The Emerging Role of Adiponectin in Female Malignancies
Abstract
1. Introduction
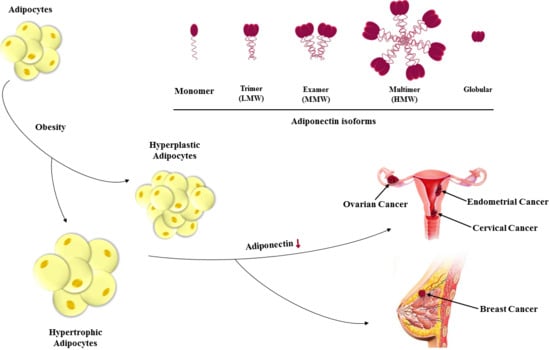
2. Adiponectin Structure and Biology
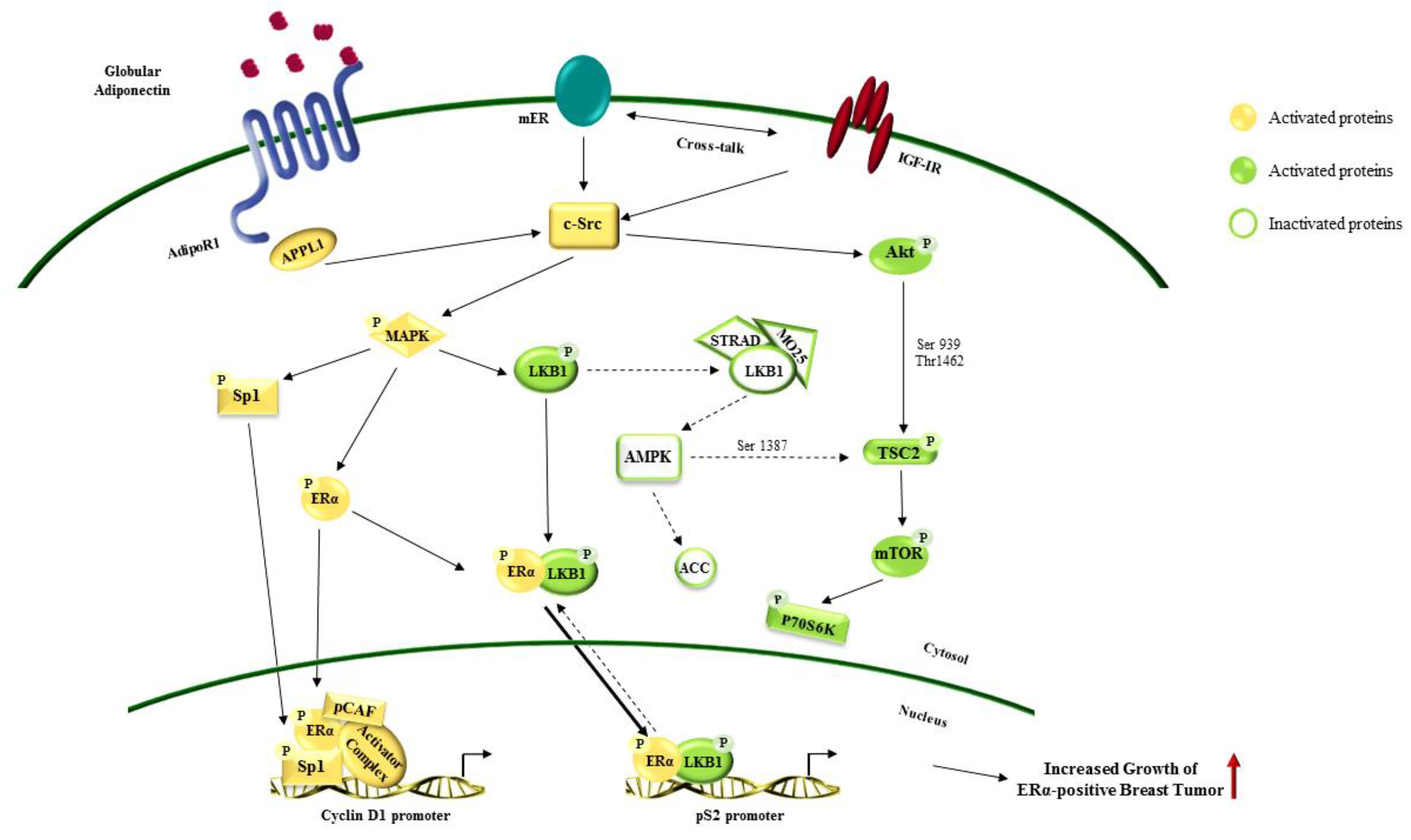
3. Adiponectin-Mediated Signaling Pathways
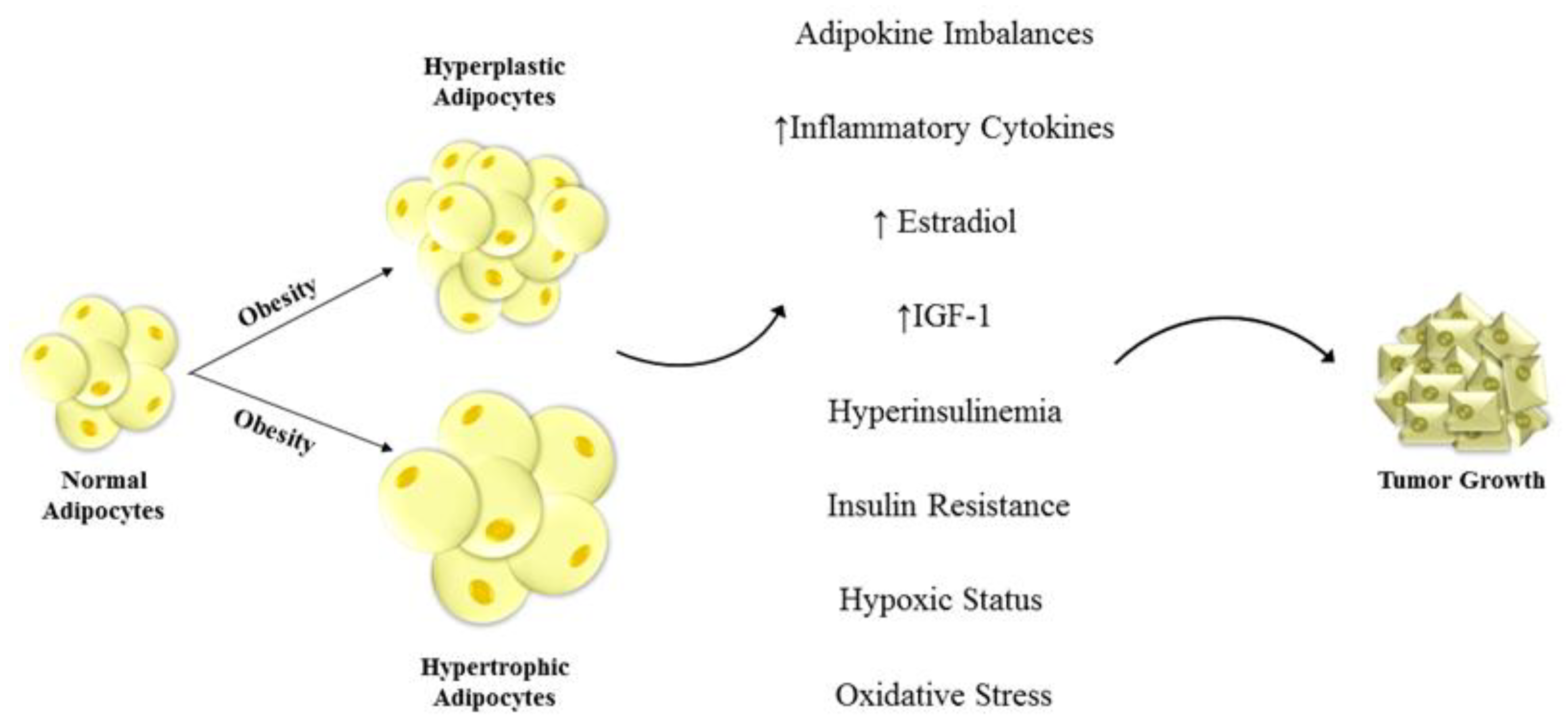
4. Adiponectin and Female Cancers
5. Adiponectin in Female Cancers
5.1. Cervical Cancer and Adiponectin
5.2. Ovarian Cancer and Adiponectin
5.3. Endometrial Cancer
5.4. Breast Cancer
6. Potential Therapeutic of Adiponectin
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| BMI | Body Mass Index |
| IGF-1 | Insulin-like Growth Factor-1 |
| fAd | full-length Adiponectin |
| LMW | Low Molecular Weight |
| MMW | Middle Molecular Weight |
| HMW | High Molecular Weight |
| gAd | globular Adiponectin |
| TNFα | Tumor Necrosis Factor α |
| PPAR-γ | Peroxisome Proliferator-Activated Receptor gamma |
| AdipoR1 | Adiponectin receptor 1 |
| AdipoR2 | Adiponectin receptor2 |
| LKB1 | Liver Kinase B 1 |
| AMPK | AMP-activated protein Kinase |
| Akt | Protein Kinase B |
| mTOR | mammalian Target of Rapamycin |
| S6K | ribosomal protein S6 Kinase |
| TSC2 | Tuberous Sclerosis Complex 2 |
| ERK1/2 | Extracellular signal-Regulated Kinases |
| PI3K | Phosphatidylinositol 3-Kinases |
| cJNK | c-Jun N-terminal kinase |
| STAT3 | Signal Transducer and Activator of Transcription |
| NF-kB | Nuclear Factor k B |
| E2 | Estradiol |
| ERα | Estrogen Receptor alpha |
| IGF1R | Insulin Growth Factor 1 Receptor |
| PR | Progesterone Receptor |
| TGF-β1 | Transforming growth factor beta 1 |
| SMAD2 | Small Mother Against Decapentaplegic 2 |
References
- Risk, N. Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar]
- Hubert, H.B.; Feinleib, M.; McNamara, P.M.; Castelli, W.P. Obesity as an independent risk factor for cardiovascular disease: A 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983, 67, 968–977. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Ford, E.S.; Bowman, B.A.; Dietz, W.H.; Vinicor, F.; Bales, V.S.; Marks, J.S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003, 289, 76–79. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; McDowell, M.A.; Tabak, C.J.; Flegal, K.M. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006, 295, 1549–1555. [Google Scholar] [CrossRef]
- Renehan, A.G.; Roberts, D.L.; Dive, C. Obesity and cancer: Pathophysiological and biological mechanisms. Arch. Physiol. Biochem. 2008, 114, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Obesity and colon and rectal cancer risk: A meta-analysis of prospective studies. Am. J. Clin. Nutr. 2007, 86, 556–565. [Google Scholar] [CrossRef]
- Hsing, A.W.; Sakoda, L.C.; Chua, S.C., Jr. Obesity, metabolic syndrome, and prostate cancer. Am. J. Clin. Nutr. 2007, 86, 843S–857S. [Google Scholar] [CrossRef]
- Basen-Engquist, K.; Chang, M. Obesity and cancer risk: Recent review and evidence. Curr. Oncol. Rep. 2011, 13, 71–76. [Google Scholar] [CrossRef]
- Goodwin, P.J.; Stambolic, V. Impact of the obesity epidemic on cancer. Annu. Rev. Med. 2015, 66, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Morley, T.S.; Kim, M.; Clegg, D.J.; Scherer, P.E. Obesity and cancer—Mechanisms underlying tumour progression and recurrence. Nat. Rev. Endocrinol. 2014, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Van Kruijsdijk, R.C.; Van Der Wall, E.; Visseren, F.L. Obesity and cancer: The role of dysfunctional adipose tissue. Cancer Epidemiol. Prev. Biomark. 2009, 18, 2569–2578. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, E.J.; LeRoith, D.; Karnieli, E. The metabolic syndrome—From insulin resistance to obesity and diabetes. Endocrinol. Metab. Clin. North Am. 2008, 37, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in obesity and type 2 diabetes: Close association with insulin resistance and hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef]
- Ouchi, N.; Kobayashi, H.; Kihara, S.; Kumada, M.; Sato, K.; Inoue, T.; Funahashi, T.; Walsh, K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004, 279, 1304–1309. [Google Scholar] [CrossRef]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.-i.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef]
- Barb, D.; Williams, C.J.; Neuwirth, A.K.; Mantzoros, C.S. Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am. J. Clin. Nutr. 2007, 86, 858S–866S. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef]
- Mantzoros, C.; Petridou, E.; Dessypris, N.; Chavelas, C.; Dalamaga, M.; Alexe, D.M.; Papadiamantis, Y.; Markopoulos, C.; Spanos, E.; Chrousos, G. Adiponectin and breast cancer risk. J. Clin. Endocrinol. Metab. 2004, 89, 1102–1107. [Google Scholar] [CrossRef]
- Petridou, E.; Mantzoros, C.; Dessypris, N.; Koukoulomatis, P.; Addy, C.; Voulgaris, Z.; Chrousos, G.; Trichopoulos, D. Plasma adiponectin concentrations in relation to endometrial cancer: A case-control study in Greece. J. Clin. Endocrinol. Metab. 2003, 88, 993–997. [Google Scholar] [CrossRef]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Polito, R.; Bartollino, S.; Nigro, E.; Porcile, C.; Bianco, A.; Daniele, A.; Moncharmont, B. Adiponectin as Link Factor between Adipose Tissue and Cancer. Int. J. Mol. Sci. 2019, 20, 839. [Google Scholar] [CrossRef]
- Ziemke, F.; Mantzoros, C.S. Adiponectin in insulin resistance: Lessons from translational research. Am. J. Clin. Nutr. 2009, 91, 258S–261S. [Google Scholar] [CrossRef]
- Brochu-Gaudreau, K.; Rehfeldt, C.; Blouin, R.; Bordignon, V.; Murphy, B.D.; Palin, M.-F. Adiponectin action from head to toe. Endocrine 2010, 37, 11–32. [Google Scholar] [CrossRef]
- Lee, C.; Woo, Y.; Wang, Y.; Yeung, C.; Xu, A.; Lam, K. Obesity, adipokines and cancer: An update. Clin. Endocrinol. 2015, 83, 147–156. [Google Scholar] [CrossRef]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPoseMost abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef]
- Fujimoto, N.; Matsuo, N.; Sumiyoshi, H.; Yamaguchi, K.; Saikawa, T.; Yoshimatsu, H.; Yoshioka, H. Adiponectin is expressed in the brown adipose tissue and surrounding immature tissues in mouse embryos. Biochim. Biophys. Acta 2005, 1731, 1–12. [Google Scholar] [CrossRef]
- Chen, J.; Tan, B.; Karteris, E.; Zervou, S.; Digby, J.; Hillhouse, E.; Vatish, M.; Randeva, H. Secretion of adiponectin by human placenta: Differential modulation of adiponectin and its receptors by cytokines. Diabetologia 2006, 49, 1292. [Google Scholar] [CrossRef]
- Fayad, R.; Pini, M.; Sennello, J.A.; Cabay, R.J.; Chan, L.; Xu, A.; Fantuzzi, G. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology 2007, 132, 601–614. [Google Scholar] [CrossRef]
- Delaigle, A.l.M.; Jonas, J.-C.; Bauche, I.B.; Cornu, O.; Brichard, S.M. Induction of adiponectin in skeletal muscle by inflammatory cytokines: In vivo and in vitro studies. Endocrinology 2004, 145, 5589–5597. [Google Scholar] [CrossRef]
- Katsiougiannis, S.; Kapsogeorgou, E.K.; Manoussakis, M.N.; Skopouli, F.N. Salivary gland epithelial cells: A new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006, 54, 2295–2299. [Google Scholar] [CrossRef]
- Kaser, S.; Moschen, A.; Cayon, A.; Kaser, A.; Crespo, J.; Pons-Romero, F.; Ebenbichler, C.; Patsch, J.; Tilg, H. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut 2005, 54, 117–121. [Google Scholar] [CrossRef]
- Nishida, M.; Funahashi, T.; Shimomura, I. Pathophysiological significance of adiponectin. Med Mol. Morphol. 2007, 40, 55–67. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Wong, G.W.; Wang, J.; Hug, C.; Tsao, T.-S.; Lodish, H.F. A family of Acrp30/adiponectin structural and functional paralogs. Proc. Natl. Acad. Sci. USA 2004, 101, 10302–10307. [Google Scholar] [CrossRef]
- Wong, G.W.; Krawczyk, S.A.; Kitidis-Mitrokostas, C.; Revett, T.; Gimeno, R.; Lodish, H.F. Molecular, biochemical and functional characterizations of C1q/TNF family members: Adipose-tissue-selective expression patterns, regulation by PPAR-γ agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem. J. 2008, 416, 161–177. [Google Scholar] [CrossRef]
- Hada, Y.; Yamauchi, T.; Waki, H.; Tsuchida, A.; Hara, K.; Yago, H.; Miyazaki, O.; Ebinuma, H.; Kadowaki, T. Selective purification and characterization of adiponectin multimer species from human plasma. Biochem. Biophys. Res. Commun. 2007, 356, 487–493. [Google Scholar] [CrossRef]
- Tsao, T.-S.; Tomas, E.; Murrey, H.E.; Hug, C.; Lee, D.H.; Ruderman, N.B.; Heuser, J.E.; Lodish, H.F. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity: Different oligomers activate different signal transduction pathways. J. Biol. Chem. 2003, 278, 50810–50817. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Knight, C.; Xu, L.Y.; Cooper, G.J. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin potential role in the modulation of its insulin-sensitizing activity. J. Biol. Chem. 2002, 277, 19521–19529. [Google Scholar] [CrossRef]
- Waki, H.; Yamauchi, T.; Kamon, J.; Ito, Y.; Uchida, S.; Kita, S.; Hara, K.; Hada, Y.; Vasseur, F.; Froguel, P. Impaired multimerization of human adiponectin mutants associated with diabetes: Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003, 278, 40352–40363. [Google Scholar] [CrossRef]
- Pajvani, U.B.; Du, X.; Combs, T.P.; Berg, A.H.; Rajala, M.W.; Schulthess, T.; Engel, J.; Brownlee, M.; Scherer, P.E. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin implications for metabolic regulation and bioactivity. J. Biol. Chem. 2003, 278, 9073–9085. [Google Scholar] [CrossRef]
- Fruebis, J.; Tsao, T.-S.; Javorschi, S.; Ebbets-Reed, D.; Erickson, M.R.S.; Yen, F.T.; Bihain, B.E.; Lodish, H.F. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 2005–2010. [Google Scholar] [CrossRef]
- Waki, H.; Yamauchi, T.; Kamon, J.; Kita, S.; Ito, Y.; Hada, Y.; Uchida, S.; Tsuchida, A.; Takekawa, S.; Kadowaki, T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology 2005, 146, 790–796. [Google Scholar] [CrossRef]
- Goldstein, B.J.; Scalia, R. Adiponectin: A novel adipokine linking adipocytes and vascular function. J. Clin. Endocrinol. Metab. 2004, 89, 2563–2568. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef]
- Cnop, M.; Havel, P.; Utzschneider, K.; Carr, D.; Sinha, M.; Boyko, E.; Retzlaff, B.; Knopp, R.; Brunzell, J.; Kahn, S.E. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: Evidence for independent roles of age and sex. Diabetologia 2003, 46, 459–469. [Google Scholar] [CrossRef]
- Swarbrick, M.M.; Havel, P.J. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab. Syndr. Relat. Disord. 2008, 6, 87–102. [Google Scholar] [CrossRef]
- Combs, T.P.; Wagner, J.A.; Berger, J.; Doebber, T.; Wang, W.-J.; Zhang, B.B.; Tanen, M.; Berg, A.H.; O’rahilly, S.; Savage, D.B. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARγ agonists: A potential mechanism of insulin sensitization. Endocrinology 2002, 143, 998–1007. [Google Scholar] [CrossRef]
- Maeda, N.; Takahashi, M.; Funahashi, T.; Kihara, S.; Nishizawa, H.; Kishida, K.; Nagaretani, H.; Matsuda, M.; Komuro, R.; Ouchi, N. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 2001, 50, 2094–2099. [Google Scholar] [CrossRef]
- Joseph, G.Y.; Javorschi, S.; Hevener, A.L.; Kruszynska, Y.T.; Norman, R.A.; Sinha, M.; Olefsky, J.M. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes 2002, 51, 2968–2974. [Google Scholar]
- Surmacz, E. Leptin and adiponectin: Emerging therapeutic targets in breast cancer. J. Mammary Gland Biol. Neoplasia 2013, 18, 321–332. [Google Scholar] [CrossRef]
- Khan, S.; Shukla, S.; Sinha, S.; Meeran, S.M. Role of adipokines and cytokines in obesity-associated breast cancer: Therapeutic targets. Cytokine Growth Factor Rev. 2013, 24, 503–513. [Google Scholar] [CrossRef]
- Takahashi, M.; Arita, Y.; Yamagata, K.; Matsukawa, Y.; Okutomi, K.; Horie, M.; Shimomura, I.; Hotta, K.; Kuriyama, H.; Kihara, S. Genomic structure and mutations in adipose-specific gene, adiponectin. Int. J. Obes. 2000, 24, 861. [Google Scholar] [CrossRef]
- Kaklamani, V.G.; Sadim, M.; Hsi, A.; Offit, K.; Oddoux, C.; Ostrer, H.; Ahsan, H.; Pasche, B.; Mantzoros, C. Variants of the adiponectin and adiponectin receptor 1 genes and breast cancer risk. Cancer Res. 2008, 68, 3178–3184. [Google Scholar] [CrossRef]
- Simpson, F.; Whitehead, J.P. Adiponectin—It’s all about the modifications. Int. J. Biochem. Cell Biol. 2010, 42, 785–788. [Google Scholar] [CrossRef]
- Trujillo, M.; Hanif, W.; Barnett, A.; McTernan, P.; Scherer, P.; Kumar, S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 2005, 48, 1084–1087. [Google Scholar]
- Trujillo, M.; Scherer, P. Adiponectin–journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J. Intern. Med. 2005, 257, 167–175. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762. [Google Scholar] [CrossRef]
- Panno, M.L.; Naimo, G.D.; Spina, E.; Andò, S.; Mauro, L. Different molecular signaling sustaining adiponectin action in breast cancer. Curr. Opin. Pharmacol. 2016, 31, 1–7. [Google Scholar] [CrossRef]
- Hug, C.; Wang, J.; Ahmad, N.S.; Bogan, J.S.; Tsao, T.-S.; Lodish, H.F. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl. Acad. Sci. USA 2004, 101, 10308–10313. [Google Scholar] [CrossRef]
- Asada, K.; Yoshiji, H.; Noguchi, R.; Ikenaka, Y.; Kitade, M.; Kaji, K.; Yoshii, J.; Yanase, K.; Namisaki, T.; Yamazaki, M. Crosstalk between high-molecular-weight adiponectin and T-cadherin during liver fibrosis development in rats. Int. J. Mol. Med. 2007, 20, 725–729. [Google Scholar]
- Chan, D.W.; Lee, J.M.; Chan, P.C.; Ng, I.O. Genetic and epigenetic inactivation of T-cadherin in human hepatocellular carcinoma cells. Int. J. Cancer 2008, 123, 1043–1052. [Google Scholar] [CrossRef]
- Lee, M.-H.; Klein, R.L.; El-Shewy, H.M.; Luttrell, D.K.; Luttrell, L.M. The adiponectin receptors AdipoR1 and AdipoR2 activate ERK1/2 through a Src/Ras-dependent pathway and stimulate cell growth. Biochemistry 2008, 47, 11682–11692. [Google Scholar] [CrossRef]
- Hebbard, L.W.; Garlatti, M.; Young, L.J.; Cardiff, R.D.; Oshima, R.G.; Ranscht, B. T-cadherin supports angiogenesis and adiponectin association with the vasculature in a mouse mammary tumor model. Cancer Res. 2008, 68, 1407–1416. [Google Scholar] [CrossRef]
- Berg, A.H.; Combs, T.P.; Scherer, P.E. ACRP30/adiponectin: An adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002, 13, 84–89. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Rajitha, B.; Aliya, S.; Kotipatruni, R.P.; Madanraj, A.S.; Hammond, A.; Park, D.; Chigurupati, S.; Alam, A.; Pattnaik, S. The role of adiponectin in obesity-associated female-specific carcinogenesis. Cytokine Growth Factor Rev. 2016, 31, 37–48. [Google Scholar] [CrossRef]
- Kharroubi, I.; Rasschaert, J.; Eizirik, D.L.; Cnop, M. Expression of adiponectin receptors in pancreatic β cells. Biochem. Biophys. Res. Commun. 2003, 312, 1118–1122. [Google Scholar] [CrossRef]
- Igata, M.; Motoshima, H.; Tsuruzoe, K.; Kojima, K.; Matsumura, T.; Kondo, T.; Taguchi, T.; Nakamaru, K.; Yano, M.; Kukidome, D. Adenosine monophosphate-activated protein kinase suppresses vascular smooth muscle cell proliferation through the inhibition of cell cycle progression. Circ. Res. 2005, 97, 837–844. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M. Adiponectin and AdipoR1 regulate PGC-1α and mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313. [Google Scholar] [CrossRef]
- Miyazaki, T.; Bub, J.D.; Uzuki, M.; Iwamoto, Y. Adiponectin activates c-Jun NH2-terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem. Biophys. Res. Commun. 2005, 333, 79–87. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.-E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
- Dieudonne, M.-N.; Bussiere, M.; Dos Santos, E.; Leneveu, M.-C.; Giudicelli, Y.; Pecquery, R. Adiponectin mediates antiproliferative and apoptotic responses in human MCF7 breast cancer cells. Biochem. Biophys. Res. Commun. 2006, 345, 271–279. [Google Scholar] [CrossRef]
- Ouchi, N.; Kihara, S.; Arita, Y.; Okamoto, Y.; Maeda, K.; Kuriyama, H.; Hotta, K.; Nishida, M.; Takahashi, M.; Muraguchi, M. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 2000, 102, 1296–1301. [Google Scholar] [CrossRef]
- Wang, Y.; Lam, J.B.; Lam, K.S.; Liu, J.; Lam, M.C.; Hoo, R.L.; Wu, D.; Cooper, G.J.; Xu, A. Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res. 2006, 66, 11462–11470. [Google Scholar] [CrossRef]
- Matafome, P.; Santos-Silva, D.; Sena, C.; Seica, R. Common mechanisms of dysfunctional adipose tissue and obesity-related cancers. Diabetes Metab. Res. Rev. 2013, 29, 285–295. [Google Scholar] [CrossRef]
- Choi, J.; Cha, Y.J.; Koo, J.S. Adipocyte biology in breast cancer: From silent bystander to active facilitator. Prog. Lipid Res. 2017. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Benedetto, C.; Salvagno, F.; Canuto, E.M.; Gennarelli, G. Obesity and female malignancies. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 528–540. [Google Scholar] [CrossRef]
- Gu, W.; Chen, C.; Zhao, K.-N. Obesity-associated endometrial and cervical cancers. Front. Biosci. (Elite Ed.) 2013, 5, 109–118. [Google Scholar] [CrossRef]
- Jee, S.H.; Yun, J.E.; Park, E.J.; Cho, E.R.; Park, I.S.; Sull, J.W.; Ohrr, H.; Samet, J.M. Body mass index and cancer risk in Korean men and women. Int. J. Cancer 2008, 123, 1892–1896. [Google Scholar] [CrossRef]
- Kemp, T.J.; Hildesheim, A.; García-Piñeres, A.; Williams, M.C.; Shearer, G.M.; Rodriguez, A.C.; Schiffman, M.; Burk, R.; Freer, E.; Bonilla, J. Elevated systemic levels of inflammatory cytokines in older women with persistent cervical human papillomavirus infection. Cancer Epidemiol. Prev. Biomark. 2010, 19, 1954–1959. [Google Scholar] [CrossRef]
- Ulmer, H.; Bjørge, T.; Concin, H.; Lukanova, A.; Manjer, J.; Hallmans, G.; Borena, W.; Häggström, C.; Engeland, A.; Almquist, M. Metabolic risk factors and cervical cancer in the metabolic syndrome and cancer project (Me–Can). Gynecol. Oncol. 2012, 125, 330–335. [Google Scholar] [CrossRef]
- Lacey, J.V., Jr.; Swanson, C.A.; Brinton, L.A.; Altekruse, S.F.; Barnes, W.A.; Gravitt, P.E.; Greenberg, M.D.; Hadjimichael, O.C.; McGowan, L.; Mortel, R. Obesity as a potential risk factor for adenocarcinomas and squamous cell carcinomas of the uterine cervix. Cancer 2003, 98, 814–821. [Google Scholar] [CrossRef]
- Maruthur, N.M.; Bolen, S.D.; Brancati, F.L.; Clark, J.M. The association of obesity and cervical cancer screening: A systematic review and meta-analysis. Obesity 2009, 17, 375–381. [Google Scholar] [CrossRef]
- Xie, L.; Wang, Y.; Wang, S.; Wu, N.; Chen, Y.; Yan, J. Adiponectin induces growth inhibition and apoptosis in cervical cancer HeLa cells. Biologia 2011, 66, 712–720. [Google Scholar] [CrossRef]
- Otsuka, I.; Kameda, S.; Hoshi, K. Early detection of ovarian and fallopian tube cancer by examination of cytological samples from the endometrial cavity. Br. J. Cancer 2013, 109, 603. [Google Scholar] [CrossRef]
- Romero, I.; Bast, R.C., Jr. Minireview: Human ovarian cancer: Biology, current management, and paths to personalizing therapy. Endocrinology 2012, 153, 1593–1602. [Google Scholar] [CrossRef]
- Nagle, C.; Dixon, S.; Jensen, A.; Kjaer, S.; Modugno, F.; Fereday, S.; Hung, J.; Johnatty, S.; Fasching, P.; Beckmann, M. Obesity and survival among women with ovarian cancer: Results from the Ovarian Cancer Association Consortium. Br. J. Cancer 2015, 113, 817. [Google Scholar] [CrossRef]
- Olsen, C.M.; Green, A.C.; Whiteman, D.C.; Sadeghi, S.; Kolahdooz, F.; Webb, P.M. Obesity and the risk of epithelial ovarian cancer: A systematic review and meta-analysis. Eur. J. Cancer 2007, 43, 690–709. [Google Scholar] [CrossRef]
- Leitzmann, M.F.; Koebnick, C.; Danforth, K.N.; Brinton, L.A.; Moore, S.C.; Hollenbeck, A.R.; Schatzkin, A.; Lacey, J.V., Jr. Body mass index and risk of ovarian cancer. Cancer 2009, 115, 812–822. [Google Scholar] [CrossRef]
- Cancer, C.G.o.E.S.o.O. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23 257 women with ovarian cancer and 87 303 controls. Lancet 2008, 371, 303–314. [Google Scholar]
- Bhaskaran, K.; Douglas, I.; Forbes, H.; dos-Santos-Silva, I.; Leon, D.A.; Smeeth, L. Body-mass index and risk of 22 specific cancers: A population-based cohort study of 5· 24 million UK adults. Lancet 2014, 384, 755–765. [Google Scholar] [CrossRef]
- Ose, J.; Fortner, R.T.; Rinaldi, S.; Schock, H.; Overvad, K.; Tjonneland, A.; Hansen, L.; Dossus, L.; Fournier, A.; Baglietto, L. Endogenous androgens and risk of epithelial invasive ovarian cancer by tumor characteristics in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2015, 136, 399–410. [Google Scholar] [CrossRef]
- Uddin, S.; Bu, R.; Ahmed, M.; Abubaker, J.; Al-Dayel, F.; Bavi, P.; Al-Kuraya, K.S. Overexpression of leptin receptor predicts an unfavorable outcome in Middle Eastern ovarian cancer. Mol. Cancer 2009, 8, 74. [Google Scholar] [CrossRef]
- Chen, C.; Chang, Y.-C.; Lan, M.S.; Breslin, M. Leptin stimulates ovarian cancer cell growth and inhibits apoptosis by increasing cyclin D1 and Mcl-1 expression via the activation of the MEK/ERK1/2 and PI3K/Akt signaling pathways. Corrigendum in/10.3892/ijo. 2016.3564. Int. J. Oncol. 2013, 42, 1113–1119. [Google Scholar] [CrossRef]
- Aune, G.; Stunes, A.K.; Lian, A.-M.; Reseland, J.E.; Tingulstad, S.; Torp, S.H.; Syversen, U. Circulating interleukin-8 and plasminogen activator inhibitor-1 are increased in women with ovarian carcinoma. Results Immunol. 2012, 2, 190–195. [Google Scholar] [CrossRef][Green Version]
- Otokozawa, S.; Tanaka, R.; Akasaka, H.; Ito, E.; Asakura, S.; Ohnishi, H.; Saito, S.; Miura, T.; Saito, T.; Mori, M. Associations of serum isoflavone, adiponectin and insulin levels with risk for epithelial ovarian cancer: Results of a case-control study. Asian Pac. J. Cancer Prev. 2015, 16, 4987–4991. [Google Scholar] [CrossRef]
- Jin, J.H.; Kim, H.-J.; Kim, C.Y.; Kim, Y.H.; Ju, W.; Kim, S.C. Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer. Obstet. Gynecol. Sci. 2016, 59, 279–285. [Google Scholar] [CrossRef]
- Wu, M.-M.; Chen, H.-C.; Chen, C.-L.; You, S.-L.; Cheng, W.-F.; Chen, C.-A.; Lee, T.-C.; Chen, C.-J. A prospective study of gynecological cancer risk in relation to adiposity factors: Cumulative incidence and association with plasma adipokine levels. PLoS ONE 2014, 9, e104630. [Google Scholar] [CrossRef]
- Diaz, E.S.; Karlan, B.Y.; Li, A.J. Obesity-associated adipokines correlate with survival in epithelial ovarian cancer. Gynecol. Oncol. 2013, 129, 353–357. [Google Scholar] [CrossRef]
- Hoffmann, M.; Gogola, J.; Ptak, A. Adiponectin Reverses the Proliferative Effects of Estradiol and IGF-1 in Human Epithelial Ovarian Cancer Cells by Downregulating the Expression of Their Receptors. Horm. Cancer 2018, 9, 166–174. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.; Fang, L.; Liu, F.; Jiang, K. Expression of adiponectin receptor-1 and prognosis of epithelial ovarian cancer patients. Med Sci. Monit. 2017, 23, 1514. [Google Scholar] [CrossRef]
- Dupont, J.; Reverchon, M.; Cloix, L.; Froment, P.; Ramé, C. Involvement of adipokines, AMPK, PI3K and the PPAR signaling pathways in ovarian follicle development and cancer. Int. J. Dev. Biol. 2013, 56, 959–967. [Google Scholar] [CrossRef]
- Huijgens, A.; Mertens, H. Factors predicting recurrent endometrial cancer. Facts Views Vis. ObGyn 2013, 5, 179. [Google Scholar]
- Arem, H.; Irwin, M. Obesity and endometrial cancer survival: A systematic review. Int. J. Obes. 2013, 37, 634. [Google Scholar] [CrossRef]
- Gunter, M.J.; Hoover, D.R.; Yu, H.; Wassertheil-Smoller, S.; Manson, J.E.; Li, J.; Harris, T.G.; Rohan, T.E.; Xue, X.; Ho, G.Y. A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer. Cancer Epidemiol. Prev. Biomark. 2008, 17, 921–929. [Google Scholar] [CrossRef]
- Berstein, L.; Kvatchevskaya, J.; Poroshina, T.; Kovalenko, I.; Tsyrlina, E.; Zimarina, T.; Ourmantcheeva, A.; Ashrafian, L.; Thijssen, J. Insulin resistance, its consequences for the clinical course of the disease, and possibilities of correction in endometrial cancer. J. Cancer Res. Clin. Oncol. 2004, 130, 687–693. [Google Scholar] [CrossRef]
- Cust, A.E.; Kaaks, R.; Friedenreich, C.; Bonnet, F.; Laville, M.; Lukanova, A.; Rinaldi, S.; Dossus, L.; Slimani, N.; Lundin, E. Plasma adiponectin levels and endometrial cancer risk in pre-and postmenopausal women. J. Clin. Endocrinol. Metab. 2007, 92, 255–263. [Google Scholar] [CrossRef]
- Ashizawa, N.; Yahata, T.; Quan, J.; Adachi, S.; Yoshihara, K.; Tanaka, K. Serum leptin–adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol. Oncol. 2010, 119, 65–69. [Google Scholar] [CrossRef]
- Gong, T.T.; Wu, Q.J.; Wang, Y.L.; Ma, X.X. Circulating adiponectin, leptin and adiponectin–leptin ratio and endometrial cancer risk: Evidence from a meta-analysis of epidemiologic studies. Int. J. Cancer 2015, 137, 1967–1978. [Google Scholar] [CrossRef]
- Li, Z.J.; Yang, X.L.; Yao, Y.; Han, W.Q.; Li, B. Circulating adiponectin levels and risk of endometrial cancer: Systematic review and meta-analysis. Exp. Ther. Med. 2016, 11, 2305–2313. [Google Scholar] [CrossRef]
- Lin, T.; Zhao, X.; Kong, W.-m. Association between adiponectin levels and endometrial carcinoma risk: Evidence from a dose–response meta-analysis. BMJ Open 2015, 5, e008541. [Google Scholar] [CrossRef]
- Zeng, F.; Shi, J.; Long, Y.; Tian, H.; Li, X.; Zhao, A.Z.; Li, R.F.; Chen, T. Adiponectin and endometrial cancer: A systematic review and meta-analysis. Cell. Physiol. Biochem. 2015, 36, 1670–1678. [Google Scholar] [CrossRef]
- Moon, H.-S.; Chamberland, J.P.; Aronis, K.; Tseleni-Balafouta, S.; Mantzoros, C.S. Direct role of adiponectin and adiponectin receptors in endometrial cancer: In vitro and ex vivo studies in humans. Mol. Cancer Ther. 2011, 10, 2234–2243. [Google Scholar] [CrossRef]
- Cong, L.; Gasser, J.; Zhao, J.; Yang, B.; Li, F.; Zhao, A.Z. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95–2. Endocr. Relat. Cancer 2007, 14, 713–720. [Google Scholar] [CrossRef]
- Harvie, M.; Hooper, L.; Howell, A. Central obesity and breast cancer risk: A systematic review. Obes. Rev. 2003, 4, 157–173. [Google Scholar] [CrossRef]
- Lahmann, P.H.; Hoffmann, K.; Allen, N.; Van Gils, C.H.; Khaw, K.T.; Tehard, B.; Berrino, F.; Tjønneland, A.; Bigaard, J.; Olsen, A. Body size and breast cancer risk: Findings from the European Prospective Investigation into Cancer And Nutrition (EPIC). Int. J. Cancer 2004, 111, 762–771. [Google Scholar] [CrossRef]
- Michels, K.B.; Terry, K.L.; Willett, W.C. Longitudinal study on the role of body size in premenopausal breast cancer. Arch. Intern. Med. 2006, 166, 2395–2402. [Google Scholar] [CrossRef]
- Barone, I.; Catalano, S.; Gelsomino, L.; Marsico, S.; Giordano, C.; Panza, S.; Bonofiglio, D.; Bossi, G.; Covington, K.R.; Fuqua, S.A. Leptin mediates tumor–stromal interactions that promote the invasive growth of breast cancer cells. Cancer Res. 2012, 72, 1416–1427. [Google Scholar] [CrossRef]
- Katira, A.; Tan, P.H. Evolving role of adiponectin in cancer-controversies and update. Cancer Biol. Med. 2016, 13, 101. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Funahashi, T.; Kihara, S.; Taguchi, T.; Tamaki, Y.; Matsuzawa, Y.; Noguchi, S. Association of serum adiponectin levels with breast cancer risk. Clin. Cancer Res. 2003, 9, 5699–5704. [Google Scholar]
- Tworoger, S.S.; Eliassen, A.H.; Kelesidis, T.; Colditz, G.A.; Willett, W.C.; Mantzoros, C.S.; Hankinson, S.E. Plasma adiponectin concentrations and risk of incident breast cancer. J. Clin. Endocrinol. Metab. 2007, 92, 1510–1516. [Google Scholar] [CrossRef]
- Oh, S.W.; Park, C.-Y.; Lee, E.S.; Yoon, Y.S.; Lee, E.S.; Park, S.S.; Kim, Y.; Sung, N.J.; Yun, Y.H.; Lee, K.S. Adipokines, insulin resistance, metabolic syndrome, and breast cancer recurrence: A cohort study. Breast Cancer Res. 2011, 13, R34. [Google Scholar] [CrossRef] [PubMed]
- Macis, D.; Guerrieri-Gonzaga, A.; Gandini, S. Circulating adiponectin and breast cancer risk: A systematic review and meta-analysis. Int. J. Epidemiol. 2014, 43, 1226–1236. [Google Scholar] [CrossRef]
- Tan, P.H.; Tyrrell, H.E.; Gao, L.; Xu, D.; Quan, J.; Gill, D.; Rai, L.; Ding, Y.; Plant, G.; Chen, Y. Adiponectin receptor signaling on dendritic cells blunts antitumor immunity. Cancer Res. 2014, 74, 5711–5722. [Google Scholar] [CrossRef]
- Kang, J.H.; Lee, Y.Y.; Yu, B.Y.; Yang, B.-S.; Cho, K.-H.; Yoon, D.K.; Roh, Y.K. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch. Pharmacal Res. 2005, 28, 1263–1269. [Google Scholar] [CrossRef]
- Dos Santos, E.; Benaitreau, D.; Dieudonne, M.-N.; Leneveu, M.-C.; Serazin, V.; Giudicelli, Y.; Pecquery, R. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol. Rep. 2008, 20, 971–977. [Google Scholar]
- Grossmann, M.; Nkhata, K.; Mizuno, N.; Ray, A.; Cleary, M. Effects of adiponectin on breast cancer cell growth and signaling. Br. J. Cancer 2008, 98, 370. [Google Scholar] [CrossRef]
- Nakayama, S.; Miyoshi, Y.; Ishihara, H.; Noguchi, S. Growth-inhibitory effect of adiponectin via adiponectin receptor 1 on human breast cancer cells through inhibition of S-phase entry without inducing apoptosis. Breast Cancer Res. Treat. 2008, 112, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Andò, S.; Gelsomino, L.; Panza, S.; Giordano, C.; Bonofiglio, D.; Barone, I.; Catalano, S. Obesity, Leptin and Breast Cancer: Epidemiological Evidence and Proposed Mechanisms. Cancers 2019, 11, 62. [Google Scholar] [CrossRef]
- Mauro, L.; Naimo, G.D.; Gelsomino, L.; Malivindi, R.; Bruno, L.; Pellegrino, M.; Tarallo, R.; Memoli, D.; Weisz, A.; Panno, M.L. Uncoupling effects of estrogen receptor α on LKB1/AMPK interaction upon adiponectin exposure in breast cancer. FASEB J. 2018, 32, 4343–4355. [Google Scholar] [CrossRef]
- Mauro, L.; Pellegrino, M.; De Amicis, F.; Ricchio, E.; Giordano, F.; Rizza, P.; Catalano, S.; Bonofiglio, D.; Sisci, D.; Panno, M.L. Evidences that estrogen receptor α interferes with adiponectin effects on breast cancer cell growth. Cell Cycle 2014, 13, 553–564. [Google Scholar] [CrossRef]
- Mauro, L.; Pellegrino, M.; Giordano, F.; Ricchio, E.; Rizza, P.; De Amicis, F.; Catalano, S.; Bonofiglio, D.; Panno, M.L.; Andò, S. Estrogen receptor-α drives adiponectin effects on cyclin D1 expression in breast cancer cells. FASEB J. 2015, 29, 2150–2160. [Google Scholar] [CrossRef]
- Pfeiler, G.H.; Buechler, C.; Neumeier, M.; Schäffler, A.; Schmitz, G.; Ortmann, O.; Treeck, O. Adiponectin effects on human breast cancer cells are dependent on 17-β estradiol. Oncol. Rep. 2008, 19, 787–793. [Google Scholar] [CrossRef]
- Lam, J.B.; Chow, K.H.; Xu, A.; Lam, K.S.; Liu, J.; Wong, N.-S.; Moon, R.T.; Shepherd, P.R.; Cooper, G.J.; Wang, Y. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS ONE 2009, 4, e4968. [Google Scholar] [CrossRef]
- Kim, K.-y.; Baek, A.; Hwang, J.-E.; Choi, Y.A.; Jeong, J.; Lee, M.-S.; Cho, D.H.; Lim, J.-S.; Kim, K.I.; Yang, Y. Adiponectin-activated AMPK stimulates dephosphorylation of AKT through protein phosphatase 2A activation. Cancer Res. 2009, 69, 4018–4026. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.B.; Chow, K.H.; Xu, A.; Lam, K.S.; Moon, R.T.; Wang, Y. Adiponectin stimulates Wnt inhibitory factor-1 expression through epigenetic regulations involving the transcription factor specificity protein 1. Carcinogenesis 2008, 29, 2195–2202. [Google Scholar] [CrossRef][Green Version]
- Taliaferro-Smith, L.; Nagalingam, A.; Knight, B.B.; Oberlick, E.; Saxena, N.K.; Sharma, D. Integral role of PTP1B in adiponectin-mediated inhibition of oncogenic actions of leptin in breast carcinogenesis. Neoplasia (New York, NY) 2013, 15, 23. [Google Scholar] [CrossRef]
- Denzel, M.S.; Hebbard, L.W.; Shostak, G.; Shapiro, L.; Cardiff, R.D.; Ranscht, B. Adiponectin deficiency limits tumor vascularization in the MMTV-PyV-mT mouse model of mammary cancer. Clin. Cancer Res. 2009, 15, 3256–3264. [Google Scholar] [CrossRef]
- Landskroner-Eiger, S.; Qian, B.; Muise, E.S.; Nawrocki, A.R.; Berger, J.P.; Fine, E.J.; Koba, W.; Deng, Y.; Pollard, J.W.; Scherer, P.E. Proangiogenic contribution of adiponectin toward mammary tumor growth in vivo. Clin. Cancer Res. 2009, 15, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.S.; Thompson, C.B. Metabolic reprogramming: A cancer hallmark even warburg did not anticipate. Cancer Cell 2012, 21, 297–308. [Google Scholar] [CrossRef]
- Luo, Z.; Zang, M.; Guo, W. AMPK as a metabolic tumor suppressor: Control of metabolism and cell growth. Future Oncol. 2010, 6, 457–470. [Google Scholar] [CrossRef]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature Cell Biol. 2011, 13, 1016. [Google Scholar] [CrossRef]
- Shackelford, D.B.; Shaw, R.J. The LKB1–AMPK pathway: Metabolism and growth control in tumour suppression. Nature Rev. Cancer 2009, 9, 563. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, M.; Tian, W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016, 49, 3–13. [Google Scholar] [CrossRef]
- Guo, W.; Gao, Y.; Li, N.; Shao, F.; Wang, C.; Wang, P.; Yang, Z.; Li, R.; He, J. Exosomes: New players in cancer. Oncol. Rep. 2017, 38, 665–675. [Google Scholar] [CrossRef]
- Lin, R.; Wang, S.; Zhao, R.C. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol. Cell. Biochem. 2013, 383, 13–20. [Google Scholar] [CrossRef]
- Gernapudi, R.; Yao, Y.; Zhang, Y.; Wolfson, B.; Roy, S.; Duru, N.; Eades, G.; Yang, P.; Zhou, Q. Targeting exosomes from preadipocytes inhibits preadipocyte to cancer stem cell signaling in early-stage breast cancer. Breast Cancer Res. Treat. 2015, 150, 685–695. [Google Scholar] [CrossRef]
- Philley, J.V.; Kannan, A.; Griffith, D.E.; Devine, M.S.; Benwill, J.L.; Wallace, R.J., Jr.; Brown-Elliott, B.A.; Thakkar, F.; Taskar, V.; Fox, J.G. Exosome secretome and mediated signaling in breast cancer patients with nontuberculous mycobacterial disease. Oncotarget 2017, 8, 18070. [Google Scholar] [CrossRef]
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y. Adiponectin/T-cadherin system enhances exosome biogenesis and decreases cellular ceramides by exosomal release. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Kang, D.-W.; Lee, J.; Suh, S.-H.; Ligibel, J.; Courneya, K.S.; Jeon, J.Y. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: A systematic review and meta-analysis. Cancer Epidemiol. Prev. Biomark. 2017, 26, 355–365. [Google Scholar] [CrossRef]
- Kriketos, A.D.; Gan, S.K.; Poynten, A.M.; Furler, S.M.; Chisholm, D.J.; Campbell, L.V. Exercise increases adiponectin levels and insulin sensitivity in humans. Diabetes Care 2004, 27, 629–630. [Google Scholar] [CrossRef]
- Saunders, T.J.; Palombella, A.; McGuire, K.A.; Janiszewski, P.M.; Després, J.-P.; Ross, R. Acute exercise increases adiponectin levels in abdominally obese men. J. Nutr. Metab. 2012, 2012. [Google Scholar] [CrossRef]
- Otvos, L., Jr.; Kovalszky, I.; Olah, J.; Coroniti, R.; Knappe, D.; Nollmann, F.I.; Hoffmann, R.; Wade, J.D.; Lovas, S.; Surmacz, E. Optimization of adiponectin-derived peptides for inhibition of cancer cell growth and signaling. Pept. Sci. 2015, 104, 156–166. [Google Scholar] [CrossRef]
- Otvos, L.; Haspinger, E.; La Russa, F.; Maspero, F.; Graziano, P.; Kovalszky, I.; Lovas, S.; Nama, K.; Hoffmann, R.; Knappe, D. Design and development of a peptide-based adiponectin receptor agonist for cancer treatment. BMC Biotechnol. 2011, 11, 90. [Google Scholar] [CrossRef]
- Okada-Iwabu, M.; Yamauchi, T.; Iwabu, M.; Honma, T.; Hamagami, K.-i.; Matsuda, K.; Yamaguchi, M.; Tanabe, H.; Kimura-Someya, T.; Shirouzu, M. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 2013, 503, 493. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Kim, J.W.; Son, Y.-J.; Ma, M.J.; Um, J.-H.; Kim, N.D.; Min, S.H.; Kim, D.I.; Kim, B.B. Discovery of a novel potent peptide agonist to adiponectin receptor 1. PLoS ONE 2018, 13, e0199256. [Google Scholar] [CrossRef]
- Wei, S.; Yang, J.; Lee, S.-L.; Kulp, S.K.; Chen, C.-S. PPARγ-independent antitumor effects of thiazolidinediones. Cancer Lett. 2009, 276, 119–124. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Med. 2001, 7, 941. [Google Scholar] [CrossRef]
- Dowling, R.J.; Zakikhani, M.; Fantus, I.G.; Pollak, M.; Sonenberg, N. Metformin inhibits mammalian target of rapamycin–dependent translation initiation in breast cancer cells. Cancer Res. 2007, 67, 10804–10812. [Google Scholar] [CrossRef]
- Brown, K.A.; Simpson, E.R. Obesity and breast cancer: Mechanisms and therapeutic implications. Front. Biosci. (Elite Ed.) 2012, 4, 2515–2524. [Google Scholar] [CrossRef]
- Renehan, A.G.; Soerjomataram, I.; Tyson, M.; Egger, M.; Zwahlen, M.; Coebergh, J.W.; Buchan, I. Incident cancer burden attributable to excess body mass index in 30 European countries. Int. J. Cancer 2010, 126, 692–702. [Google Scholar] [CrossRef]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.; Vieira, A.; Aune, D.; Bandera, E.; Greenwood, D.; McTiernan, A.; Navarro Rosenblatt, D.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef] [PubMed]
- Taubes, G. Unraveling the Obesity-Cancer Connection; American Association for the Advancement of Science: Washington, DC, USA, 2012. [Google Scholar]
- Tahergorabi, Z.; Khazaei, M.; Moodi, M.; Chamani, E. From obesity to cancer: A review on proposed mechanisms. Cell Biochem. Funct. 2016, 34, 533–545. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gelsomino, L.; Naimo, G.D.; Catalano, S.; Mauro, L.; Andò, S. The Emerging Role of Adiponectin in Female Malignancies. Int. J. Mol. Sci. 2019, 20, 2127. https://doi.org/10.3390/ijms20092127
Gelsomino L, Naimo GD, Catalano S, Mauro L, Andò S. The Emerging Role of Adiponectin in Female Malignancies. International Journal of Molecular Sciences. 2019; 20(9):2127. https://doi.org/10.3390/ijms20092127
Chicago/Turabian StyleGelsomino, Luca, Giuseppina Daniela Naimo, Stefania Catalano, Loredana Mauro, and Sebastiano Andò. 2019. "The Emerging Role of Adiponectin in Female Malignancies" International Journal of Molecular Sciences 20, no. 9: 2127. https://doi.org/10.3390/ijms20092127
APA StyleGelsomino, L., Naimo, G. D., Catalano, S., Mauro, L., & Andò, S. (2019). The Emerging Role of Adiponectin in Female Malignancies. International Journal of Molecular Sciences, 20(9), 2127. https://doi.org/10.3390/ijms20092127