Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells
Abstract
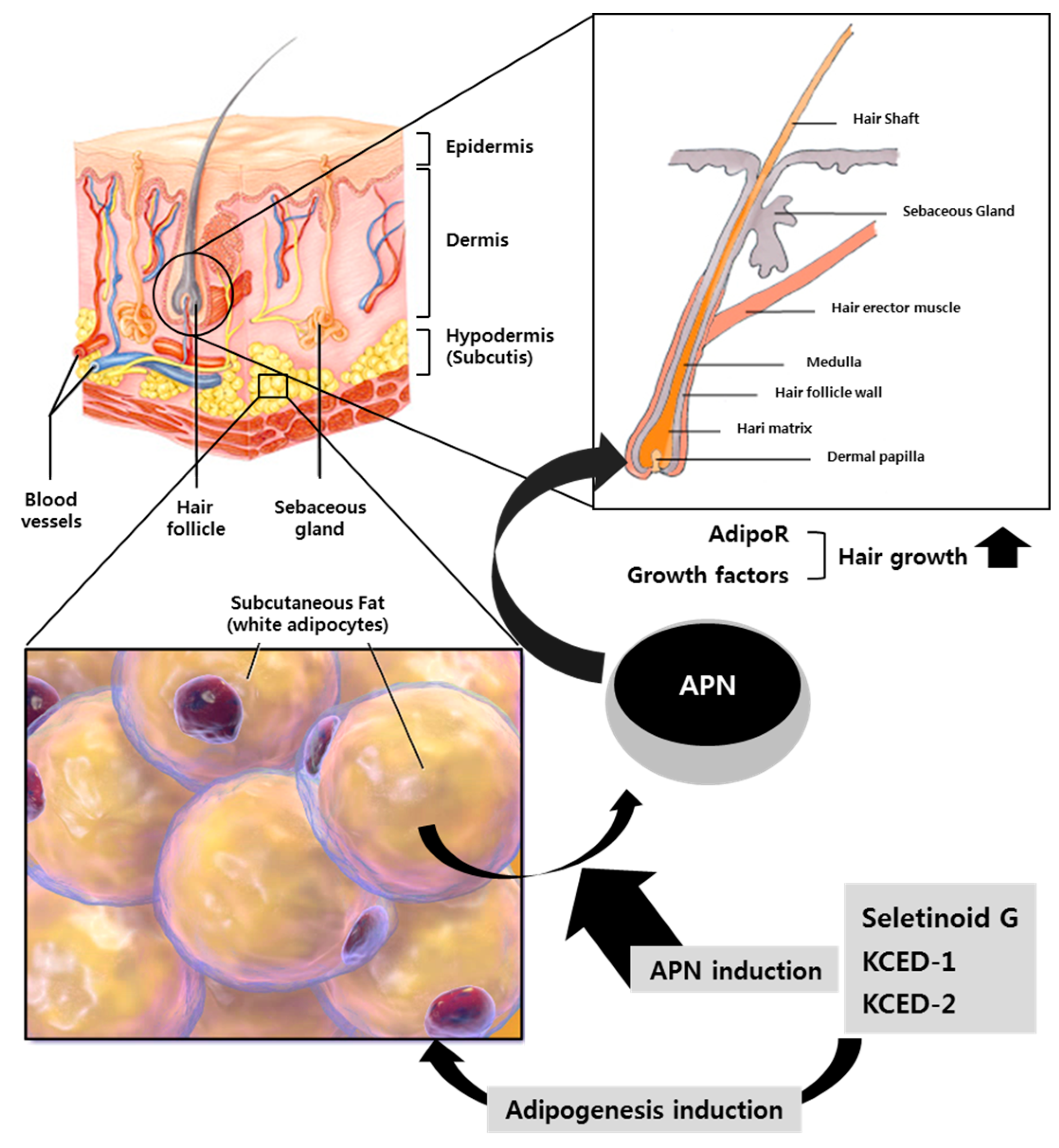
1. Introduction
2. Results and Discussion
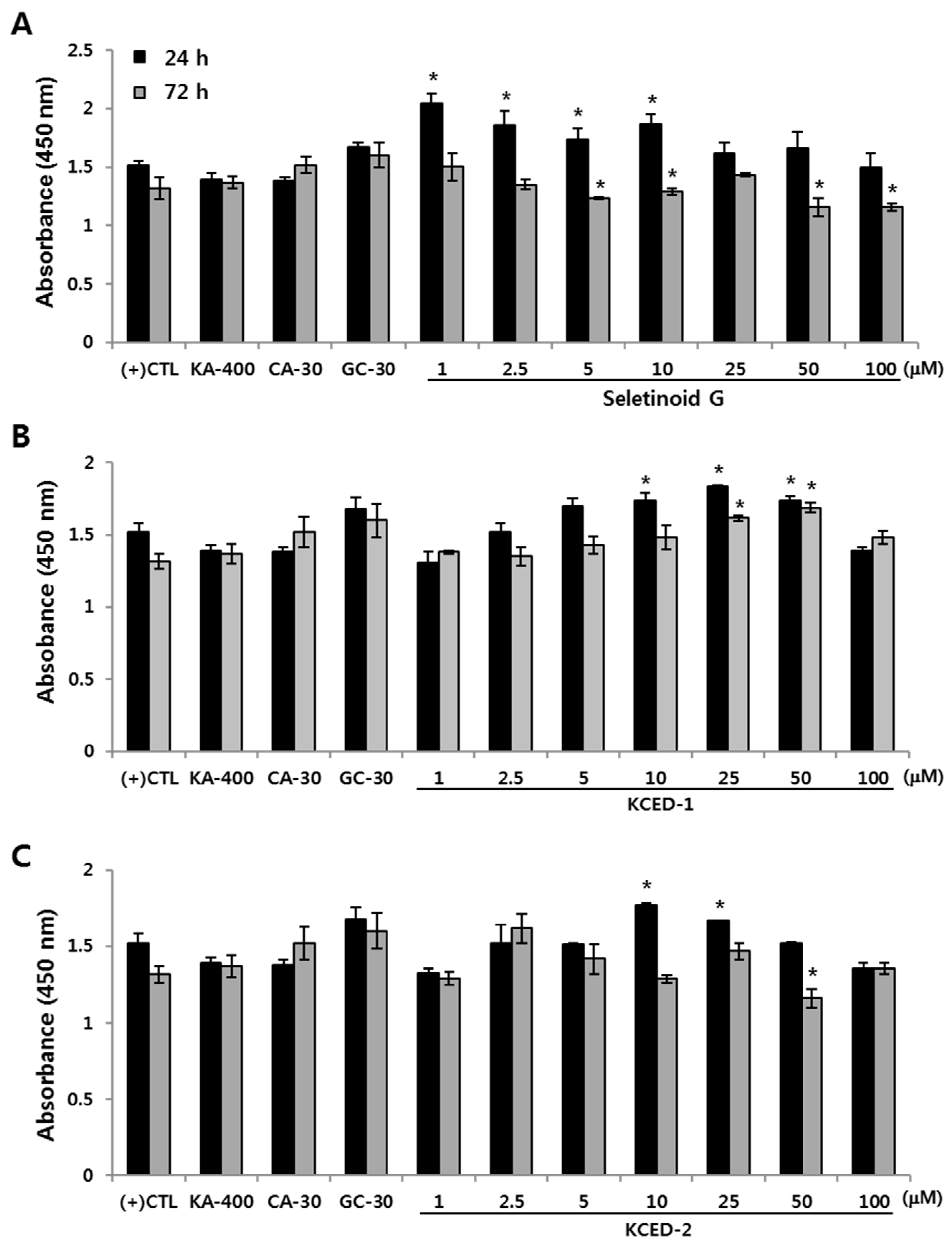
2.1. Selected Kojyl Cinnamate Ester Derivatives Show No Significant Effect On hSCF Viability
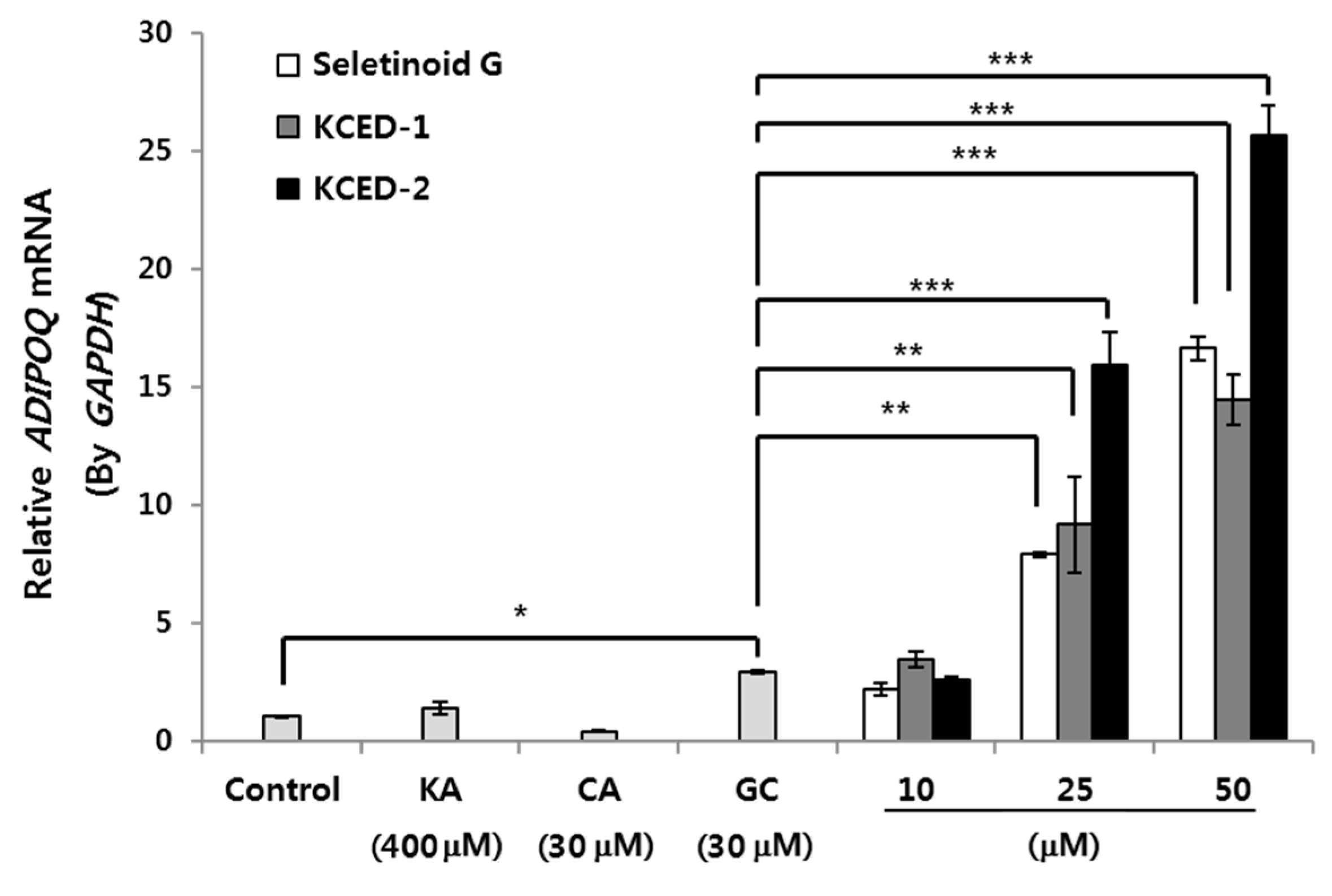
2.2. Selected Kojyl Cinnamate Ester Derivatives Induce mRNA Expression of APN
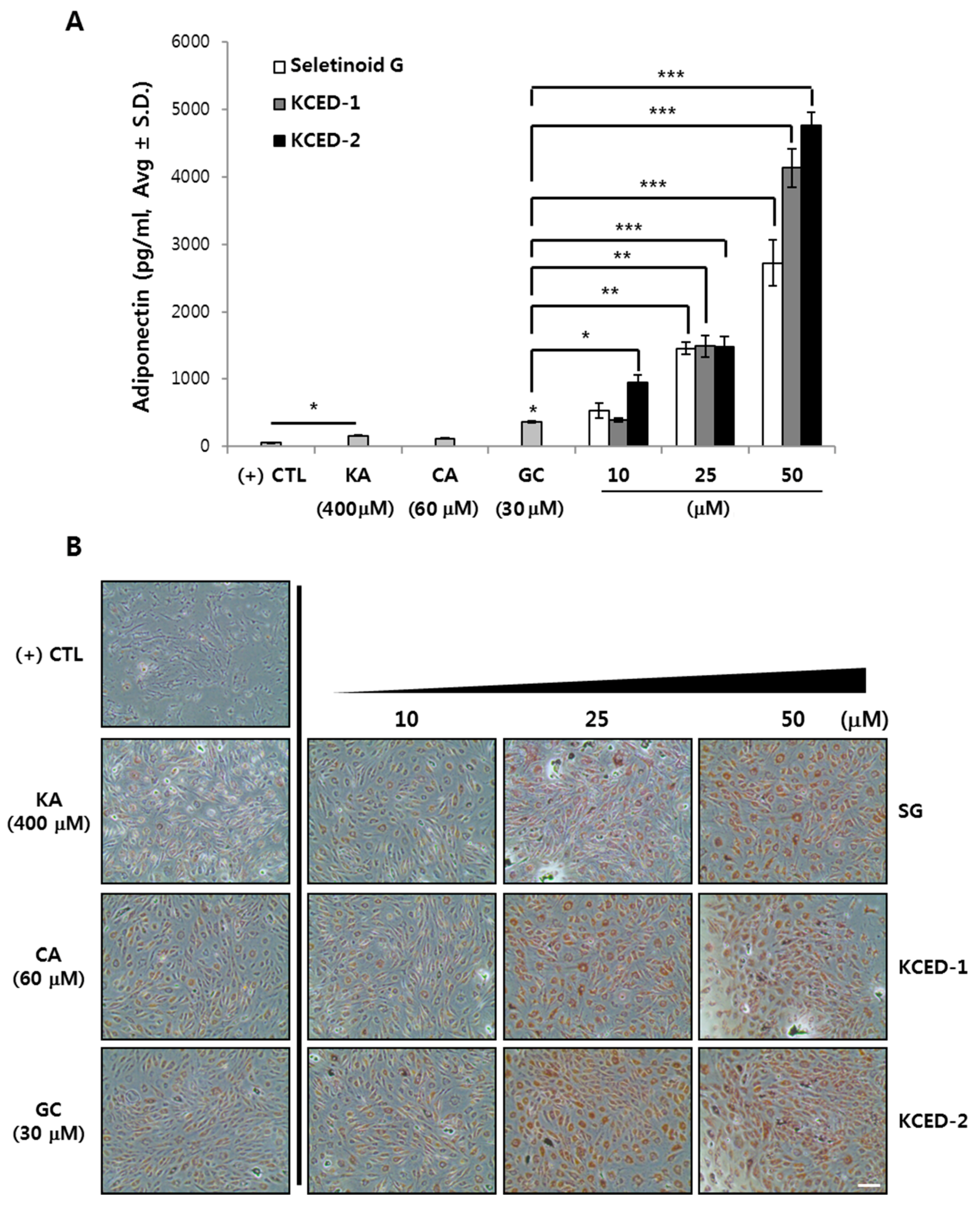
2.3. Selected Kojyl Cinnamate Ester Derivatives Promote APN Secretion and Stimulate Adipogenesis in hSCFs
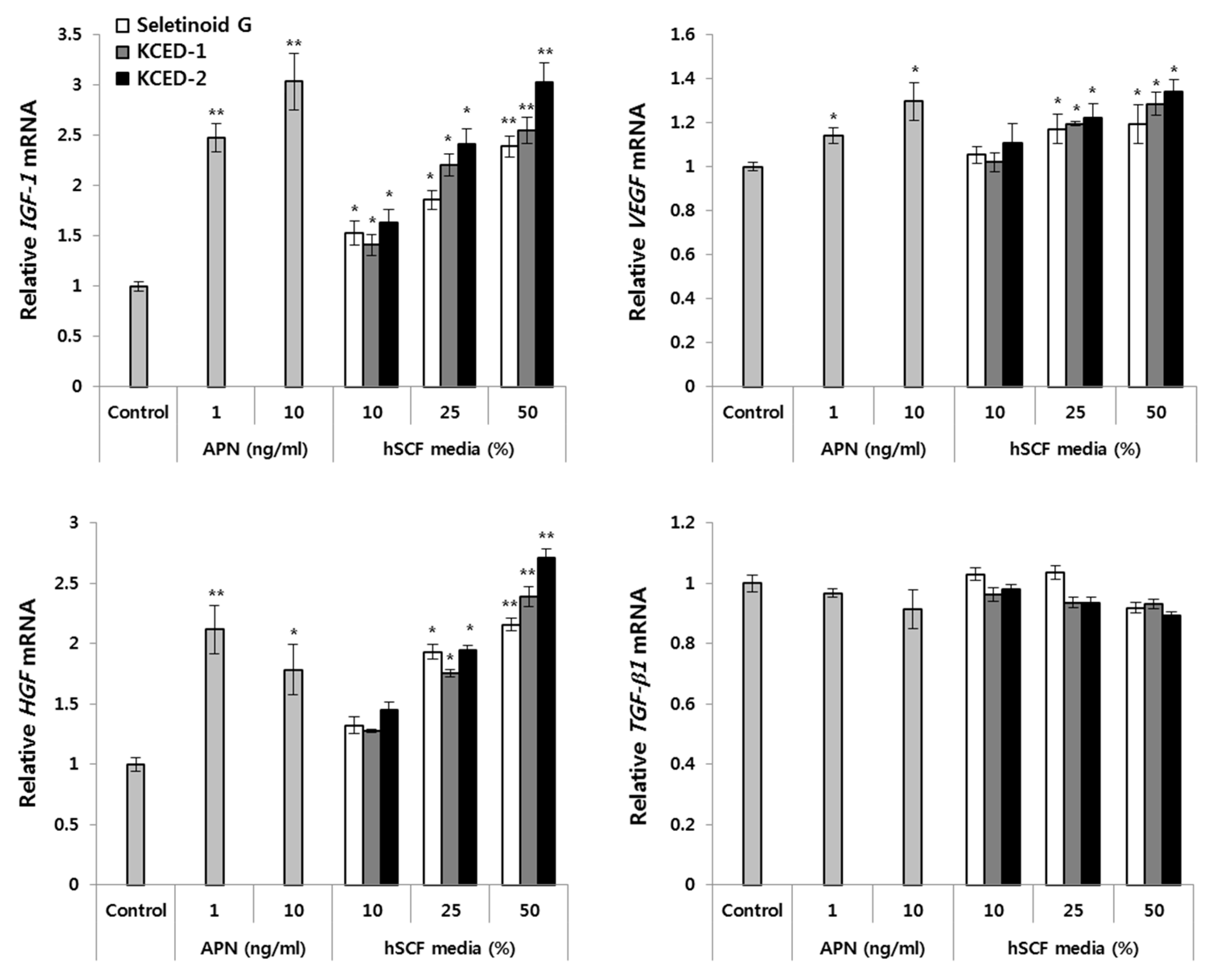
2.4. APN-Containing Culture Supernatants Induce the Expression of Hair Growth-related Factors in Human Follicular Dermal Papilla Cells
3. Materials and Methods
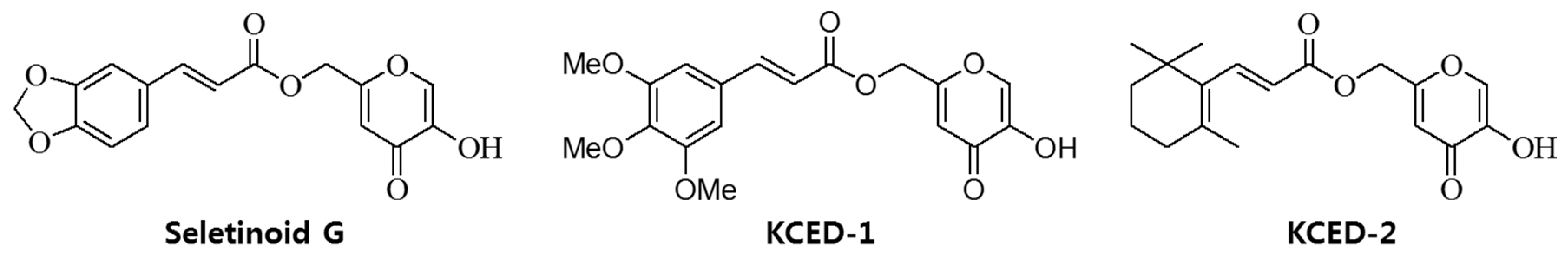
3.1. Compounds
3.2. Cell Culture, Differentiation, and Compound Treatment
3.3. Cell Viability Assay
3.4. Quantitative Real-time PCR (RT-qPCR)
3.5. ELISA Assay for Secreted Adiponectin
3.6. Oil Red O Staining
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APN | Adiponectin |
| KCED | Kojyl cinnamate ester derivative |
| hSCFs | Human subcutaneous fat cells |
| AdioR | Adiponectin receptor |
| hDPCs | Human follicular dermal papilla cells |
| SG | Seletinoid G |
| ADSCs | Adipose tissue-derived stem cells |
| KA | Kojic acid |
| CA | Cinnamic acid |
| GC | Glibenclamide |
| IGF-1 | Insulin-like growth factor 1 |
| VEGF | Vascular endothelial growth factor |
| HGF | Hepatocyte growth factor |
| TGF-β1 | Transforming growth factor β-1 |
| HF | Hair follicle |
| dWAT | Dermal white adipose tissue |
| sWAT | Subcutaneous white adipose tissue |
References
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Avram, M.M.; Avram, A.S.; James, W.D. Subcutaneous fat in normal and diseased states: 1. Introduction. J. Am. Acad. Dermatol. 2005, 53, 663–670. [Google Scholar] [CrossRef]
- Yosipovitch, G.; DeVore, A.; Dawn, A. Obesity and the skin: Skin physiology and skin manifestations of obesity. J. Am. Acad. Dermatol. 2007, 56, 901–916, quiz 917–920. [Google Scholar] [CrossRef] [PubMed]
- Ezure, T.; Amano, S. Influence of subcutaneous adipose tissue mass on dermal elasticity and sagging severity in lower cheek. Skin Res. Technol. 2010, 16, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Lafontan, M.; Viguerie, N. Role of adipokines in the control of energy metabolism: Focus on adiponectin. Curr. Opin. Pharmacol. 2006, 6, 580–585. [Google Scholar] [CrossRef]
- Yamauchi, T.; Iwabu, M.; Okada-Iwabu, M.; Kadowaki, T. Adiponectin receptors: A review of their structure, function and how they work. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Won, C.H.; Yoo, H.G.; Park, K.Y.; Shin, S.H.; Park, W.S.; Park, P.J.; Chung, J.H.; Kwon, O.S.; Kim, K.H. Hair growth-promoting effects of adiponectin in vitro. J. Invest. Dermatol. 2012, 132, 2849–2851. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.C.; Rho, H.S.; Baek, H.S.; Ahn, S.M.; Woo, B.Y.; Hong, Y.D.; Cheon, J.W.; Heo, J.M.; Shin, S.S.; Park, Y.H.; et al. Depigmenting activity of new kojic acid derivative obtained as a side product in the synthesis of cinnamate of kojic acid. Bioorg. Med. Chem. Lett. 2012, 22, 2004–2007. [Google Scholar] [CrossRef]
- Rho, H.S.; Hong, S.H.; Park, J.; Jung, H.I.; Park, Y.H.; Lee, J.H.; Shin, S.S.; Noh, M. Kojyl cinnamate ester derivatives promote adiponectin production during adipogenesis in human adipose tissue-derived mesenchymal stem cells. Bioorg. Med. Chem. Lett. 2014, 24, 2141–2145. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Lee, S.; Rho, H.S.; Kim, D.H.; Chang, I.S.; Chung, J.H. The effects of a novel synthetic retinoid, seletinoid G, on the expression of extracellular matrix proteins in aged human skin in vivo. Clin. Chim. Acta 2005, 362, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Khalili, M.; Khatami, K.; Farsadrooh, M.; Nahri-Niknafs, B. Synthesis and investigating hypoglycemic and hypolipidemic activities of some glibenclamide analogues in rats. Mini Rev. Med. Chem. 2014, 14, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Guerre-Millo, M. Adiponectin: An update. Diabetes Metab. 2008, 34, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 regulate PGC1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Waki, H.; Iwabu, M.; Okada-Iwabu, M.; Nakamura, M. Adiponectin, adiponectin receptors, and epigenetic regulation of adipogenesis. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Sifuentes, A.; Holland, W.L. Regulation of glucose and lipid homeostasis by adiponectin: Effects on hepatocytes, pancreatic β cells and adipocytes. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 43–58. [Google Scholar] [CrossRef]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef]
- Guerrero-Juarez, C.F.; Plikus, M.V. Emerging nonmetabolic functions of skin fat. Nat. Rev. Endocrinol. 2018, 14, 163–173. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Scherer, P.E. Dermal adipocytes and hair cycling: Is spatial heterogeneity a characteristic feature of the dermal adipose tissue depot? Exp. Dermatol. 2016, 25, 258–262. [Google Scholar] [CrossRef]
- Kruglikov, I.L.; Zhang, Z.; Scherer, P.E. The Role of Immature and Mature Adipocytes in Hair Cycling. Trends Endocrinol. Metab. 2019, 30, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Kruglikov, I.L.; Scherer, P.E. Skin aging: Are adipocytes the next target? Aging (Albany NY) 2016, 8, 1457–1469. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, P.J.; Cho, E.-G. Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2019, 20, 1859. https://doi.org/10.3390/ijms20081859
Park PJ, Cho E-G. Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells. International Journal of Molecular Sciences. 2019; 20(8):1859. https://doi.org/10.3390/ijms20081859
Chicago/Turabian StylePark, Phil June, and Eun-Gyung Cho. 2019. "Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells" International Journal of Molecular Sciences 20, no. 8: 1859. https://doi.org/10.3390/ijms20081859
APA StylePark, P. J., & Cho, E.-G. (2019). Kojyl Cinnamate Ester Derivatives Increase Adiponectin Expression and Stimulate Adiponectin-Induced Hair Growth Factors in Human Dermal Papilla Cells. International Journal of Molecular Sciences, 20(8), 1859. https://doi.org/10.3390/ijms20081859





