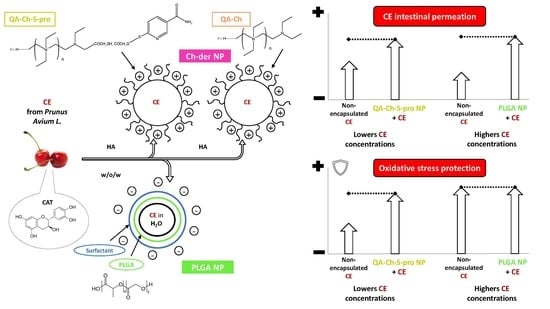
Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(lactic-co-glycolic acid) Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
2.1. Nanoparticle Characterization
2.2. Ex Vivo Mucoadhesivity Studies
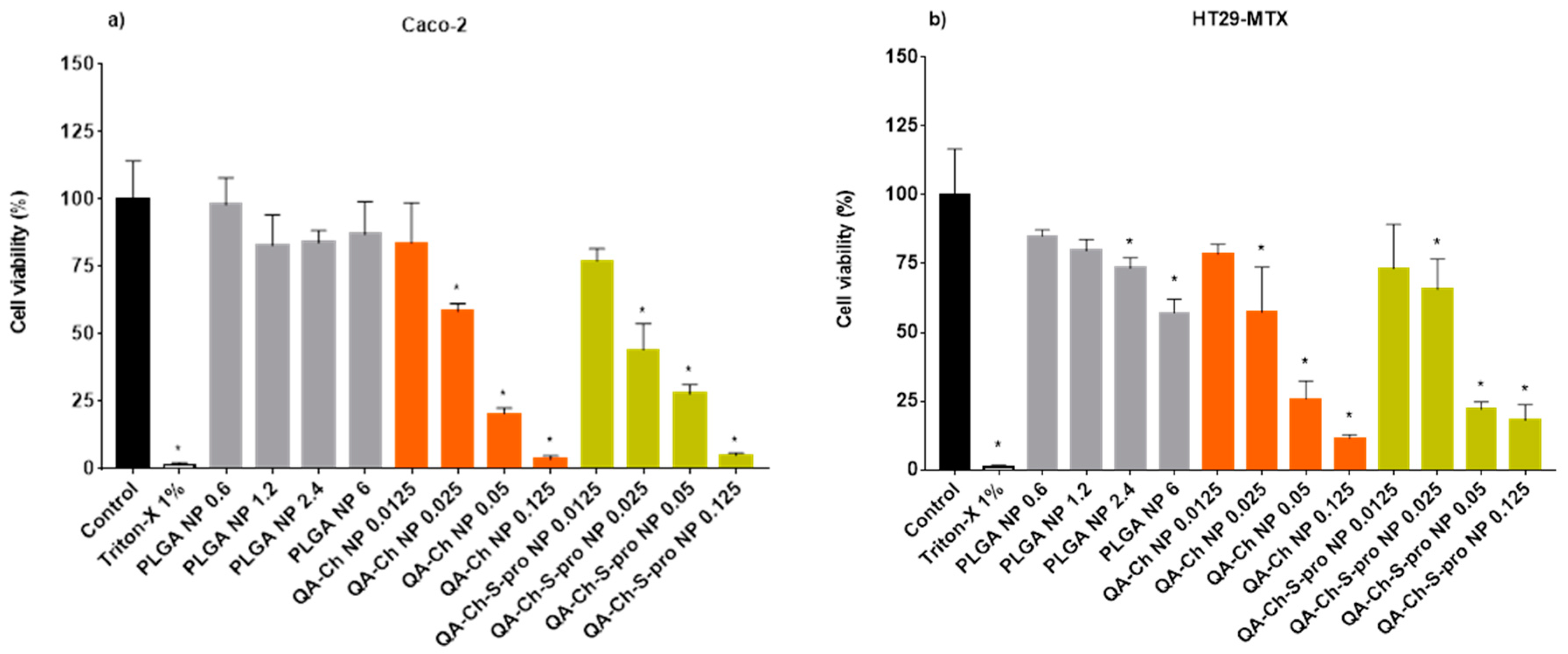
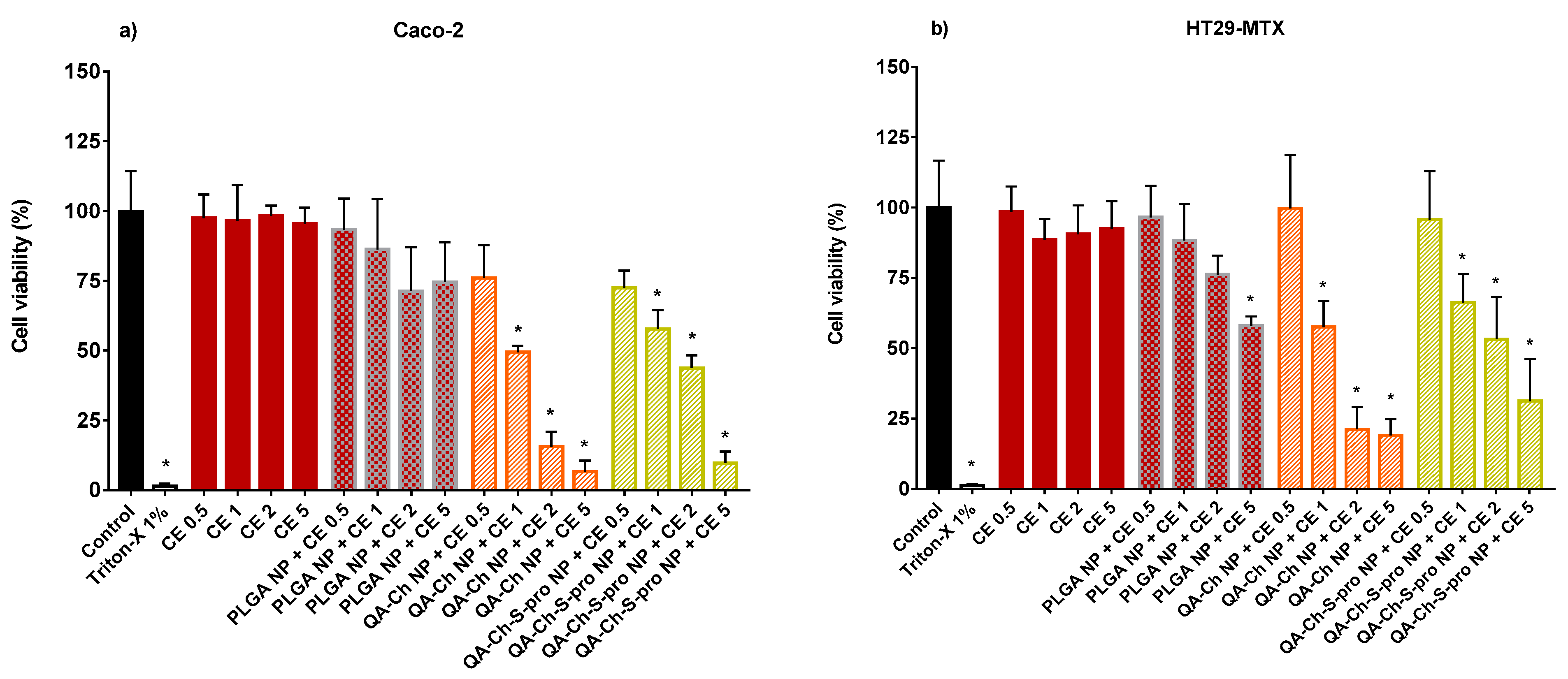
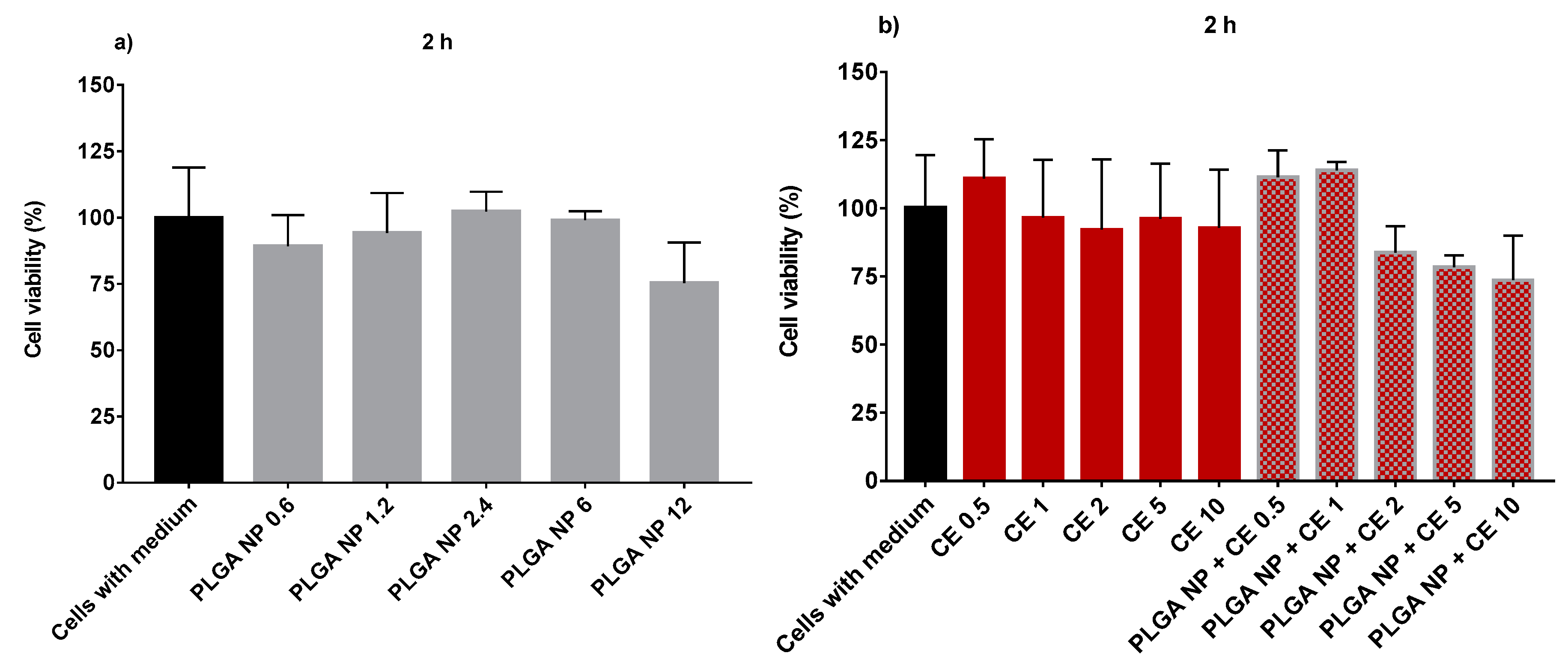
2.3. Cell Viability
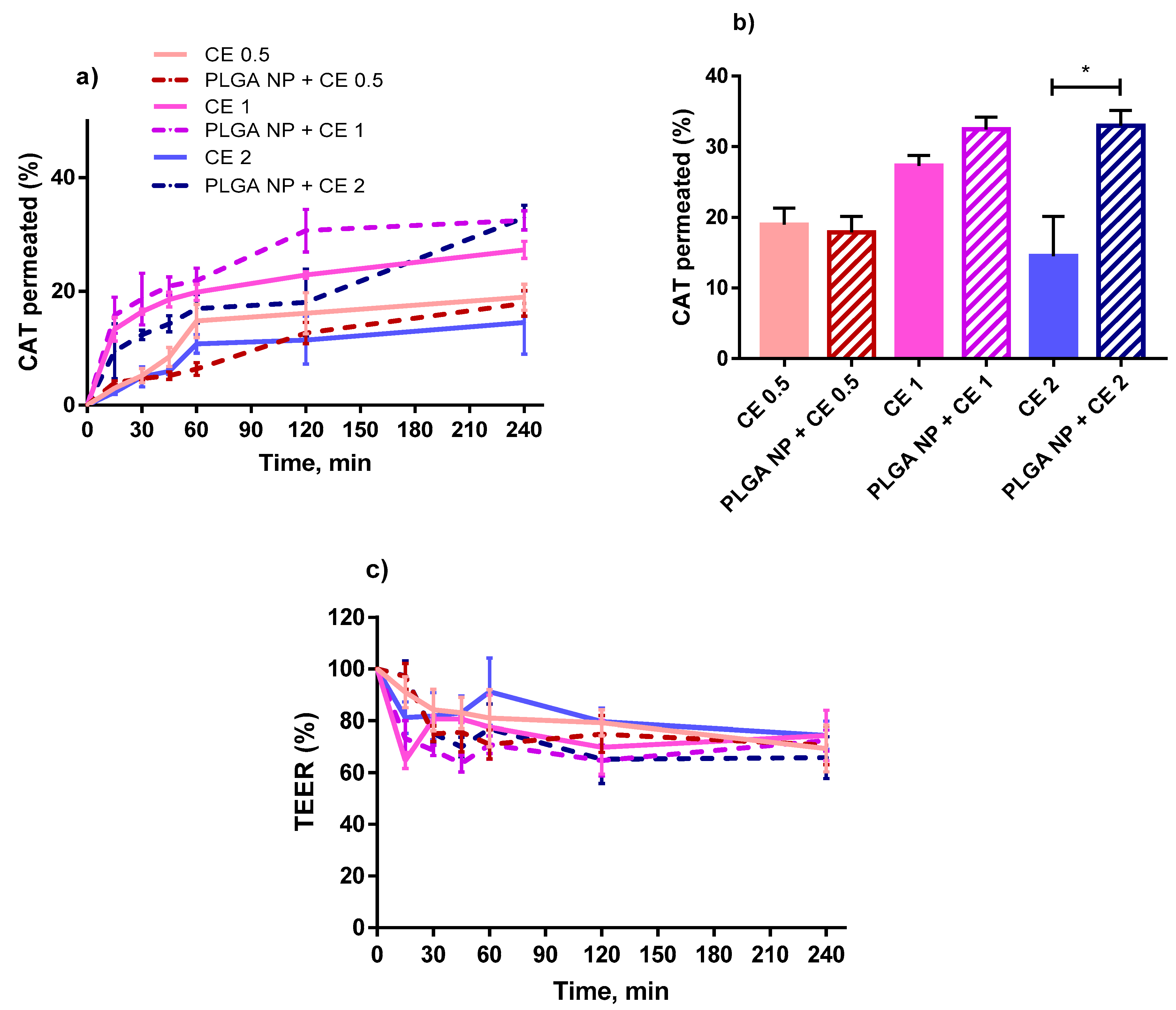
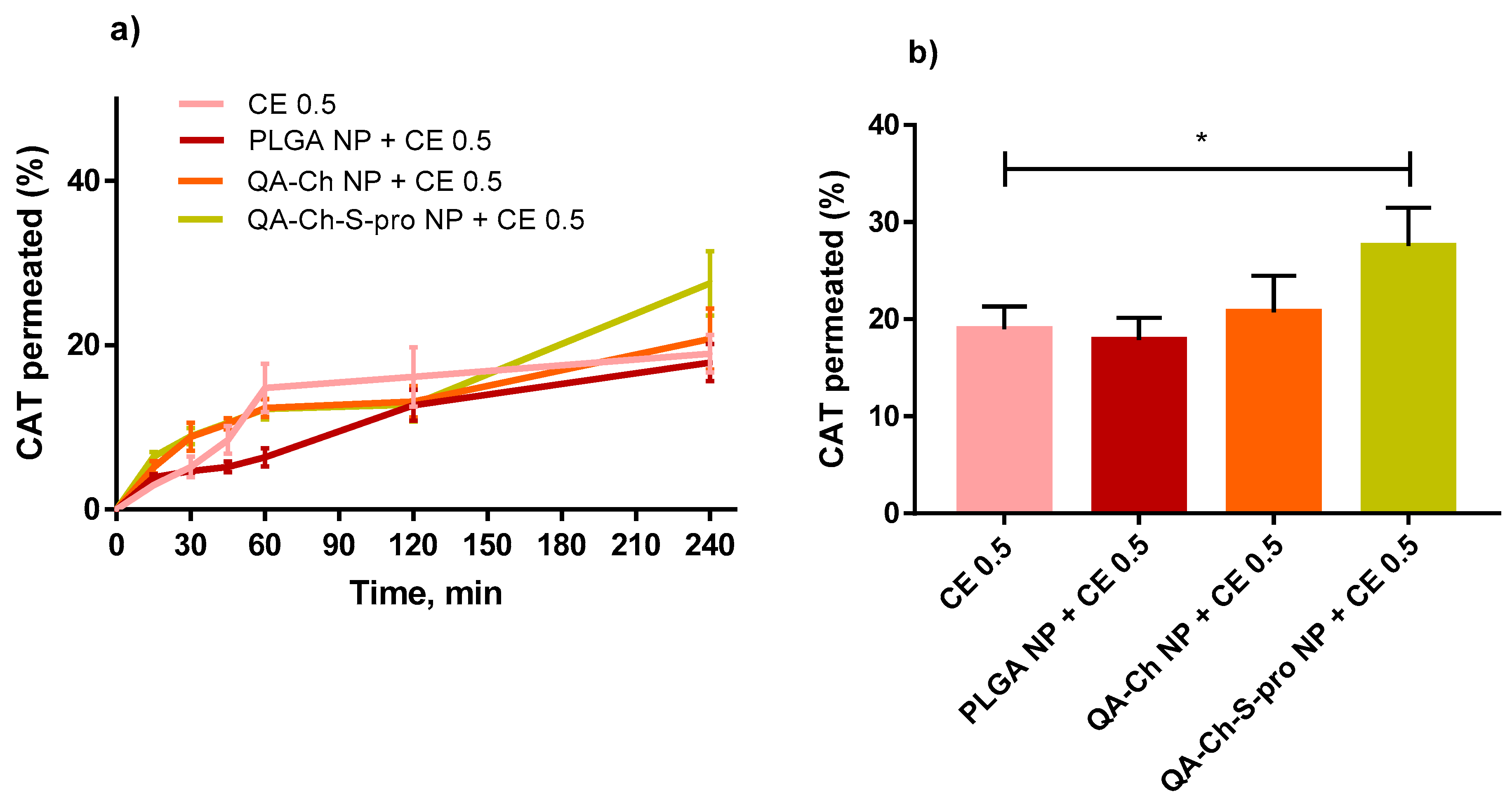
2.4. Permeability Studies in a Triple Co-Culture Intestine Cell Model
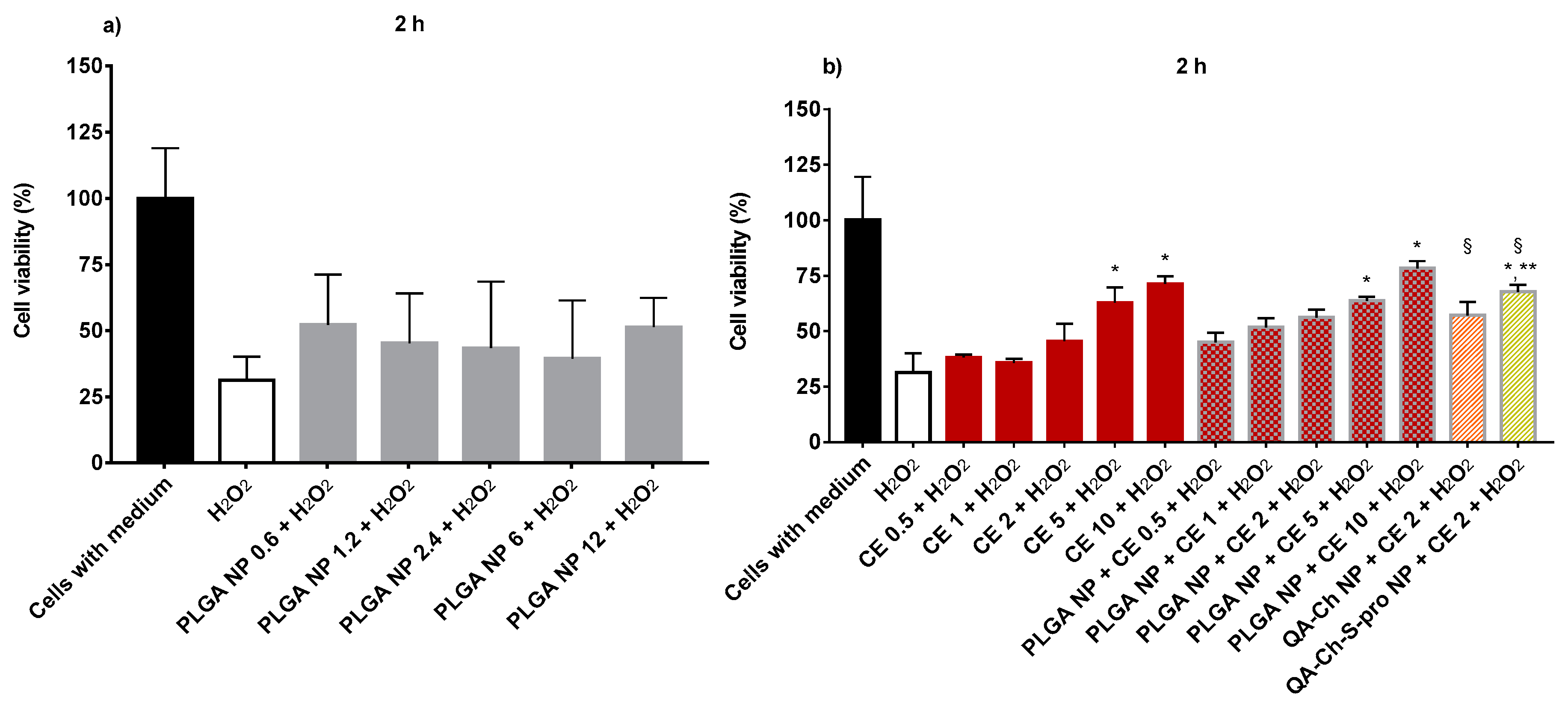
2.5. Protective Effect of Non-Encapsulated CE or NP-Loaded CE from Oxidative Stress
3. Materials and Methods
3.1. Catechin Determination
3.2. Preparation and Characterization of PLGA NPs
3.3. FITC Labeling of QA-Ch, QA-Ch-S-pro, and PLGA
3.4. Ex Vivo Mucoadhesivity Studies
3.5. Cell Viability Assay on Caco-2 and HT29-MTX
3.6. Permeability Studies in a Triple-Cell Co-Culture Model
3.7. Effect of Empty NPs or CE-Loaded NPs on HUVEC Viability
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ballistreri, G.; Continella, A.; Gentile, A.; Amenta, M.; Fabroni, S.; Rapisarda, P. Fruit quality and bioactive compounds relevant to human health of sweet cherry (Prunus avium L.) cultivars grown in Italy. Food Chem. 2013, 140, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Bacchetti, T.; Belleggia, A.; Neri, D. Cherry antioxidants: From farm to table. Molecules 2010, 15, 6993–7005. [Google Scholar] [CrossRef] [PubMed]
- McCune, L.M.; Kubota, C.; Stendell-Hollis, N.R.; Thomson, C.A. Cherries and Health: A Review. Crit. Rev. Food Sci. Nutr. 2010, 51, 1–12. [Google Scholar] [CrossRef]
- Chockchaisawasdee, S.; Golding, J.B.; Vuong, Q.V.; Papoutsis, K.; Stathopoulos, C.E. Sweet cherry: Composition, postharvest preservation, processing and trends for its future use. Trends Food Sci. Technol. 2016, 55, 72–83. [Google Scholar] [CrossRef]
- Usenik, V.; Fabčič, J.; Štampar, F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.). Food Chem. 2008, 107, 185–192. [Google Scholar] [CrossRef]
- Reis, J.F.; Monteiro, V.V.S.; de Souza Gomes, R.; do Carmo, M.M.; da Costa, G.V.; Ribera, P.C.; Monteiro, M.C. Action mechanism and cardiovascular effect of anthocyanins: A systematic review of animal and human studies. J. Transl. Med. 2016, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Bastos, C.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Queiroz, M.J.R.; Santos-Buelga, C.; Ferreira, I.C. Chemical characterisation and bioactive properties of Prunus Avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015, 173, 1045–1053. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-Based Nanoparticles Containing Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef]
- Berni, R.; Cantini, C.; Romi, M.; Hausman, J.F.; Guerriero, G.; Cai, G. Agrobiotechnology goes wild: Ancient local varieties as sources of bioactives. Int. J. Mol. Sci. 2018, 19, 2248. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.; Apone, F.; Abdel-Salam, E.; Qahtan, A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef]
- Berni, R.; Romi, M.; Cantini, C.; Hausman, J.F.; Guerriero, G.; Cai, G. Functional Molecules in Locally-Adapted Crops: The Case Study of Tomatoes, Onions, and Sweet Cherry Fruits from Tuscany in Italy. Front. Plant Sci. 2018, 9, 1983. [Google Scholar] [CrossRef]
- Felice, F.; Zambito, Y.; Belardinelli, E.; D’Onofrio, C.; Fabiano, A.; Balbarini, A.; Di Stefano, R. Delivery of natural polyphenols by polymeric nanoparticles improves the resistance of endothelial progenitor cells to oxidative stress. Eur. J. Pharm. Sci. 2013, 50, 393–399. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2018, 240, 396–404. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, A.K.; Raj, V.; Rai, A.; Keshari, A.K.; Kumar, D.; Maity, B.; Prakash, A.; Maiti, S.; Saha, S. Poly(lactic-co-glycolic acid)-loaded nanoparticles of betulinic acid for improved treatment of hepatic cancer: Characterization, in vitro and in vivo evaluations. Int. J. Nanomed. 2018, 13, 975. [Google Scholar] [CrossRef]
- Silva, L.M.; Hill, L.E.; Figueiredo, E.; Gomes, C.L. Delivery of phytochemicals of tropical fruit by-products using poly (DL-lactide-co-glycolide) (PLGA) nanoparticles: Synthesis, characterization, and antimicrobial activity. Food Chem. 2014, 165, 362–370. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Angonese, M.; Ferreira, S.R.S.; Gomes, C.L. Nanoencapsulation of passion fruit by-products extracts for enhanced antimicrobial activity. Food Bioprod. Process. 2017, 104, 137–146. [Google Scholar] [CrossRef]
- Sousa, F.; Cruz, A.; Fonte, P.; Pinto, I.M.; Neves-Petersen, M.T.; Sarmento, B. A new paradigm for antiangiogenic therapy through controlled release of bevacizumab from PLGA nanoparticles. Sci. Rep. 2017, 7, 3736. [Google Scholar] [CrossRef]
- Nair, H.B.; Sung, B.; Yadav, V.R.; Kannappan, R.; Chaturvedi, M.M.; Aggarwal, B.B. Delivery of antiinflammatory nutraceuticals by nanoparticles for the prevention and treatment of cancer. Biochem. Pharmacol. 2010, 80, 1833–1843. [Google Scholar] [CrossRef]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef]
- Fabiano, A.; Zambito, Y.; Bernkop-Schnürch, A. About the impact of water movement on the permeation behaviour of nanoparticles in mucus. Int. J. Pharm. 2017, 517, 279–285. [Google Scholar] [CrossRef]
- Fabiano, A.; Piras, A.M.; Uccello-Barretta, G.; Balzano, F.; Cesari, A.; Testai, L.; Citi, V.; Zambito, Y. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur. J. Pharm. Biopharm. 2018, 130, 281–289. [Google Scholar] [CrossRef]
- Zambito, Y.; Colo, G. Di Thiolated quaternary ammonium-chitosan conjugates for enhanced precorneal retention, transcorneal permeation and intraocular absorption of dexamethasone. Eur. J. Pharm. Biopharm. 2010, 75, 194–199. [Google Scholar] [CrossRef]
- Silva, D.S.; Almeida, A.; Prezotti, F.G.; Facchinatto, W.M.; Colnago, L.A.; Campana-Filho, S.P.; Sarmento, B. Self-aggregates of 3,6-O,O’-dimyristoylchitosan derivative are effective in enhancing the solubility and intestinal permeability of camptothecin. Carbohydr. Polym. 2017, 177, 178–186. [Google Scholar] [CrossRef]
- Sgorla, D.; Lechanteur, A.; Almeida, A.; Sousa, F.; Melo, E.; Bunhak, É.; Mainardes, R.; Khalil, N.; Cavalcanti, O.; Sarmento, B. Development and characterization of lipid-polymeric nanoparticles for oral insulin delivery. Expert Opin. Drug Deliv. 2018, 15, 213–222. [Google Scholar] [CrossRef]
- Bazes, A.; Nollevaux, G.; Coco, R.; Joly, A.; Sergent, T.; Schneider, Y.J. Development of a triculture based system for improved benefit/risk assessment in pharmacology and human food. BMC Proc. 2011, 5, 67. [Google Scholar] [CrossRef]
- Tariq, M.; Alam, M.A.; Singh, A.T.; Iqbal, Z.; Panda, A.K.; Talegaonkar, S. Biodegradable polymeric nanoparticles for oral delivery of epirubicin: In vitro, ex vivo, and in vivo investigations. Colloids Surf. B Biointerfaces 2015, 128, 448–456. [Google Scholar] [CrossRef]
- Yin Win, K.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- Kafedjiiski, K.; Hoffer, M.; Werle, M.; Bernkop-Schnürch, A. Improved synthesis and in vitro characterization of chitosan-thioethylamidine conjugate. Biomaterials 2006, 27, 127–135. [Google Scholar] [CrossRef]
- Zambito, Y.; Felice, F.; Fabiano, A.; Di Stefano, R.; Di Colo, G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr. Polym. 2013, 92, 33–39. [Google Scholar] [CrossRef]
- Zambito, Y.; Uccello-Barretta, G.; Zaino, C.; Balzano, F.; Di Colo, G. Novel transmucosal absorption enhancers obtained by aminoalkylation of chitosan. Eur. J. Pharm. Sci. 2006, 29, 460–469. [Google Scholar] [CrossRef]
- Zambito, Y.; Fogli, S.; Zaino, C.; Stefanelli, F.; Breschi, M.C.; Di Colo, G. Synthesis, characterization and evaluation of thiolated quaternary ammonium-chitosan conjugates for enhanced intestinal drug permeation. Eur. J. Pharm. Sci. 2009, 38, 112–120. [Google Scholar] [CrossRef]
- Ferreira-Nunes, R.; Angelo, T.; da Silva, S.M.M.; Magalhães, P.O.; Gratieri, T.; da Cunha-Filho, M.S.S.; Gelfuso, G.M. Versatile chromatographic method for catechin determination in development of topical formulations containing natural extracts. Biomed. Chromatogr. 2018, 32, e4062. [Google Scholar] [CrossRef]
- Araújo, F.; Sarmento, B. Towards the characterization of an in vitro triple co-culture intestine cell model for permeability studies. Int. J. Pharm. 2013, 458, 128–134. [Google Scholar] [CrossRef]
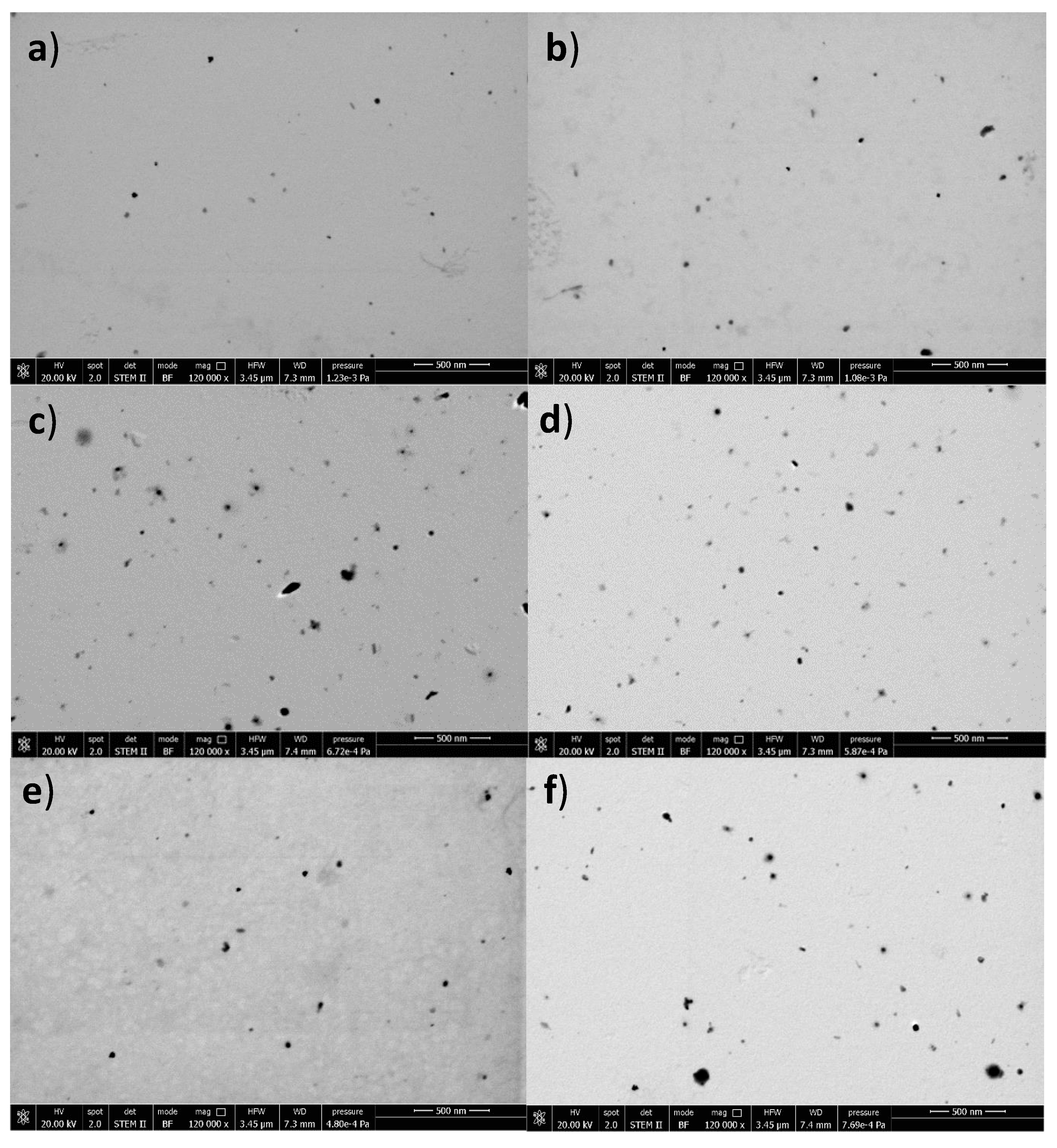
| NP Type | Nanoparticle Size, nm (Polydispersity Index) | Zeta Potential (ZP), mV | EE % (Direct Method) | EE % (Indirect Method) |
|---|---|---|---|---|
| PLGA NP + CE 100 | 216.0 ± 2.6 (0.06 ± 0.03) | −11.0 ± 1.23 | - | - |
| PLGA NP + CE 250 | 216.8 ± 4.9 (0.06 ± 0.02) | −10.9 ± 1.04 | 64.8 ± 2.3 | 51.7 ± 8.9 |
| PLGA NP + CE 420 | 208.4 ± 4.9 (0.05 ± 0.02) | −12.6 ± 0.87 | 43.9 ± 4.6 | 70.8 ± 5.5 |
| PLGA NP + CE 840 | 206.1 ± 1.8 (0.06 ± 0.03) | −8.36 ± 1.07 | 79.8 ± 6.2 | 88.6 ± 6.2 |
| Formulation | Adsorbed Mass Fraction, % |
|---|---|
| QA-Ch | 23.5 ± 3.1 a,b |
| QA-Ch-S-pro | 40.7 ± 2.0 b,c |
| PLGA | ND |
| QA-Ch NP | 39.7 ± 4.6 a,d |
| QA-Ch-S-pro NP | 80.5 ± 6.4 c,d |
| PLGA NP | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beconcini, D.; Fabiano, A.; Di Stefano, R.; Macedo, M.H.; Felice, F.; Zambito, Y.; Sarmento, B. Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(lactic-co-glycolic acid) Nanoparticles. Int. J. Mol. Sci. 2019, 20, 1759. https://doi.org/10.3390/ijms20071759
Beconcini D, Fabiano A, Di Stefano R, Macedo MH, Felice F, Zambito Y, Sarmento B. Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(lactic-co-glycolic acid) Nanoparticles. International Journal of Molecular Sciences. 2019; 20(7):1759. https://doi.org/10.3390/ijms20071759
Chicago/Turabian StyleBeconcini, Denise, Angela Fabiano, Rossella Di Stefano, Maria Helena Macedo, Francesca Felice, Ylenia Zambito, and Bruno Sarmento. 2019. "Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(lactic-co-glycolic acid) Nanoparticles" International Journal of Molecular Sciences 20, no. 7: 1759. https://doi.org/10.3390/ijms20071759
APA StyleBeconcini, D., Fabiano, A., Di Stefano, R., Macedo, M. H., Felice, F., Zambito, Y., & Sarmento, B. (2019). Cherry Extract from Prunus avium L. to Improve the Resistance of Endothelial Cells to Oxidative Stress: Mucoadhesive Chitosan vs. Poly(lactic-co-glycolic acid) Nanoparticles. International Journal of Molecular Sciences, 20(7), 1759. https://doi.org/10.3390/ijms20071759