Multiplex Analysis Platform for Endocrine Disruption Prediction Using Zebrafish
Abstract
:1. Introduction
2. Results and Discussion
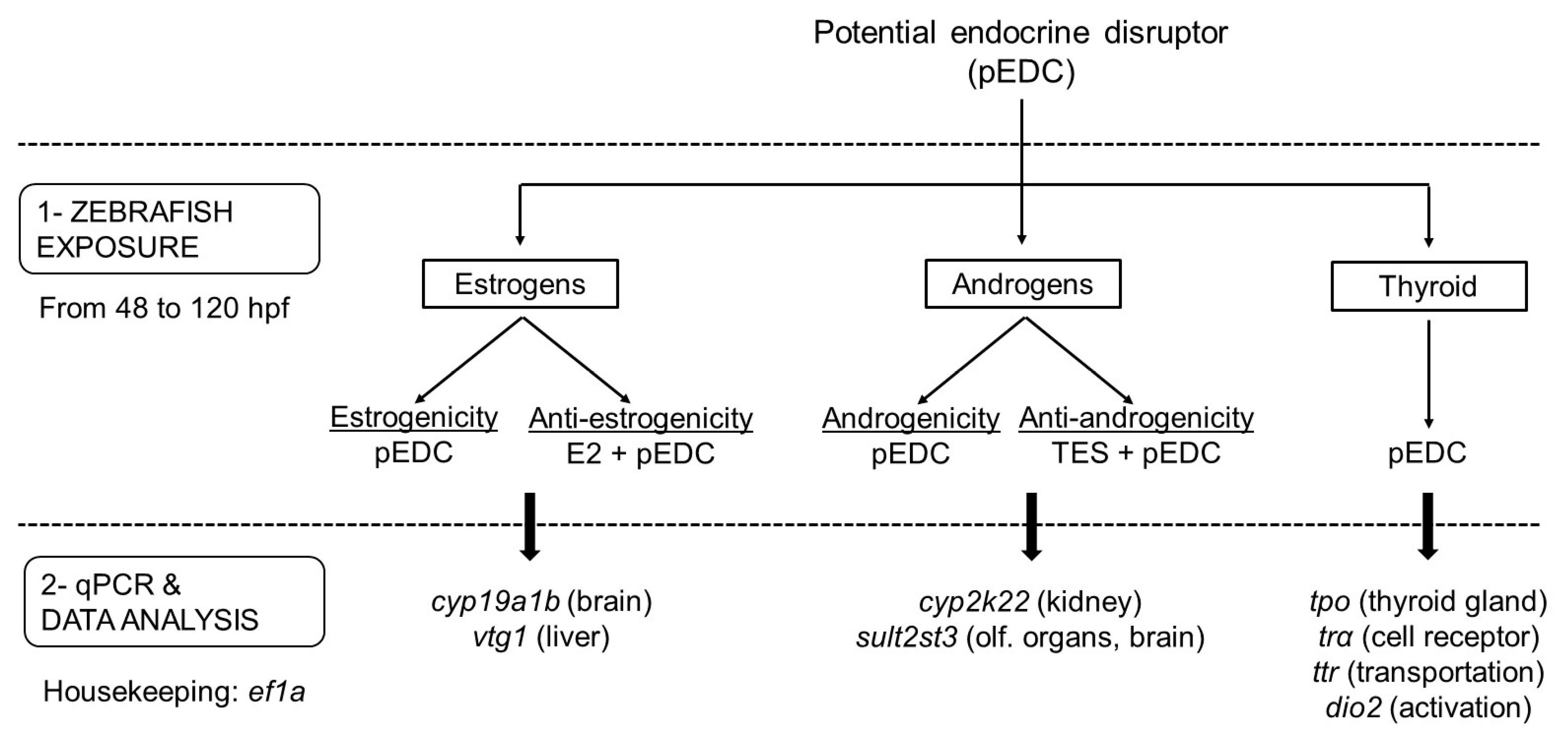
2.1. Experimental Setup
2.2. Gene Biomarker Validation
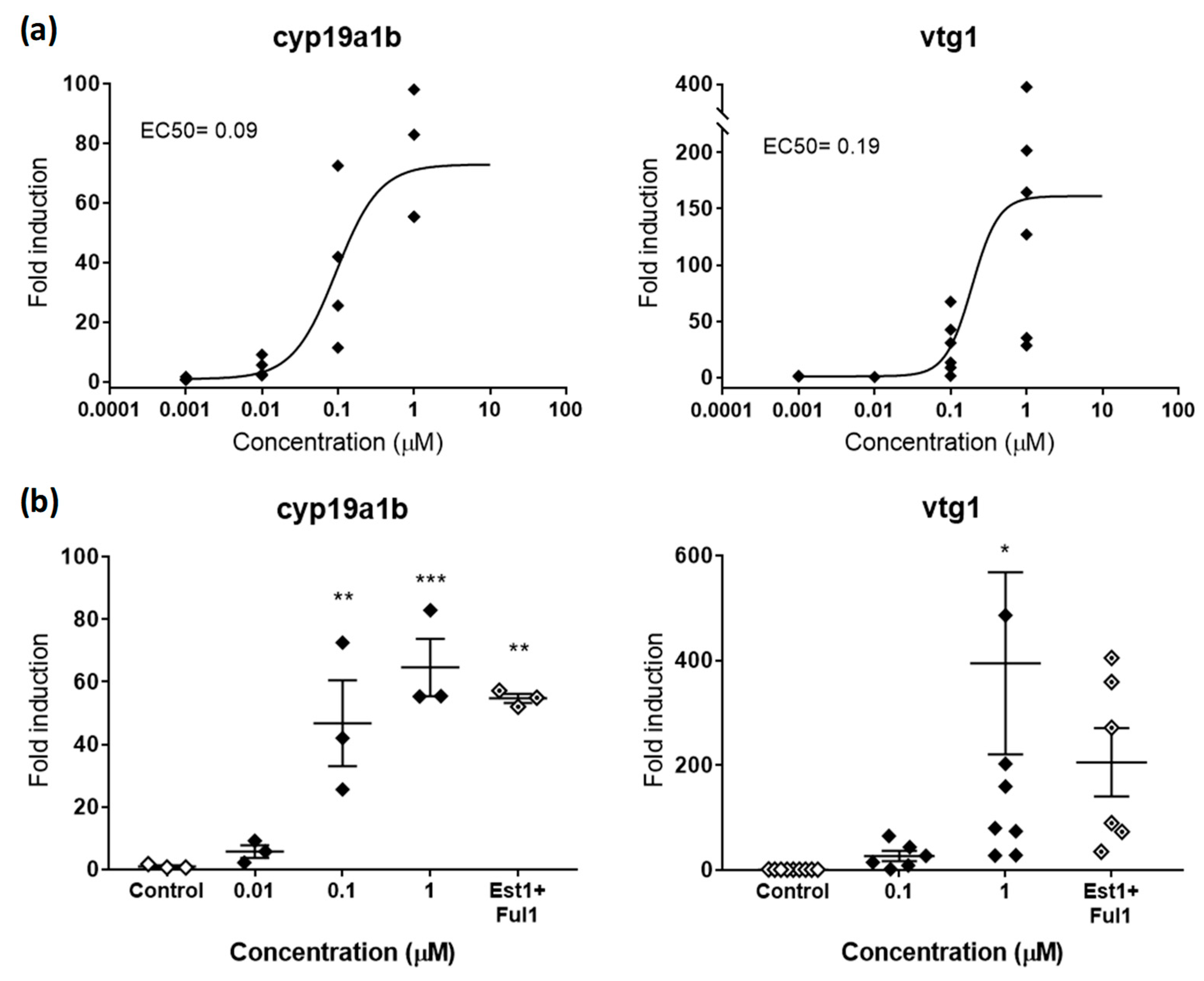
2.2.1. Estrogenic and Antiestrogenic Potencies
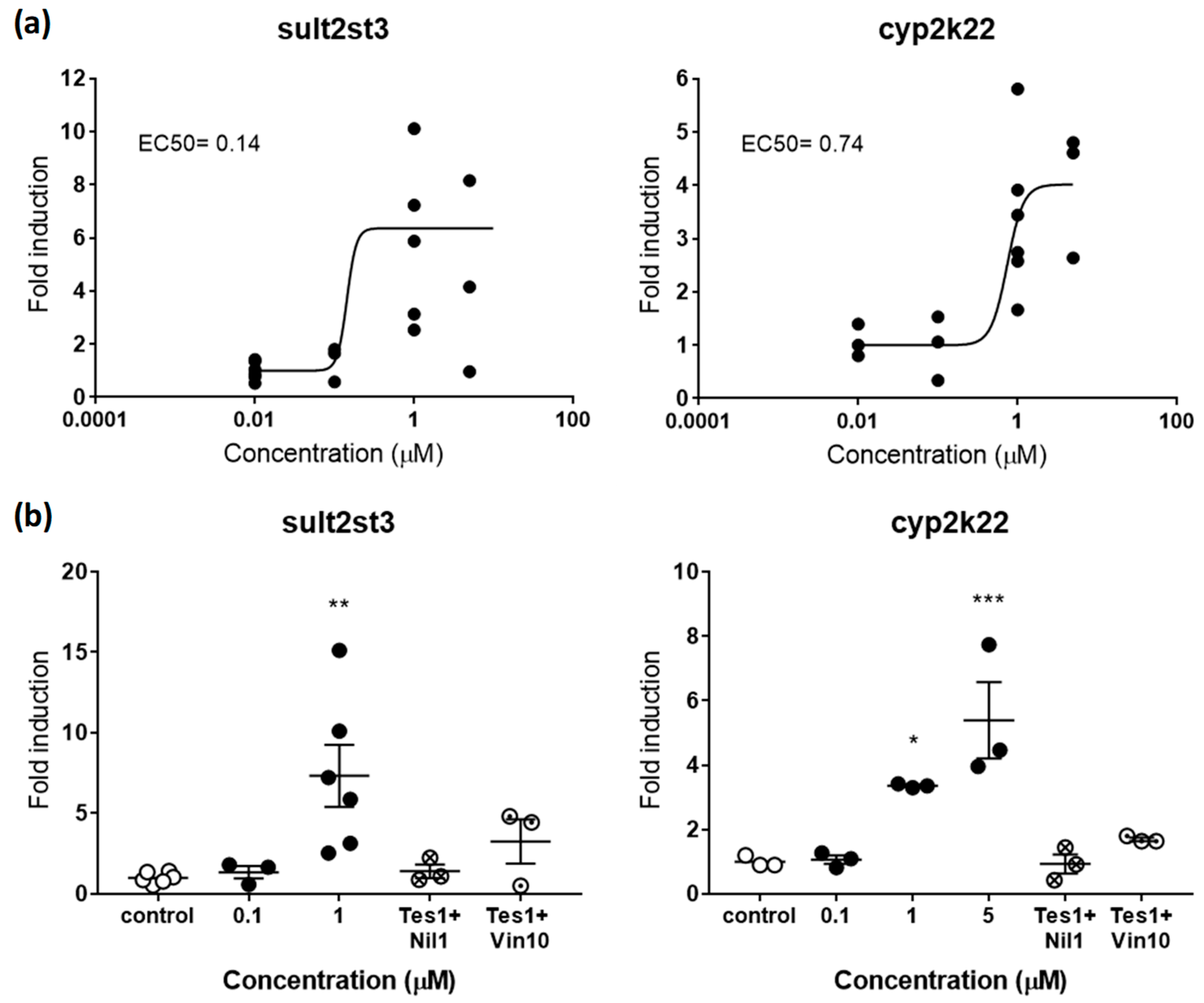
2.2.2. Androgenic and Antiandrogenic Potencies
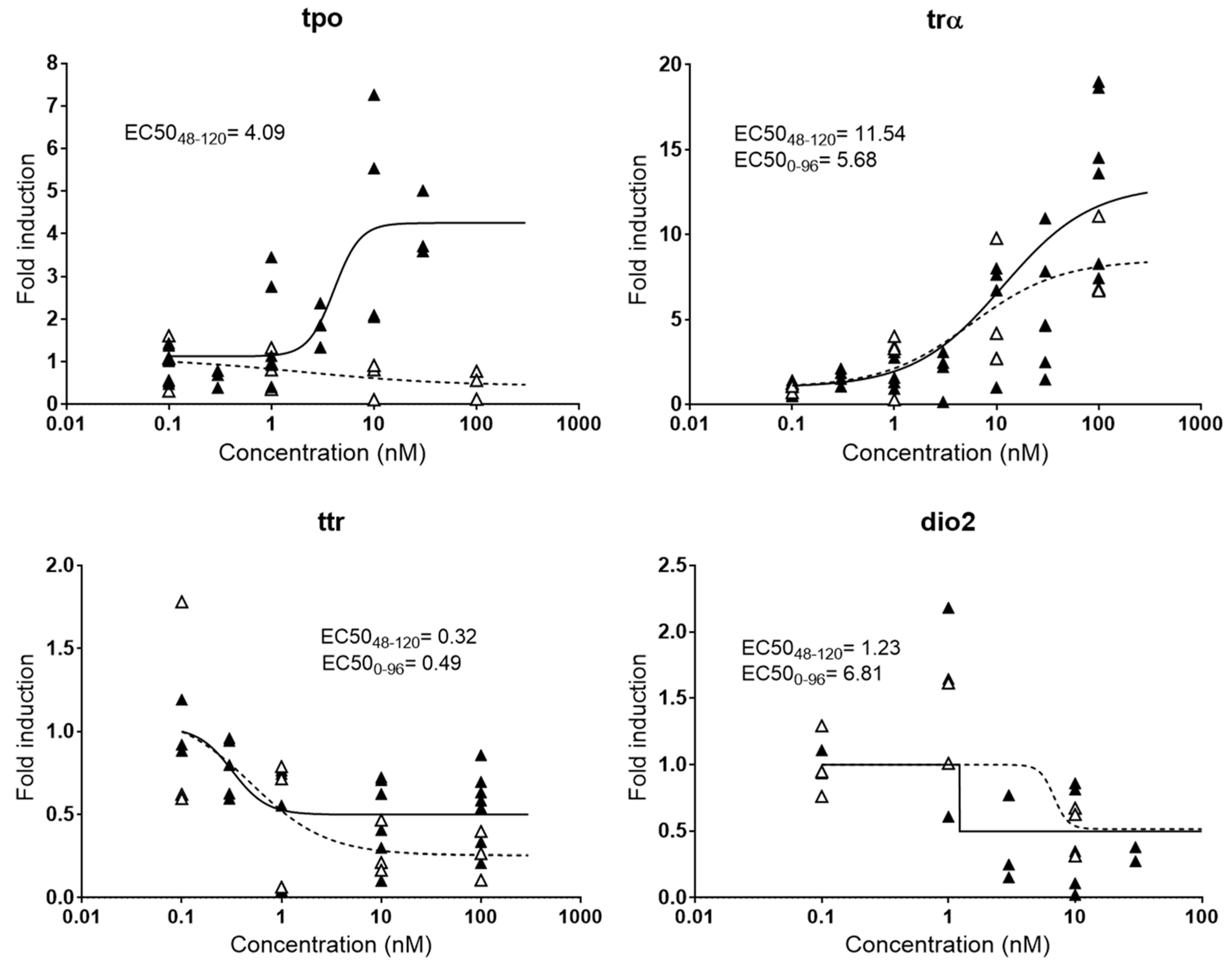
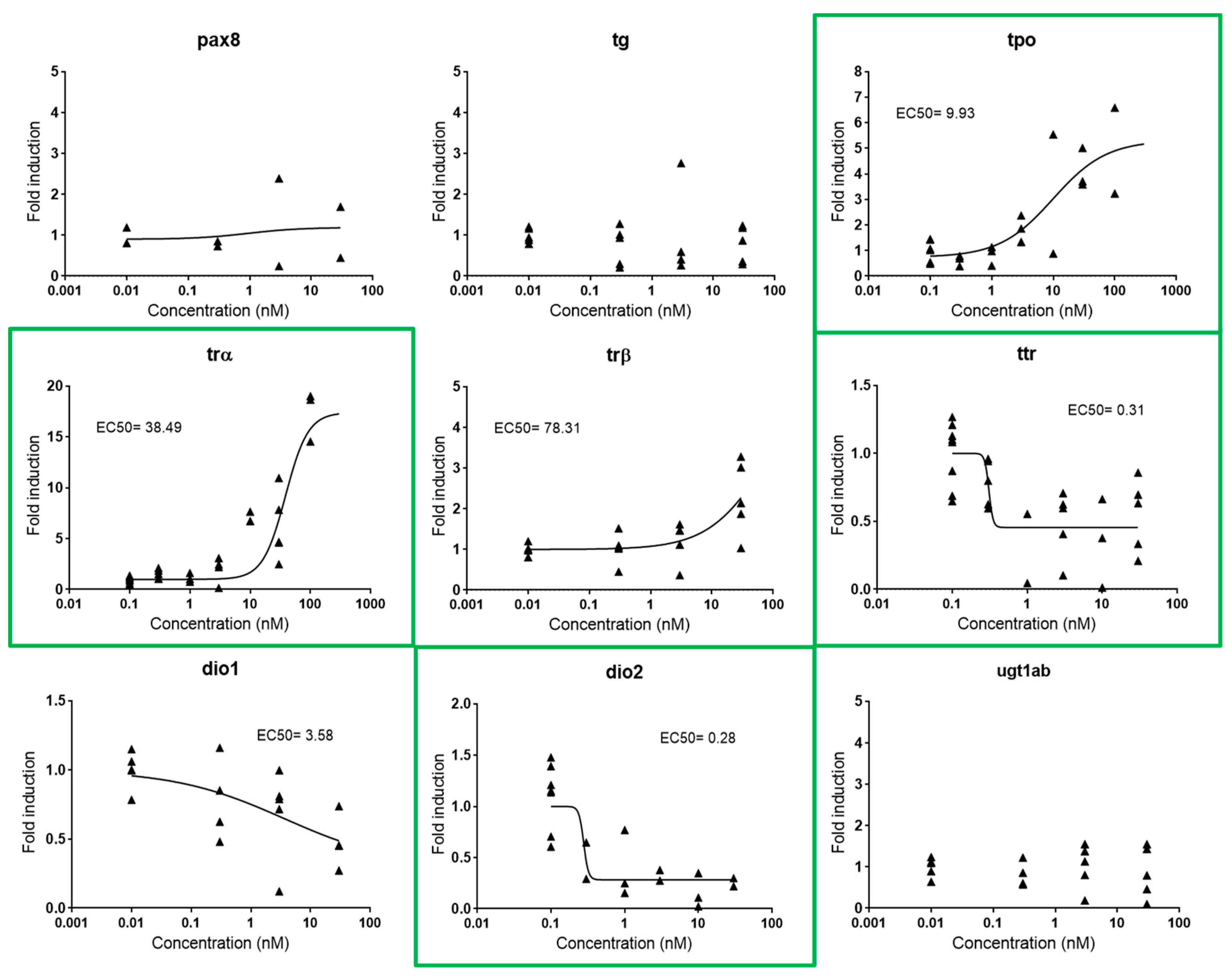
2.2.3. Thyroid
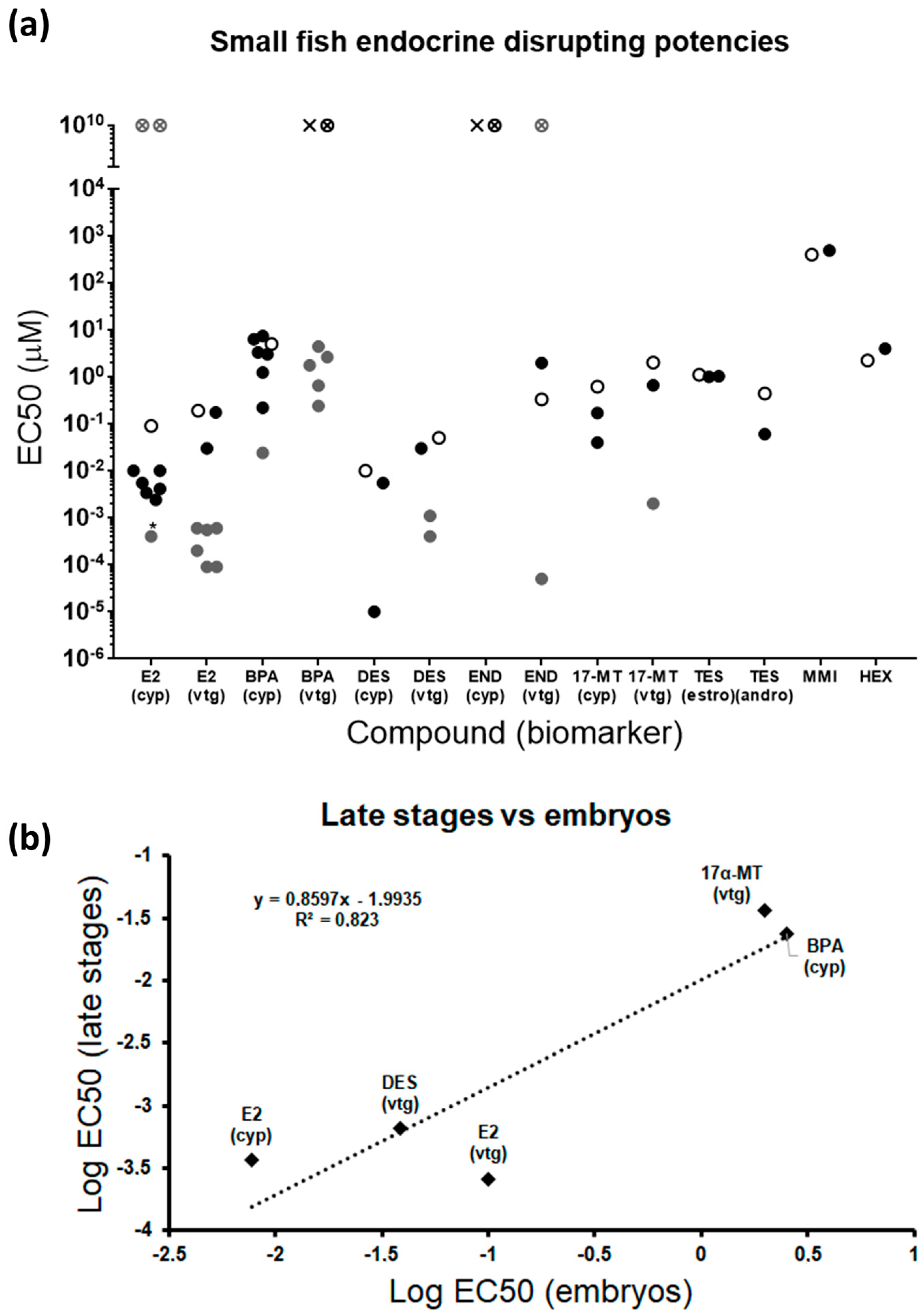
2.3. Screening of Compounds
2.4. Cross-Talk Effects
2.5. Zebrafish Embryos vs. Juveniles and Adults
3. Materials and Methods
3.1. Chemicals
3.2. Zebrafish Maintenance and Breeding
3.3. Toxicity Tests
3.4. EDCs Exposure Tests
3.5. Gene Expression Analysis by qPCR
3.6. Statistical Analysis
3.7. Ethical Study Approval
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EDC | endocrine disrupting compound |
| HPT | hypothalamus-pituitary-thyroid axis |
| TH | thyroid hormone |
| LC50 | half maximal lethal concentration |
| EC50 | half maximal effective concentration |
| LOEC | lowest observed effect concentration |
| NOEC | no observed effect concentration |
| ER | estrogen receptor |
| AR | androgen receptor |
| FI | fold induction |
| BPA | bisphenol A |
| pEDC | potential endocrine disrupting compound |
| DES | Diethylstilbestrol |
| END | Endosulfan |
| E2 | 17β-estradiol |
| FUL | Fulvestrant |
| HEX | Hexaconazole |
| MMI | Methimazole |
| 17-αMT | 17α-methyltestosterone |
| NAN | Nandrolone |
| NIL | Nilutamide |
| TES | Testosterone |
| T3 | 3,3′,5-triiodo-L-thyronine |
| VIN | Vinclozolin |
| DMSO | dimethyl sulfoxide |
| E3 | control media |
References
- Le Magueresse-Battistoni, B.; Vidal, H.; Naville, D. Environmental Pollutants and Metabolic Disorders: The Multi-Exposure Scenario of Life. Front. Endocrinol. 2018, 9, 582. [Google Scholar] [CrossRef] [PubMed]
- Sfakianakis, D.G.; Renieri, E.; Kentouri, M.; Tsatsakis, A.M. Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 2015, 137, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Li, H.; Ma, R.; Yu, Z.; Li, L.; Xiang, M.; Chen, X.; Hua, X.; Yu, Y. A review of toxicity induced by persistent organic pollutants (POPs) and endocrine-disrupting chemicals (EDCs) in the nematode Caenorhabditis elegans. J. Environ. Manag. 2019, 237, 519–525. [Google Scholar] [CrossRef]
- De Coster, S.; van Larebeke, N. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J. Environ. Public Health 2012, 2012, 713696. [Google Scholar] [CrossRef]
- Matthiessen, P.; Wheeler, J.R.; Weltje, L. A review of the evidence for endocrine disrupting effects of current-use chemicals on wildlife populations. Crit. Rev. Toxicol. 2018, 48, 195–216. [Google Scholar] [CrossRef]
- Brown, S.B.; Adams, B.A.; Cyr, D.G.; Eales, J.G. Contaminant effects on the teleost fish thyroid. Environ. Toxicol. Chem. 2004, 23, 1680. [Google Scholar] [CrossRef] [PubMed]
- WHO. Global Assessment of the State-of-the-Science of Endocrine Disruptors; Damstra, T., Barlow, S., Bergman, A., Kavlock, R., Van der Kraak, G., Eds.; WHO/PCS/EDC/02.2; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Jarque, S.; Bittner, M.; Blaha, L.; Hilscherova, K. Yeast Biosensors for Detection of Environmental Pollutants: Current State and Limitations. Trends Biotechnol. 2016, 34. [Google Scholar] [CrossRef]
- Petersen, K.; Fetter, E.; Kah, O.; Brion, F.; Scholz, S.; Tollefsen, K.E. Transgenic (cyp19a1b-GFP) zebrafish embryos as a tool for assessing combined effects of oestrogenic chemicals. Aquat. Toxicol. 2013, 138–139, 88–97. [Google Scholar] [CrossRef]
- Paul, K.B.; Hedge, J.M.; Rotroff, D.M.; Hornung, M.W.; Crofton, K.M.; Simmons, S.O. Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem. Res. Toxicol. 2014, 27, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Ankley, G.T.; Johnson, R.D. Small fish models for identifying and assessing the effects of endocrine-disrupting chemicals. ILAR J. 2004, 45, 469–483. [Google Scholar] [CrossRef]
- Scholz, S.; Mayer, I. Molecular biomarkers of endocrine disruption in small model fish. Mol. Cell. Endocrinol. 2008, 293, 57–70. [Google Scholar] [CrossRef]
- OECD. Test No. 230: 21-Day Fish Assay; OECD: Paris, France, 2009. [Google Scholar] [CrossRef]
- OECD. Test No. 229: Fish Short Term Reproduction Assay; OECD: Paris, France, 2012. [Google Scholar]
- OECD. Test No. 234: Fish Sexual Development Test [Internet]. 2011. Available online: https://www.oecd-ilibrary.org/environment/test-no-220-enchytraeid-reproduction-test_9789264264472-en (accessed on 12 February 2019).
- Kishida, M.; McLellan, M.; Miranda, J.A.; Callard, G.V. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 261–268. [Google Scholar] [CrossRef]
- Brion, F.; Le Page, Y.; Piccini, B.; Cardoso, O.; Tong, S.-K.; Chung, B.; Kah, O. Screening Estrogenic Activities of Chemicals or Mixtures In Vivo Using Transgenic (cyp19a1b-GFP) Zebrafish Embryos. PLoS ONE 2012, 7, e36069. [Google Scholar] [CrossRef]
- Green, J.M.; Metz, J.; Lee, O.; Trznadel, M.; Takesono, A.; Brown, A.R.; Owen, S.F.; Kudoh, T.; Tyler, C.R. High-Content and Semi-Automated Quantification of Responses to Estrogenic Chemicals Using a Novel Translucent Transgenic Zebrafish. Environ. Sci. Technol. 2016, 50, 6536–6545. [Google Scholar] [CrossRef]
- Fetter, E.; Smetanová, S.; Baldauf, L.; Lidzba, A.; Altenburger, R.; Schüttler, A.; Scholz, S. Identification and Characterization of Androgen-Responsive Genes in Zebrafish Embryos. Environ. Sci. Technol. 2015, 49, 11789–11798. [Google Scholar] [CrossRef]
- Sébillot, A.; Damdimopoulou, P.; Ogino, Y.; Spirhanzlova, P.; Miyagawa, S.; Du Pasquier, D.; Mouatassim, N.; Iguchi, T.; Lemkine, G.F.; Demeneix, B.A.; et al. Rapid fluorescent detection of (anti)androgens with spiggin-gfp medaka. Environ. Sci. Technol. 2014, 48, 10919–10928. [Google Scholar] [CrossRef]
- Thienpont, B.; Tingaud-Sequeira, A.; Prats, E.; Barata, C.; Babin, P.J.; Raldúa, D. Zebrafish eleutheroembryos provide a suitable vertebrate model for screening chemicals that impair thyroid hormone synthesis. Environ. Sci. Technol. 2011, 45, 7525–7532. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Zhang, X. Zebrafish embryos/larvae for rapid determination of effects on hypothalamic-pituitary-thyroid (HPT) and hypothalamic-pituitary-interrenal (HPI) axis: mRNA expression. Chemosphere 2013, 93, 2327–2332. [Google Scholar] [CrossRef]
- Yu, L.; Chen, M.; Liu, Y.; Gui, W.; Zhu, G. Thyroid endocrine disruption in zebrafish larvae following exposure to hexaconazole and tebuconazole. Aquat. Toxicol. 2013, 138–139C, 35–42. [Google Scholar] [CrossRef]
- Terrien, X.; Fini, J.-B.; Demeneix, B.A.; Schramm, K.-W.; Prunet, P. Generation of fluorescent zebrafish to study endocrine disruption and potential crosstalk between thyroid hormone and corticosteroids. Aquat. Toxicol. 2011, 105, 13–20. [Google Scholar] [CrossRef]
- Jarque, S.; Fetter, E.; Veneman, W.J.; Spaink, H.P.; Peravali, R.; Strähle, U.; Scholz, S. An automated screening method for detecting compounds with goitrogenic activity using transgenic zebrafish embryos. PLoS ONE 2018, 13, e0203087. [Google Scholar] [CrossRef]
- Jarque, S.; Piña, B. Deiodinases and thyroid metabolism disruption in teleost fish. Environ. Res. 2014, 135, 361–375. [Google Scholar] [CrossRef]
- Lassiter, C.S.; Linney, E. Embryonic Expression And Steroid Regulation of Brain Aromatase cyp19a1b in Zebrafish (Danio rerio). Zebrafish 2007, 4, 49–58. [Google Scholar] [CrossRef]
- Alt, B.; Reibe, S.; Feitosa, N.M.; Elsalini, O.A.; Wendl, T.; Rohr, K.B. Analysis of origin and growth of the thyroid gland in zebrafish. Dev. Dyn. 2006, 235, 1872–1883. [Google Scholar] [CrossRef]
- Fetter, E.; Baldauf, L.; Da Fonte, D.F.; Ortmann, J.; Scholz, S. Comparative analysis of goitrogenic effects of phenylthiourea and methimazole in zebrafish embryos. Reprod. Toxicol. 2015, 57, 10–20. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar] [CrossRef]
- García-Reyero, N.; Raldúa, D.; Quirós, L.; Llaveria, G.; Cerdà, J.; Barceló, D.; Grimalt, J.O.; Piña, B. Use of vitellogenin mRNA as a biomarker for endocrine disruption in feral and cultured fish. Anal. Bioanal. Chem. 2004, 378, 670–675. [Google Scholar] [CrossRef]
- Yang, M.; Hu, J.; Li, S.; Ma, Y.; Gui, W.; Zhu, G. Thyroid endocrine disruption of acetochlor on zebrafish (Danio rerio) larvae. J. Appl. Toxicol. 2016, 36, 844–852. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, E.; Yang, Z. Waterborne exposure to BPS causes thyroid endocrine disruption in zebrafish larvae. PLoS ONE 2017, 12, e0176927. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Pang, S.; Wang, C.; Wang, L.; Sun, Y.; Song, M.; Liang, Y. Evaluating estrogenic and anti-estrogenic effect of endocrine disrupting chemicals (EDCs) by zebrafish (Danio rerio) embryo-based vitellogenin 1 (vtg1) mRNA expression. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 204, 45–50. [Google Scholar] [CrossRef]
- Brian, J.V.; Harris, C.A.; Scholze, M.; Backhaus, T.; Booy, P.; Lamoree, M.; Pojana, G.; Jonkers, N.; Runnalls, T.; Bonfà, A.; et al. Accurate Prediction of the Response of Freshwater Fish to a Mixture of Estrogenic Chemicals. Environ. Health Perspect. 2005, 113, 721–728. [Google Scholar] [CrossRef]
- Hall, J.M.; Couse, J.F.; Korach, K.S. The multifaceted mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 2001, 276, 36869–36872. [Google Scholar] [CrossRef]
- Fent, K.; Siegenthaler, P.F.; Schmid, A.A. Transcriptional effects of androstenedione and 17α-hydroxyprogesterone in zebrafish embryos. Aquat. Toxicol. 2018, 202, 1–5. [Google Scholar] [CrossRef]
- Molina-Molina, J.-M.; Hillenweck, A.; Jouanin, I.; Zalko, D.; Cravedi, J.-P.; Fernández, M.-F.; Pillon, A.; Nicolas, J.C.; Olea, N.; Balaguer, P. Steroid receptor profiling of vinclozolin and its primary metabolites. Toxicol. Appl. Pharmacol. 2006, 216, 44–54. [Google Scholar] [CrossRef]
- Urushibara, M.; Ishioka, J.; Hyochi, N.; Kihara, K.; Hara, S.; Singh, P.; Isaacs, J.T.; Kageyama, Y. Effects of steroidal and non-steroidal antiandrogens on wild-type and mutant androgen receptors. Prostate 2007, 67, 799–807. [Google Scholar] [CrossRef]
- Le Fol, V.; Aït-Aïssa, S.; Sonavane, M.; Porcher, J.-M.; Balaguer, P.; Cravedi, J.-P.; Zalko, D.; Brion, F. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicol. Environ. Saf. 2017, 142, 150–156. [Google Scholar] [CrossRef]
- Nesan, D.; Sewell, L.C.; Kurrasch, D.M. Opening the black box of endocrine disruption of brain development: Lessons from the characterization of Bisphenol A. Horm. Behav. 2018, 101, 50–58. [Google Scholar] [CrossRef]
- Han, Z.; Jiao, S.; Kong, D.; Shan, Z.; Zhang, X. Effects of β-endosulfan on the growth and reproduction of zebrafish (Danio rerio). Environ. Toxicol. Chem. 2011, 30, 2525–2531. [Google Scholar] [CrossRef]
- Petit, F.; Le Goff, P.; Cravédi, J.P.; Valotaire, Y.; Pakdel, F. Two complementary bioassays for screening the estrogenic potency of xenobiotics: Recombinant yeast for trout estrogen receptor and trout hepatocyte cultures. J. Mol. Endocrinol. 1997, 19, 321–335. [Google Scholar] [CrossRef]
- Smeets, J.M.; van Holsteijn, I.; Giesy, J.P.; Seinen, W.; van den Berg, M. Estrogenic potencies of several environmental pollutants, as determined by vitellogenin induction in a carp hepatocyte assay. Toxicol. Sci. 1999, 50, 206–213. [Google Scholar] [CrossRef]
- Maskey, E.; Crotty, H.; Wooten, T.; Khan, I.A. Disruption of oocyte maturation by selected environmental chemicals in zebrafish. Toxicol. In Vitro 2019, 54, 123–129. [Google Scholar] [CrossRef]
- Chakravorty, S.; Lal, B.; Singh, T.P. Effect of endosulfan (thiodan) on vitellogenesis and its modulation by different hormones in the vitellogenic catfish Clarias batrachus. Toxicology 1992, 75, 191–198. [Google Scholar] [CrossRef]
- Hemmer, M.J.; Hemmer, B.L.; Bowman, C.J.; Kroll, K.J.; Folmar, L.C.; Marcovich, D.; Hoglund, M.D.; Denslow, N.D. Effects of p-nonylphenol, methoxychlor, and endosulfan on vitellogenin induction and expression in sheepshead minnow (Cyprinodon variegatus). Environ. Toxicol. Chem. 2001, 20, 336–343. [Google Scholar] [CrossRef]
- Fostier, A.; Jalabert, B.; Billard, R.; Breton, B.; Zohar, Y. The Gonadal Steroids. In Reproduction Endocrine Tissues and Hormones; Hoar, W.S., Randall, D.J., Donaldson, E.M., Eds.; Academic Press: New York, NY, USA, 1983; pp. 277–372. [Google Scholar]
- Li, W.; Zhu, L.; Zha, J.; Wang, Z. Effects of decabromodiphenyl ether (BDE-209) on mRNA transcription of thyroid hormone pathway and spermatogenesis associated genes in Chinese rare minnow (Gobiocypris rarus). Environ. Toxicol. 2014, 29, 1–9. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, X.; Deng, J.; Hecker, M.; Al-Khedhairy, A.; Giesy, J.P.; Zhou, B. Effects of prochloraz or propylthiouracil on the cross-talk between the HPG, HPA, and HPT axes in zebrafish. Environ. Sci. Technol. 2011, 45, 769–775. [Google Scholar] [CrossRef]
- Hornung, M.W.; Jensen, K.M.; Korte, J.J.; Kahl, M.D.; Durhan, E.J.; Denny, J.S.; Henry, T.R.; Ankley, G.T. Mechanistic basis for estrogenic effects in fathead minnow (Pimephales promelas) following exposure to the androgen 17α-methyltestosterone: Conversion of 17α-methyltestosterone to 17α-methylestradiol. Aquat. Toxicol. 2004, 66, 15–23. [Google Scholar] [CrossRef]
- Sirianni, R.; Capparelli, C.; Chimento, A.; Panza, S.; Catalano, S.; Lanzino, M.; Pezzi, V.; Andò, S. Nandrolone and stanozolol upregulate aromatase expression and further increase IGF-I-dependent effects on MCF-7 breast cancer cell proliferation. Mol. Cell. Endocrinol. 2012, 363, 100–110. [Google Scholar] [CrossRef]
- Kim, B.-H.; Takemura, A.; Kim, S.J.; Lee, Y.-D. Vitellogenin synthesis via androgens in primary cultures of tilapia hepatocytes. Gen. Comp. Endocrinol. 2003, 132, 248–255. [Google Scholar] [CrossRef]
- Mori, T.; Matsumoto, H.; Yokota, H. Androgen-induced vitellogenin gene expression in primary cultures of rainbow trout hepatocytes. J. Steroid Biochem. Mol. Biol. 1998, 67, 133–141. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Ying, G.-G.; Yang, Y.-Y.; Liu, W.-R.; Liu, S.-S.; Chen, J.; Liu, Y.S.; Zhao, J.L.; Zhang, Q.Q. Occurrence, fate and risk assessment of androgens in ten wastewater treatment plants and receiving rivers of South China. Chemosphere 2018, 201, 644–654. [Google Scholar] [CrossRef]
- Freitas, J.; Cano, P.; Craig-Veit, C.; Goodson, M.L.; Furlow, J.D.; Murk, A.J. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicol. In Vitro 2011, 25, 257–266. [Google Scholar] [CrossRef]
- Lu, L.; Zhan, T.; Ma, M.; Xu, C.; Wang, J.; Zhang, C.; Liu, W.; Zhuang, S. Thyroid Disruption by Bisphenol S Analogues via Thyroid Hormone Receptor β: In Vitro, in Vivo, and Molecular Dynamics Simulation Study. Environ. Sci. Technol. 2018, 52, 6617–6625. [Google Scholar] [CrossRef]
- Berto-Júnior, C.; Santos-Silva, A.P.; Ferreira, A.C.F.; Graceli, J.B.; de Carvalho, D.P.; Soares, P.; Romeiro, N.C.; Miranda-Alves, L. Unraveling molecular targets of bisphenol A and S in the thyroid gland. Environ. Sci. Pollut. Res. 2018, 25, 26916–26926. [Google Scholar] [CrossRef]
- Dammann, A.A.; Shappell, N.W.; Bartell, S.E.; Schoenfuss, H.L. Comparing biological effects and potencies of estrone and 17β-estradiol in mature fathead minnows, Pimephales promelas. Aquat. Toxicol. 2011, 105, 559–568. [Google Scholar] [CrossRef]
- Hinfray, N.; Palluel, O.; Turies, C.; Cousin, C.; Porcher, J.M.; Brion, F. Brain and gonadal aromatase as potential targets of endocrine disrupting chemicals in a model species, the zebrafish (Danio rerio). Environ. Toxicol. 2006, 21, 332–337. [Google Scholar] [CrossRef]
- Kallivretaki, E.; Eggen, R.; Neuhauss, S.; Alberti, M.; Kausch, U.; Segner, H. Aromatase in zebrafish: A potential target for endocrine disrupting chemicals. Mar. Environ. Res. 2006, 62, S187–S190. [Google Scholar] [CrossRef]
- Halm, S.; Pounds, N.; Maddix, S.; Rand-Weaver, M.; Sumpter, J.; Hutchinson, T.; Tyler, C.R. Exposure to exogenous 17β-oestradiol disrupts P450aromB mRNA expression in the brain and gonad of adult fathead minnows (Pimephales promelas). Aquat. Toxicol. 2002, 60, 285–299. [Google Scholar] [CrossRef]
- Pawlowski, S.; Sauer, A.; Shears, J.; Tyler, C.; Braunbeck, T. Androgenic and estrogenic effects of the synthetic androgen 17α-methyltestosterone on sexual development and reproductive performance in the fathead minnow (Pimephales promelas) determined using the gonadal recrudescence assay. Aquat. Toxicol. 2004, 68, 277–291. [Google Scholar] [CrossRef]
- Kang, I.J.; Yokota, H.; Oshima, Y.; Tsuruda, Y.; Shimasaki, Y.; Honjo, T. The effects of methyltestosterone on the sexual development and reproduction of adult medaka (Oryzias latipes). Aquat. Toxicol. 2008, 87, 37–46. [Google Scholar] [CrossRef]
- Chung, E.; Genco, M.C.; Megrelis, L.; Ruderman, J.V. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proc. Natl. Acad. Sci. USA 2011, 108, 17732–17737. [Google Scholar] [CrossRef]
- Muncke, J.; Eggen, R.I.L. Vitellogenin 1 mRNA as an early molecular biomarker for endocrine disruption in developing zebrafish (Danio rerio). Environ. Toxicol. Chem. 2006, 25, 2734–2741. [Google Scholar] [CrossRef]
- Rose, J.; Holbech, H.; Lindholst, C.; Nørum, U.; Povlsen, A.; Korsgaard, B.; Bjerregaard, P. Vitellogenin induction by 17beta-estradiol and 17alpha-ethinylestradiol in male zebrafish (Danio rerio). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2002, 131, 531–539. [Google Scholar] [CrossRef]
- Van den Belt, K.; Berckmans, P.; Vangenechten, C.; Verheyen, R.; Witters, H. Comparative study on the in vitro/in vivo estrogenic potencies of 17β-estradiol, estrone, 17α-ethynylestradiol and nonylphenol. Aquat. Toxicol. 2004, 66, 183–195. [Google Scholar] [CrossRef]
- Parks, L.G.; Cheek, A.O.; Denslow, N.D.; Heppell, S.A.; McLachlan, J.A.; LeBlanc, G.A.; Sullivan, C.V. Fathead minnow (Pimephales promelas) vitellogenin: Purification, characterization and quantitative immunoassay for the detection of estrogenic compounds. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1999, 123, 113–125. [Google Scholar] [CrossRef]
- Zeng, Z.; Shan, T.; Tong, Y.; Lam, S.H.; Gong, Z. Development of Estrogen-Responsive Transgenic Medaka for Environmental Monitoring of Endocrine Disrupters. Environ. Sci. Technol. 2005, 39, 9001–9008. [Google Scholar] [CrossRef]
- Saeed, A.; Hashmi, I.; Zare, A.; Mehrabani-Zeinabad, M.; Achari, G.; Habibi, H.R. Efficacy of UV-C photolysis of bisphenol A on transcriptome alterations of genes in zebrafish embryos. J. Environ. Sci. Health Part A 2016, 51, 877–883. [Google Scholar] [CrossRef]
- Molina, A.; Abril, N.; Morales-Prieto, N.; Monterde, J.; Ayala, N.; Lora, A.; Moyano, R. Hypothalamic-pituitary-ovarian axis perturbation in the basis of bisphenol A (BPA) reproductive toxicity in female zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2018, 156, 116–124. [Google Scholar] [CrossRef]
- Sohoni, P.C.R.T.; Tyler, C.R.; Hurd, K.; Caunter, J.; Hetheridge, M.; Williams, T.; Woods, C.; Evans, M.; Toy, R.; Gargas, M.; et al. Reproductive Effects of Long-Term Exposure to Bisphenol A in the Fathead Minnow (Pimephales promelas). Environ. Sci. Technol. 2001, 35, 2917–2925. [Google Scholar] [CrossRef]
- Zhong, X.; Xu, Y.; Liang, Y.; Liao, T.; Wang, J. Vitellogenin in rare minnow (Gobiocypris rarus): Identification and induction by waterborne diethylstilbestrol. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2004, 137, 291–298. [Google Scholar] [CrossRef]
- Yin, P.; Li, Y.-W.; Chen, Q.-L.; Liu, Z.-H. Diethylstilbestrol, flutamide and their combination impaired the spermatogenesis of male adult zebrafish through disrupting HPG axis, meiosis and apoptosis. Aquat. Toxicol. 2017, 185, 129–137. [Google Scholar] [CrossRef]
- Moon, Y.-S.; Jeon, H.-J.; Nam, T.-H.; Choi, S.-D.; Park, B.-J.; Ok, Y.S.; Lee, S.E. Acute toxicity and gene responses induced by endosulfan in zebrafish (Danio rerio) embryos. Chem. Speciat. Bioavailab. 2016, 28, 103–109. [Google Scholar] [CrossRef]
- Mouriec, K.; Gueguen, M.-M.; Manuel, C.; Percevault, F.; Thieulant, M.-L.; Pakdel, F.; Kah, O. Androgens Upregulate cyp19a1b (Aromatase B) Gene Expression in the Brain of Zebrafish (Danio rerio) Through Estrogen Receptors1. Biol. Reprod. 2009, 80, 889–896. [Google Scholar] [CrossRef]
- Trant, J.M.; Gavasso, S.; Ackers, J.; Chung, B.C.; Place, A.R. Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Danio rerio). J. Exp. Zool. 2001, 290, 475–483. [Google Scholar] [CrossRef]
- Ankley, G.T.; Jensen, K.M.; Kahl, M.D.; Korte, J.J.; Makynen, E.A. Description and evaluation of a short-term reproduction test with the fathead minnow (Pimephales promelas). Environ. Toxicol. Chem. 2001, 20, 1276–1290. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book; A Guide for the Laboratory Use of Zebrafish (Brachydanio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Tonyushkina, K.N.; Shen, M.-C.; Ortiz-Toro, T.; Karlstrom, R.O. Embryonic exposure to excess thyroid hormone causes thyrotrope cell death. J. Clin. Investig. 2014, 124, 321–327. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- McCurley, A.T.; Callard, G.V. Characterization of housekeeping genes in zebrafish: Male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008, 9, 102. [Google Scholar] [CrossRef]

| Parental Pathway | Compound | Estrogen Axis | Androgen Axis | Thyroid Axis |
|---|---|---|---|---|
| Estrogens | E2 | cyp19a1b (0.09) vtg 1 (0.19) | ||
| BPA | cyp19a1b (4.99) | |||
| END | vtg1 (0.33) | tpo (0.06) | ||
| DES | cyp19a1b (0.01) vtg1 (0.05) | |||
| Androgens | TES | cyp19a1b (1.11) vtg1 (1.46) | sult2st3 (0.14) cyp2k22 (0.74) | tpo (0.02) |
| NAN | cyp19a1b (0.2) vtg1 (1.72) | sult2st3 (0.13) cyp2k22 (0.11) | dio2 (0.08) | |
| 17α-MT | cyp19a1b (0.62) vtg1 (2) | sult2st3 (0.10) cyp2k22 (0.05) | dio2 (0.09) | |
| Thyroid | T3 | not evaluated | tpo (0.01) trα (0.038) ttr (0.0003) dio2 (0.0003) | |
| MMI | tpo (397) | |||
| HEX | not evaluated | dio2 (2.22) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarque, S.; Ibarra, J.; Rubio-Brotons, M.; García-Fernández, J.; Terriente, J. Multiplex Analysis Platform for Endocrine Disruption Prediction Using Zebrafish. Int. J. Mol. Sci. 2019, 20, 1739. https://doi.org/10.3390/ijms20071739
Jarque S, Ibarra J, Rubio-Brotons M, García-Fernández J, Terriente J. Multiplex Analysis Platform for Endocrine Disruption Prediction Using Zebrafish. International Journal of Molecular Sciences. 2019; 20(7):1739. https://doi.org/10.3390/ijms20071739
Chicago/Turabian StyleJarque, Sergio, Jone Ibarra, Maria Rubio-Brotons, Jessica García-Fernández, and Javier Terriente. 2019. "Multiplex Analysis Platform for Endocrine Disruption Prediction Using Zebrafish" International Journal of Molecular Sciences 20, no. 7: 1739. https://doi.org/10.3390/ijms20071739
APA StyleJarque, S., Ibarra, J., Rubio-Brotons, M., García-Fernández, J., & Terriente, J. (2019). Multiplex Analysis Platform for Endocrine Disruption Prediction Using Zebrafish. International Journal of Molecular Sciences, 20(7), 1739. https://doi.org/10.3390/ijms20071739





