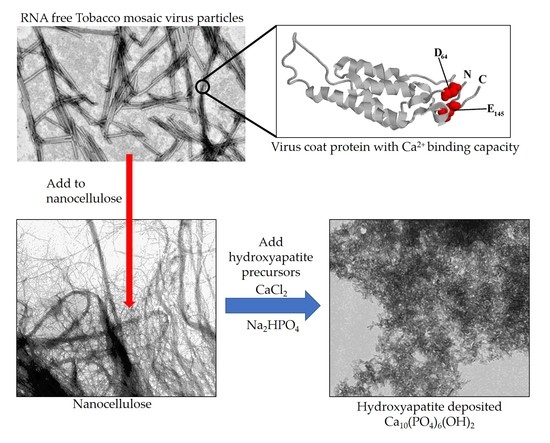
Virus-Like Particle Facilitated Deposition of Hydroxyapatite Bone Mineral on Nanocellulose after Exposure to Phosphate and Calcium Precursors
Abstract
1. Introduction
2. Results
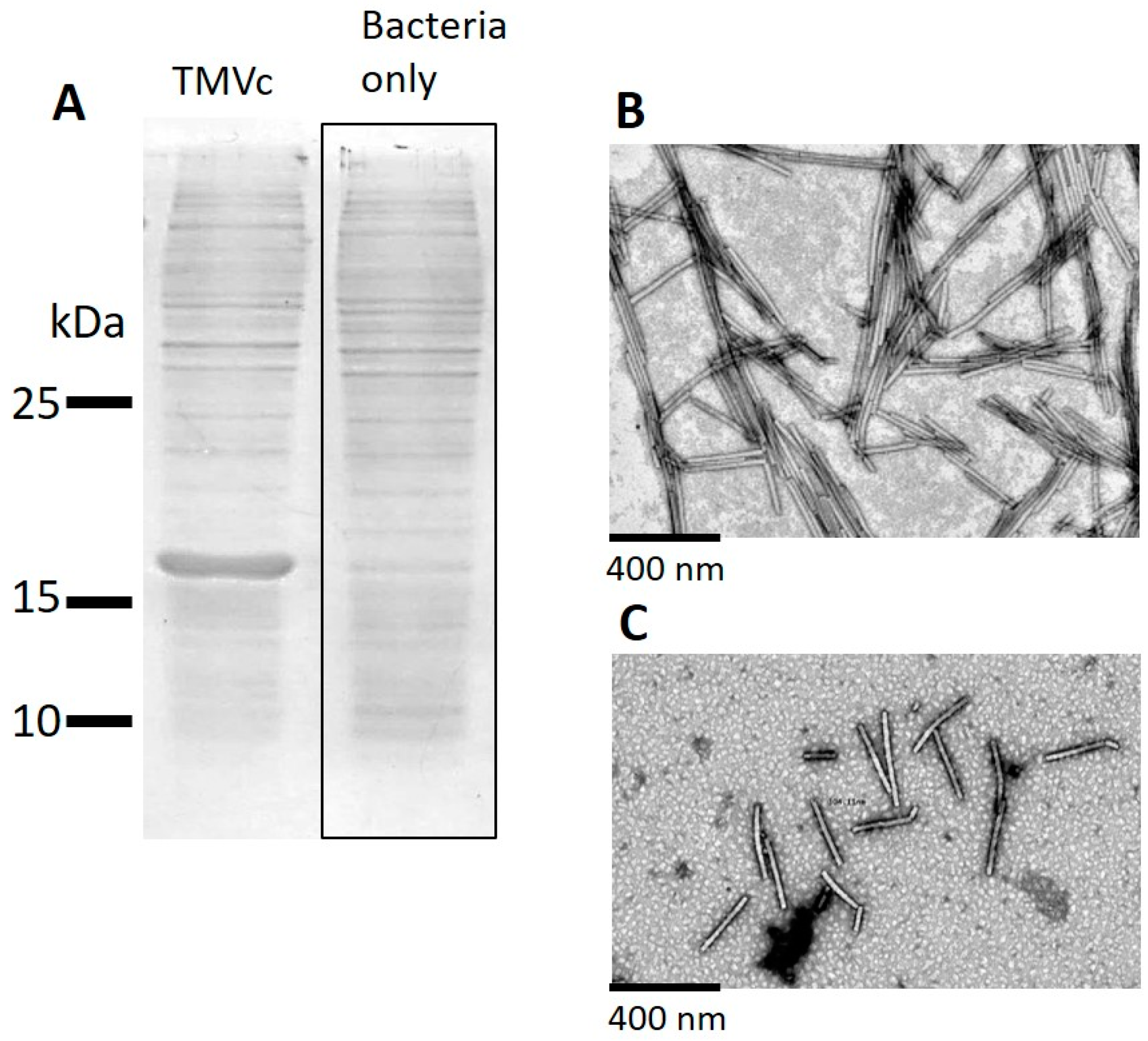
2.1. Production and Isolation of Tobacco Mosaic Virus Derived Virus-Like Particles (TMV VLPs)
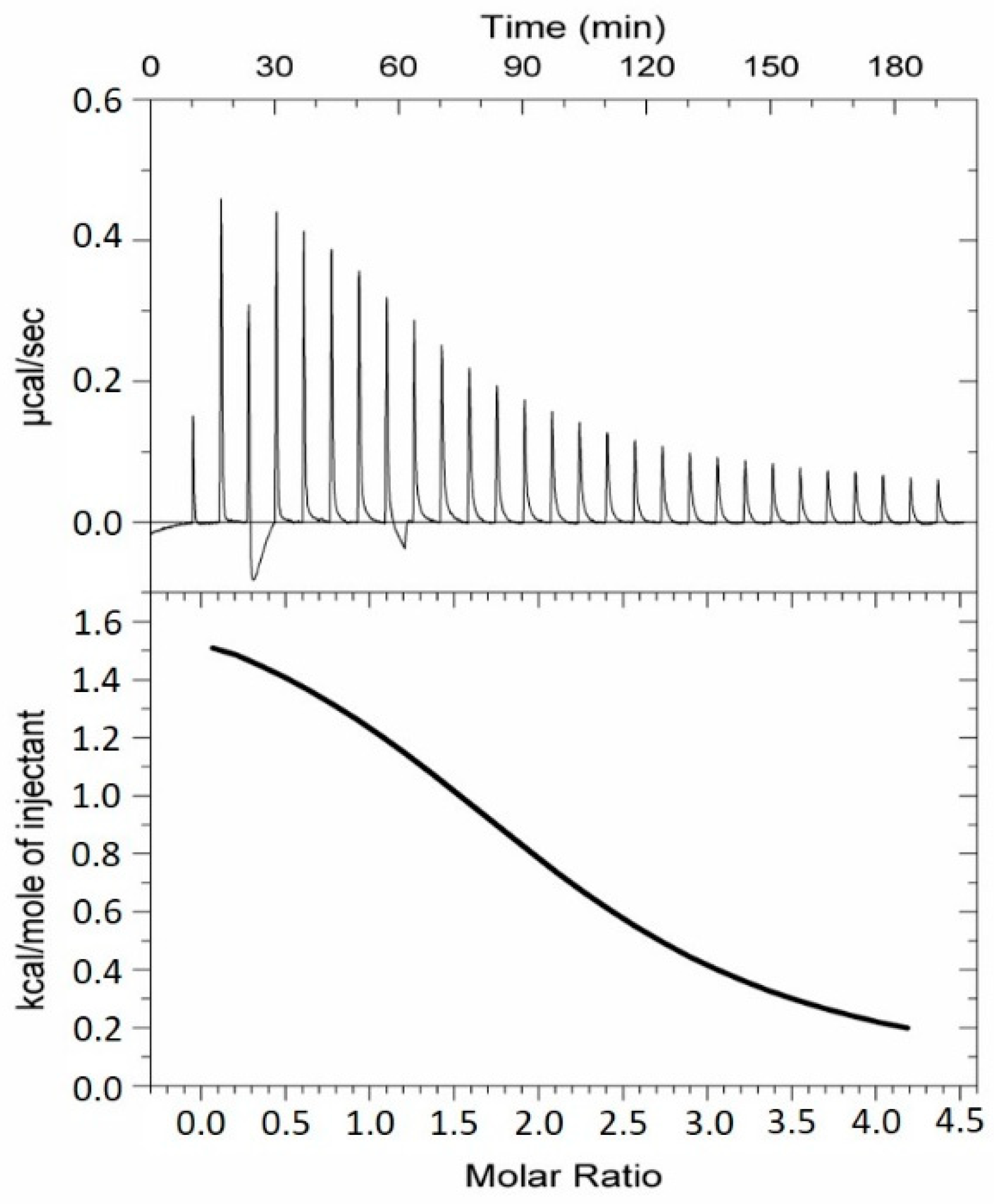
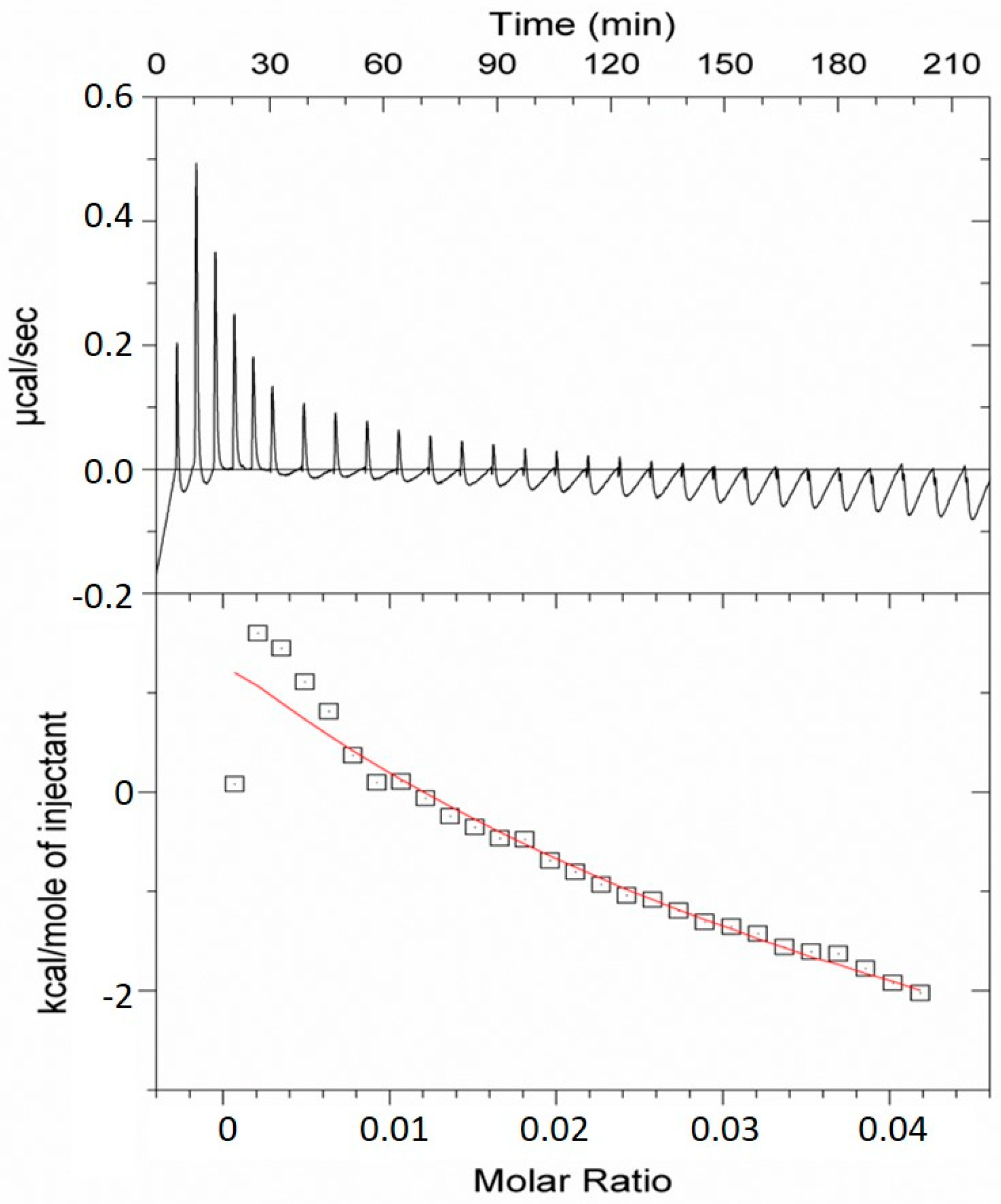
2.2. Analysis of the TMV VLP Surface Charges and Association with Calcium
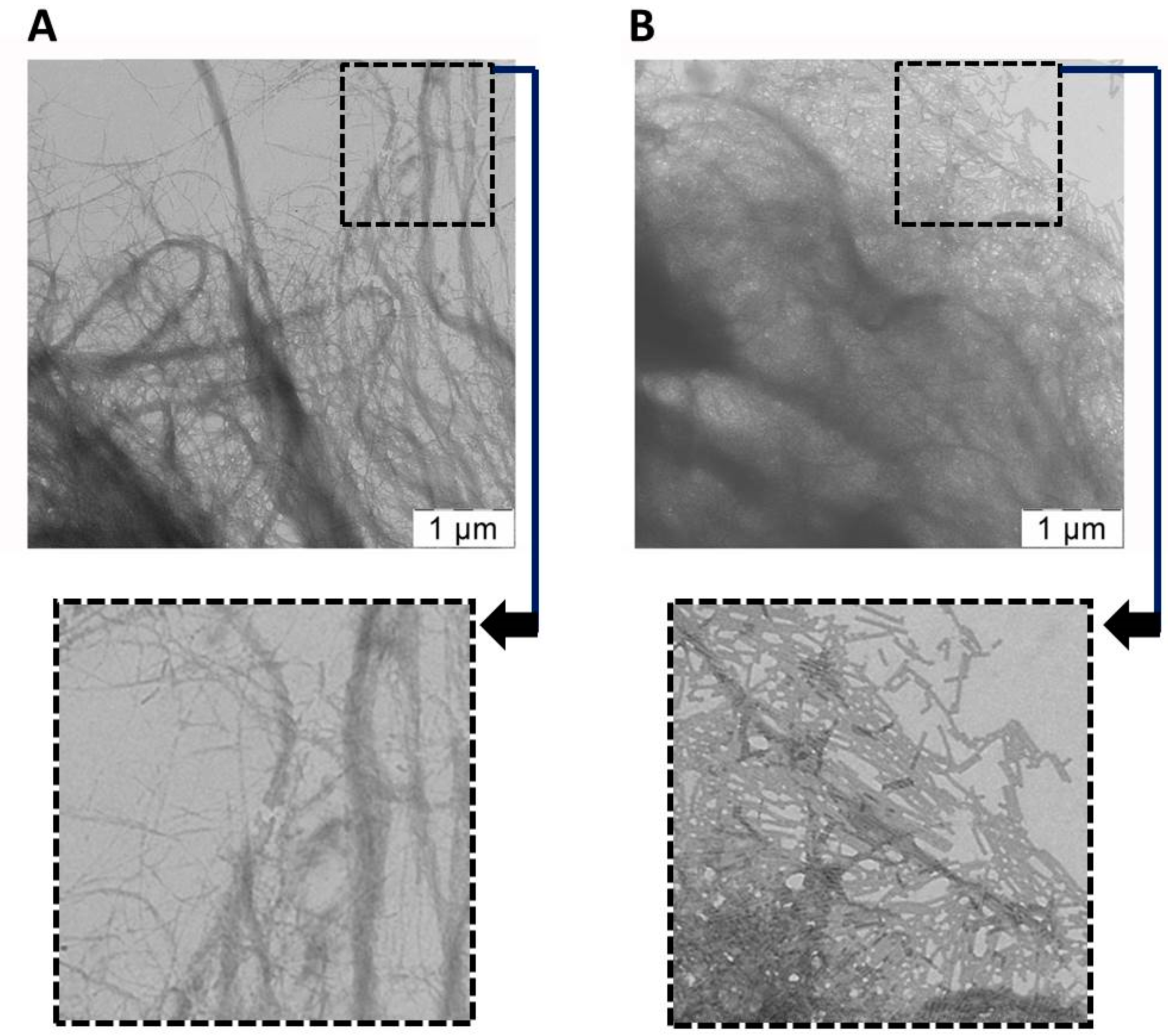
2.3. Analysis of the Attachment of TMV VLPs to Nanocellulose
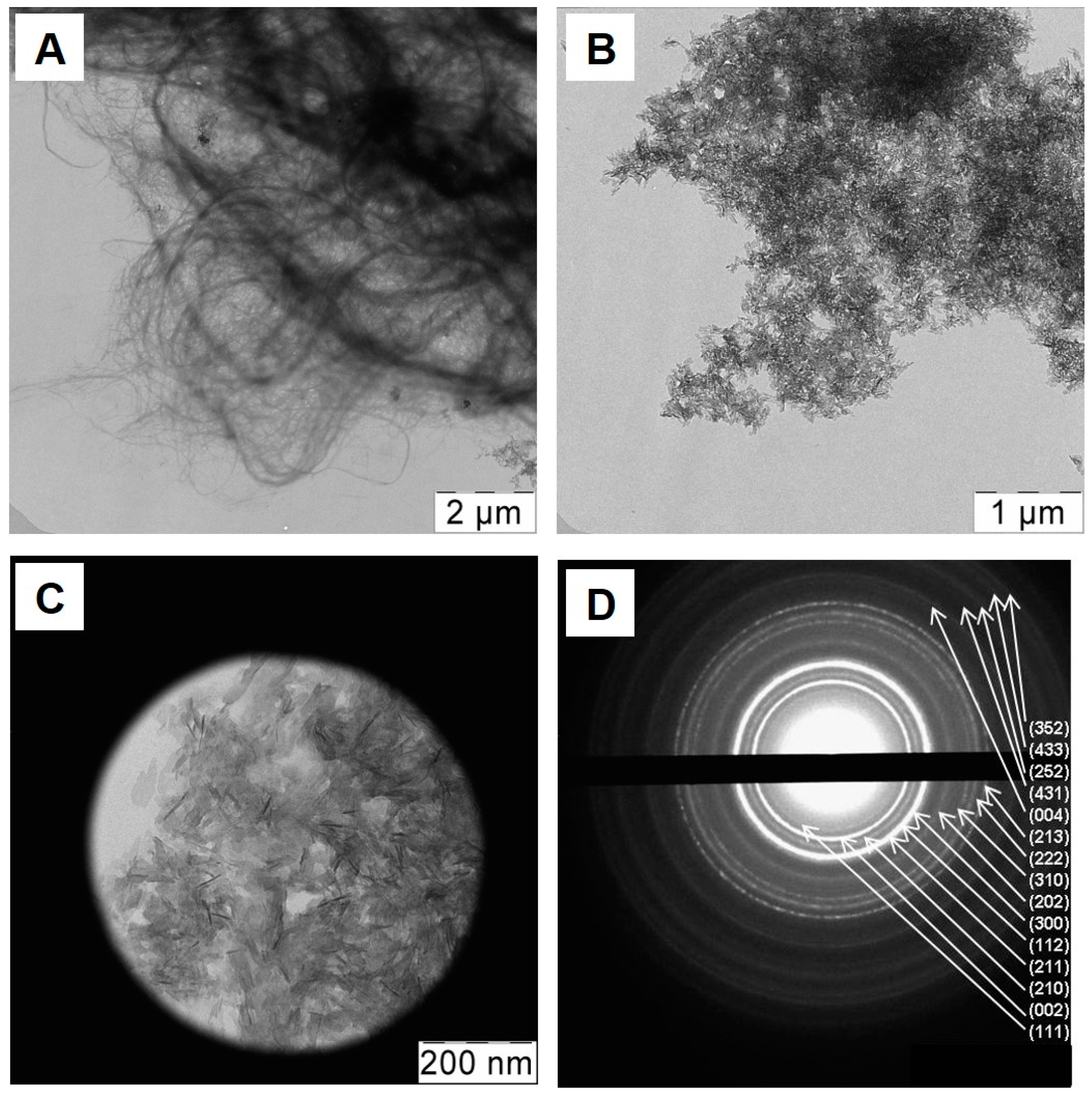
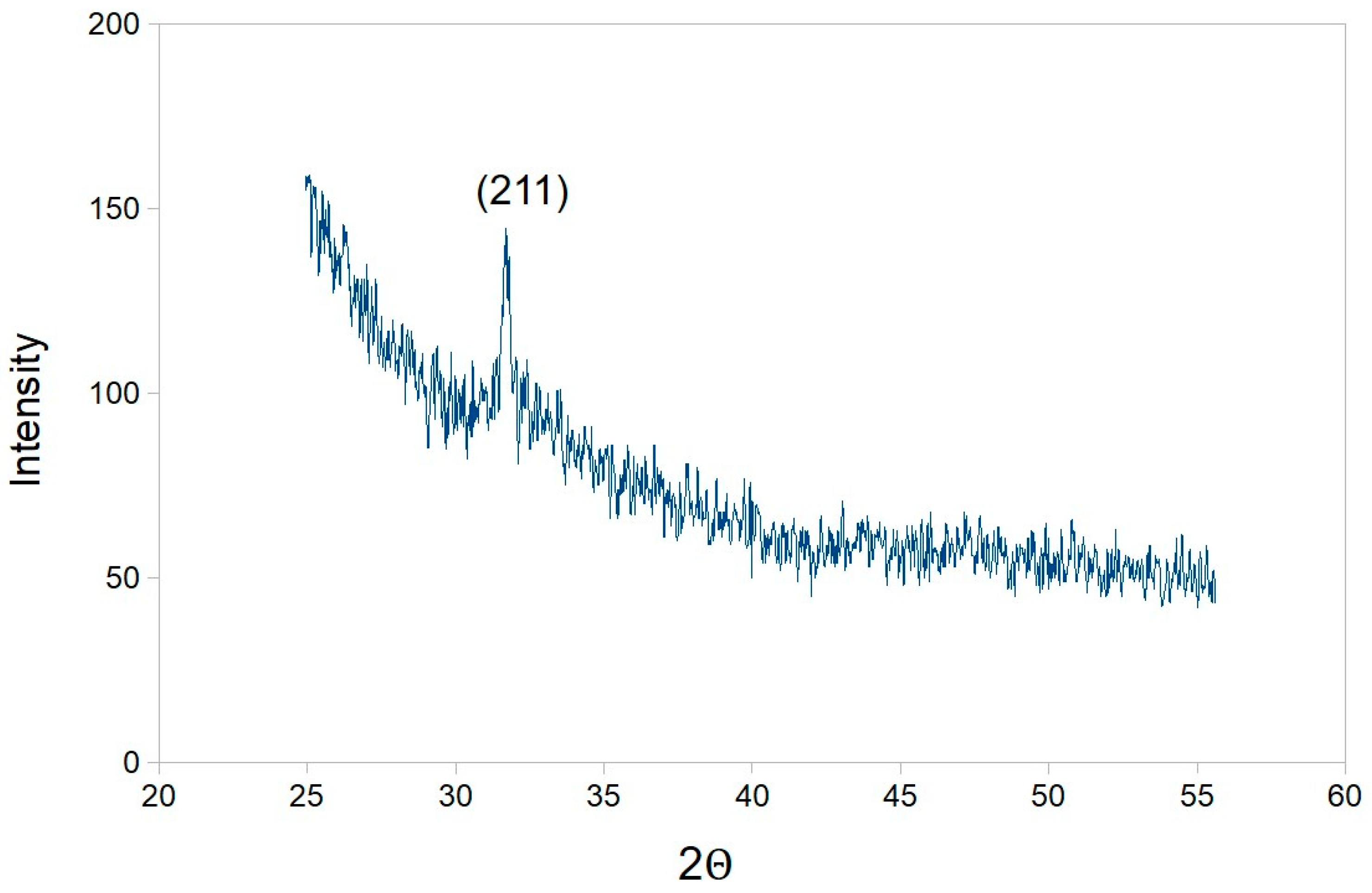
2.4. Assessment of Hydroxyapatite Formation on Nanocellulose and Nanocellulose-TMV VLP Composites
3. Discussion
3.1. Surface Chemistry and Mechanisms of Calcium Binding Activities of the TMV VLPs
3.2. Potential Mechanisms of Interaction between Nanocellulose and TMV VLPs
3.3. Formation of New Mineralized TMV VLP-Nanocellulose Composites and Their Future Applications
4. Materials and Methods
4.1. Sequences and Plasmids
4.2. Expression in Bacteria and Isolation of VLPs
4.3. SDS-PAGE Analysis
4.4. Nanocellulose Formulation
4.5. Hydroxyapatite Mineralization Reactions
4.6. Transmission Electron Microscopy, Selected Area Diffraction, X-ray Diffraction and Zeta Potential Analysis
4.7. Isothermal Titration Calorimetry
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AFM | Atomic Force Microscopy |
| CP | Coat protein |
| HA | Hydroxyapatite |
| IPTG | Isopropyl β-d-1-thiogalactopyranoside |
| ITC | Isothermal Titration Calorimetry |
| PVX | Potato virus X |
| SDS PAGE | Sodium dodecyl sulphate polyacrylamide gel electrophoresis |
| SAED | Selected Area Electron Diffraction |
| TEM | Transmission Electron Microscopy |
| TMV | Tobacco mosaic virus |
| VLP | Virus-like particle |
| XRD | X-ray diffraction |
References
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef] [PubMed]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Tardy, B.L.; Yokota, S.; Ago, M. Nanocellulose-surfactant interactions. Curr. Opin. Colloid Interface Sci. 2017, 29, 57–67. [Google Scholar] [CrossRef]
- Uddin, K.M.A.; Orelma, H.; Mohammadi, P.; Borghei, M.; Laine, J.; Linder, M.; Rojas, O.J. Retention of lysozyme activity by physical immobilization in nanocellulose aerogels and antibacterial effects. Cellulose 2017, 24, 2837–2848. [Google Scholar] [CrossRef]
- Haghpanah, J.S.; Tu, R.; Da Silva, S.; Yan, D.; Mueller, S.; Weder, C.; Foster, E.J.; Sacui, I.; Gilman, J.W.; Montclare, J.K. Bionanocomposites: Differential effects of cellulose nanocrystals on protein diblock copolymers. Biomacromolecules 2013, 14, 4360–4367. [Google Scholar] [CrossRef]
- Orelma, H.; Filpponen, I.; Johansson, L.; Österberg, M.; Rojas, O.J. Surface functionalized nanofibrillar cellulose (NFC) film as a platform for immunoassays and diagnostics. Biointerphases 2012, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Butchosa, N.; Jayawardena, H.S.N.; Park, J.; Zhou, Q.; Yan, M.D.; Ramstrom, O. Synthesis of multifunctional cellulose nanocrystals for lectin recognition and bacterial imaging. Biomacromolecules 2015, 16, 1426–1432. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef] [PubMed]
- Oshima, T.; Taguchi, S.; Ohe, K.; Baba, Y. Phosphorylated bacterial cellulose for adsorption of proteins. Carbohydr. Polym. 2011, 83, 953–958. [Google Scholar] [CrossRef]
- Beladi, F.; Saber-Samandari, S.; Saber-Samadari, S. Cellular compatibility of nanocomposite scaffolds based on hydroxyapatite entrapped in cellulose network for bone repair. Mater. Sci. Eng. 2017, 75, 385–392. [Google Scholar] [CrossRef]
- Ao, C.; Niu, Y.; Zhang, X.; He, X.; Zhang, W.; Lu, C. Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 2017, 97, 568–573. [Google Scholar] [CrossRef]
- Zhong, Z.; Qin, J.; Ma, J. Cellulose acetate/hydroxyapatite/chitosan coatings for improved corrosion resistance and bioactivity. Mater. Sci. Eng. C 2015, 49, 251–255. [Google Scholar] [CrossRef]
- Ishikawa, M.; Oaki, Y.; Tanaka, Y.; Kakisawa, H.; Salazar- Alvarez, G.; Imai, H. Fabrication of nanocellulose-hydroxyapatite composites and their application as water-resistant transparent coatings. J. Mater. Chem. B 2015, 3, 5858–5863. [Google Scholar] [CrossRef]
- Gonzalez, M.; Hernandez, E.; Ascencio, J.A.; Pacheco, F.; Pacheco, S.; Rodriguez, R. Hydroxyapatite crystals grown on a cellulose matrix using titanium alkoxide as a coupling agent. J. Mater. Chem. 2003, 13, 2948–2951. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Hong, L.; Jia, S.R.; Huang, Y.; Zhu, Y.; Wang, Y.L.; Jiang, H.J. Synthesis and characterization of hydroxyapatite-bacterial cellulose nanocomposites. Compos. Sci. Technol. 2006, 66, 1825–1832. [Google Scholar] [CrossRef]
- Brown, A.D.; Naves, L.; Wang, X.; Ghodssi, R.; Culver, J.N. Carboxylate-directed in vivo assembly of virus-like nanorods and tubes for the display of functional peptides and residues. Biomacromolecules 2013, 14, 3123–3129. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, W.H.; Gastfriend, H.H.; Lauffer, M.A. Ion binding by TMV, TMV protein and TMV-RNA. Biophys. J. 1983, 41, A238. [Google Scholar]
- Nedoluzhko, A.; Douglas, T. Ordered association of tobacco mosaic virus in the presence of divalent metal ions. J. Inorg. Biochem. 2001, 84, 233–240. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lee, J.Y.; Suh, J.S.; Lee, G.; Chung, C.P.; Park, Y.J. The mineralization inducing peptide derived from dentin sialophosphoprotein for bone regeneration. J. Biomed. Mater. Res. A 2013, 101, 590–598. [Google Scholar] [CrossRef]
- He, J.; Niu, Z.; Tangirala, R.; Wan, J.Y.; Wei, X.Y.; Kaur, G.; Wang, Q.; Jutz, G.; Boker, A.; Lee, B.; et al. Self-assembly of tobacco mosaic virus at oil/water interfaces. Langmuir 2009, 9, 4979–4987. [Google Scholar] [CrossRef]
- Tavafoghi, M.; Cerruti, M. The role of amino acids in hydroxyapatite mineralization. J. R. Soc. Interface 2016, 13, 20160462. [Google Scholar] [CrossRef]
- Benesch, J.; Mano, J.; Reis, R. Proteins and their peptide motifs in acellular apatite mineralization of scaffolds for tissue engineering. Tissue Eng. 2008, 14, 433–445. [Google Scholar] [CrossRef]
- Palmer, L.; Newcombe, C.; Kaltz, S.; Spoerke, E.; Stupp, S. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–8473. [Google Scholar] [CrossRef]
- Lauria, I.; Dickmeis, C.; Röder, J.; Beckers, M.; Rütten, S.; Lin, Y.Y.; Commandeur, U.; Fischer, H. Engineered Potato virus X nanoparticles support hydroxyapatite nucleation for improved bone tissue replacement. Acta Biomater. 2017, 62, 317–327. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. Arg-Gly-Asp- A versatile cell recognition signal. Cell 1986, 44, 517–518. [Google Scholar] [CrossRef]
- Yamane, C.; Aoyagi, T.; Ago, M.; Sato, K.; Okajima, K.; Takahashi, T. Two different surface properties of regenerated cellulose due to structural anisotropy. Polym. J. 2006, 38, 819–882. [Google Scholar] [CrossRef]
- Yamane, C. Structure formation of regenerated cellulose from its solution and resultant features of high wettability: A review. Nordic Pulp Pap. Res. J. 2015, 30, 78–91. [Google Scholar] [CrossRef]
- Khazraji, A.C.; Robert, S. Self-assembly and intermolecular forces when cellulose and water interact using molecular modeling. J. Nanomater. 2013, 2013, 745979. [Google Scholar] [CrossRef]
- Knez, M.; Sumser, M.P.; Bittner, A.M.; Wege, C.; Jeske, H.; Hoffmann, D.M.P.; Kuhnke, K.; Kern, K. Binding the tobacco mosaic virus to inorganic surfaces. Langmuir 2004, 2, 441–447. [Google Scholar] [CrossRef]
- Britt, D.W.; Buijs, J.; Hlady, V. Tobacco mosaic virus adsorption on self-assembled and Langmuir-Blodget monolayers studied by TIRF and SFM. Thin Solid Films 1998, 327–329, 824–828. [Google Scholar] [CrossRef]
- Li, L.; Zhao, M.; Li, J.; Zuo, Y.; Zou, Q.; Li, Y. Preparation and cell infiltration of lotus-type porous nano-hydroxyapatite/ polyurethane scaffold for bone tissue regeneration. Mater. Lett. 2015, 149, 25–28. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Saber-Samandari, S.; Gazi, M.; Cebeci, F.C.; Talasaz, E. Synthesis, characterization and application of cellulose based nano-biocomposite hydrogels. J. Macromol. Sci. Pure Appl. Chem. 2013, 50, 1133–1141. [Google Scholar] [CrossRef]
- Sitasuwan, P.; Lee, L.A.; Bo, P.; Davis, E.N.; Lin, Y.; Wang, Q. A plant virus substrate induces early upregulation of BMP2 for rapid bone formation. Int. Biol. 2012, 4, 651–660. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinitsyna, O.V.; Makarov, V.V.; McGeachy, K.; Bukharova, T.; Whale, E.; Hepworth, D.; Yaminsky, I.V.; Kalinina, N.O.; Taliansky, M.E.; Love, A.J. Virus-Like Particle Facilitated Deposition of Hydroxyapatite Bone Mineral on Nanocellulose after Exposure to Phosphate and Calcium Precursors. Int. J. Mol. Sci. 2019, 20, 1814. https://doi.org/10.3390/ijms20081814
Sinitsyna OV, Makarov VV, McGeachy K, Bukharova T, Whale E, Hepworth D, Yaminsky IV, Kalinina NO, Taliansky ME, Love AJ. Virus-Like Particle Facilitated Deposition of Hydroxyapatite Bone Mineral on Nanocellulose after Exposure to Phosphate and Calcium Precursors. International Journal of Molecular Sciences. 2019; 20(8):1814. https://doi.org/10.3390/ijms20081814
Chicago/Turabian StyleSinitsyna, Olga V., Valentine V. Makarov, Kara McGeachy, Tatyana Bukharova, Eric Whale, David Hepworth, Igor V. Yaminsky, Natalia O. Kalinina, Michael E. Taliansky, and Andrew J. Love. 2019. "Virus-Like Particle Facilitated Deposition of Hydroxyapatite Bone Mineral on Nanocellulose after Exposure to Phosphate and Calcium Precursors" International Journal of Molecular Sciences 20, no. 8: 1814. https://doi.org/10.3390/ijms20081814
APA StyleSinitsyna, O. V., Makarov, V. V., McGeachy, K., Bukharova, T., Whale, E., Hepworth, D., Yaminsky, I. V., Kalinina, N. O., Taliansky, M. E., & Love, A. J. (2019). Virus-Like Particle Facilitated Deposition of Hydroxyapatite Bone Mineral on Nanocellulose after Exposure to Phosphate and Calcium Precursors. International Journal of Molecular Sciences, 20(8), 1814. https://doi.org/10.3390/ijms20081814