukuri, K.; Kato, Y.; Sasaki, S.; Mukaida, N.; Maehata, Y.; Miyamoto, C.; Akasaka, T.; Yang, X.; Nagashima, Y.; et al. Suppressed rate of carcinogenesis and decreases in tumour volume and lung metastasis in CXCL14/BRAK transgenic mice. Sci. Rep. 2015, 5, 9083. [Google Scholar] [CrossRef]
- Yang, X.Y.; Miyamoto, C.; Akasaka, T.; Izukuri, K.; Maehata, Y.; Ikoma, T.; Ozawa, S.; Hata, R. Chemokine CXCL14 is a multistep tumor suppressor. J. Oral Biosci. 2016, 58, 16–22. [Google Scholar] [CrossRef]
- Ikoma, T.; Ozawa, S.; Suzuki, K.; Kondo, T.; Maehata, Y.; Lee, M.C.; Hata, R.; Kubota, E. Calcium-calmodulin signaling induced by epithelial cell differentiation upregulates BRAK/CXCL14 expression via the binding of SP1 to the BRAK promoter region. Biochem. Biophys. Res. Commun. 2012, 420, 217–222. [Google Scholar] [CrossRef]
- Maerki, C.; Meuter, S.; Liebi, M.; Muhlemann, K.; Frederick, M.J.; Yawalkar, N.; Moser, B.; Wolf, M. Potent and broad-spectrum antimicrobial activity of CXCL14 suggests an immediate role in skin infections. J. Immunol. 2009, 182, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, G.; Dinesh Kumar, S.; Nam, J.; Jeon, D.; Kim, Y.; Lee, C.W.; Park, I.S.; Shin, S.Y. Antimicrobial and anti-inflammatory activities of chemokine CXCL14-derived antimicrobial peptide and its analogs. Biochim. Biophys. Acta Biomembr. 2018, 1861, 256–267. [Google Scholar] [CrossRef]
- Veldkamp, C.T.; Ziarek, J.J.; Su, J.; Basnet, H.; Lennertz, R.; Weiner, J.J.; Peterson, F.C.; Baker, J.E.; Volkman, B.F. Monomeric structure of the cardioprotective chemokine SDF-1/CXCL12. Protein Sci. 2009, 18, 1359–1369. [Google Scholar] [CrossRef] [PubMed]
- Sawai, M.V.; Jia, H.P.; Liu, L.; Aseyev, V.; Wiencek, J.M.; McCray, P.B., Jr.; Ganz, T.; Kearney, W.R.; Tack, B.F. The NMR structure of human beta-defensin-2 reveals a novel alpha-helical segment. Biochemistry 2001, 40, 3810–3816. [Google Scholar] [CrossRef]
- Ozawa, S.; Ito, S.; Kato, Y.; Kubota, E.; Hata, R. Human p38 delta MAP kinase mediates UV irradiation induced up-regulation of the gene expression of chemokine BRAK/CXCL14. Biochem. Biophys. Res. Commun. 2010, 396, 1060–1064. [Google Scholar] [CrossRef]
- Komori, R.; Ozawa, S.; Kato, Y.; Shinji, H.; Kimoto, S.; Hata, R. Functional characterization of proximal promoter of gene for human BRAK/CXCL14, a tumor-suppressing chemokine. Biomed. Res. 2010, 31, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Tanegashima, K.; Takahashi, R.; Nuriya, H.; Iwase, R.; Naruse, N.; Tsuji, K.; Shigenaga, A.; Otaka, A.; Hara, T. CXCL14 acts as a specific carrier of CpG DNA into dendritic cells and activates Toll-like receptor 9-mediated adaptive immunity. EBioMedicine 2017, 24, 247–256. [Google Scholar] [CrossRef]
- Chen, L.; Guo, L.; Tian, J.; He, H.; Marinova, E.; Zhang, P.; Zheng, B.; Han, S. Overexpression of CXC chemokine ligand 14 exacerbates collagen-induced arthritis. J. Immunol. 2010, 184, 4455–4459. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.-Y.; Ozawa, S.; Kato, Y.; Maehata, Y.; Izukuri, K.; Ikoma, T.; Kanamori, K.; Akasaka, T.; Suzuki, K.; Iwabuchi, H.; et al. C-X-C Motif Chemokine Ligand 14 is a Unique Multifunctional Regulator of Tumor Progression. Int. J. Mol. Sci. 2019, 20, 1872. https://doi.org/10.3390/ijms20081872
Yang X-Y, Ozawa S, Kato Y, Maehata Y, Izukuri K, Ikoma T, Kanamori K, Akasaka T, Suzuki K, Iwabuchi H, et al. C-X-C Motif Chemokine Ligand 14 is a Unique Multifunctional Regulator of Tumor Progression. International Journal of Molecular Sciences. 2019; 20(8):1872. https://doi.org/10.3390/ijms20081872
Chicago/Turabian StyleYang, Xiao-Yan, Shigeyuki Ozawa, Yasumasa Kato, Yojiro Maehata, Kazuhito Izukuri, Takeharu Ikoma, Keisuke Kanamori, Tetsu Akasaka, Kenji Suzuki, Hiroshi Iwabuchi, and et al. 2019. "C-X-C Motif Chemokine Ligand 14 is a Unique Multifunctional Regulator of Tumor Progression" International Journal of Molecular Sciences 20, no. 8: 1872. https://doi.org/10.3390/ijms20081872
APA StyleYang, X.-Y., Ozawa, S., Kato, Y., Maehata, Y., Izukuri, K., Ikoma, T., Kanamori, K., Akasaka, T., Suzuki, K., Iwabuchi, H., Kurata, S.-I., Katoh, I., Sakurai, T., Kiyono, T., & Hata, R.-I. (2019). C-X-C Motif Chemokine Ligand 14 is a Unique Multifunctional Regulator of Tumor Progression. International Journal of Molecular Sciences, 20(8), 1872. https://doi.org/10.3390/ijms20081872




