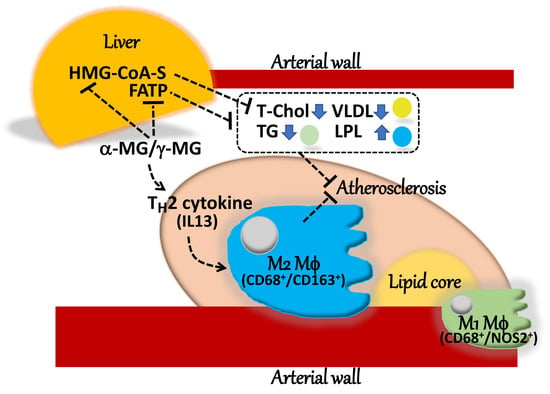
Crude α-Mangostin Suppresses the Development of Atherosclerotic Lesions in Apoe-Deficient Mice by a Possible M2 Macrophage-Mediated Mechanism
Abstract
1. Introduction
2. Results
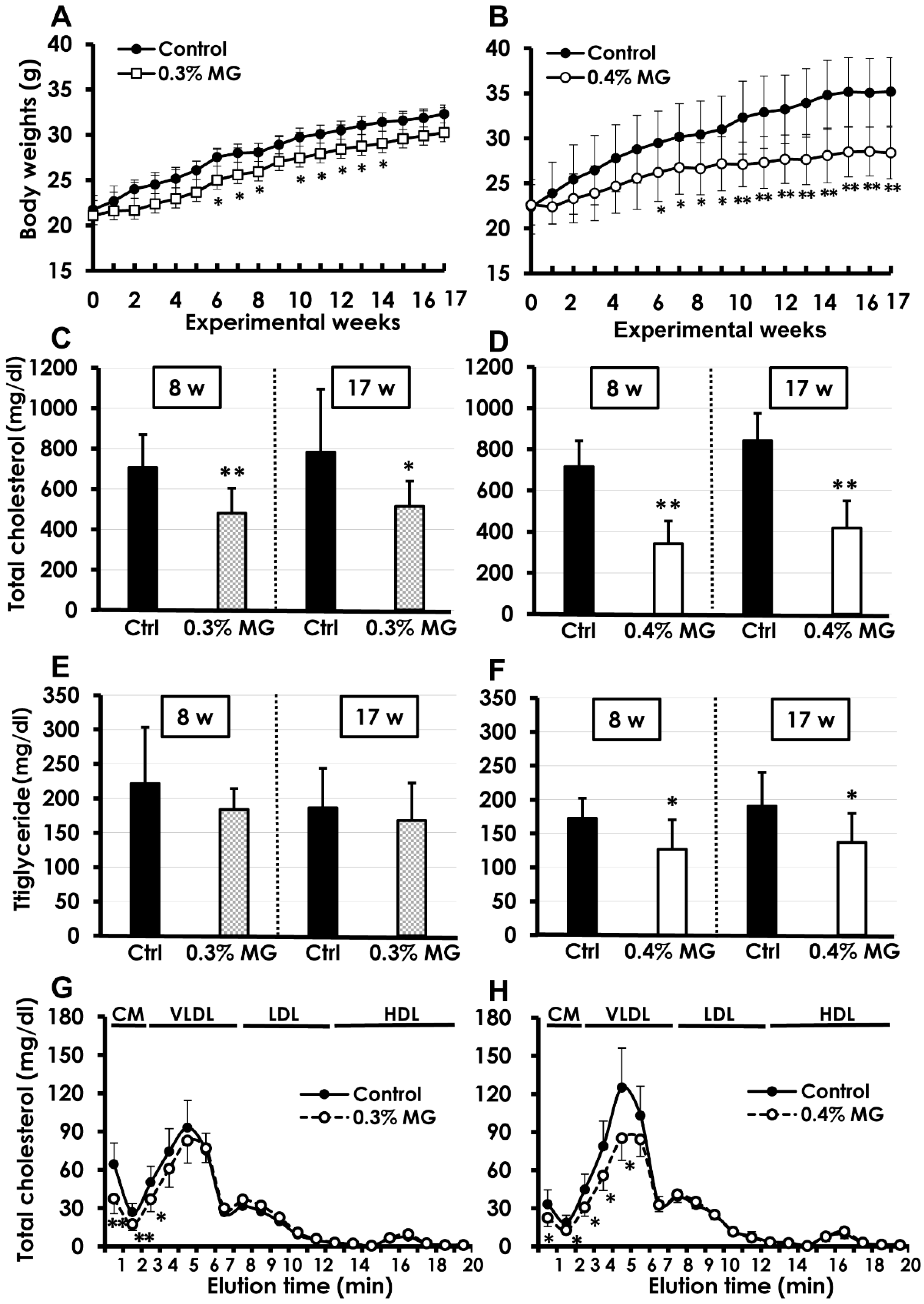
2.1. Survival and Body Weight
2.2. Blood Biochemistry
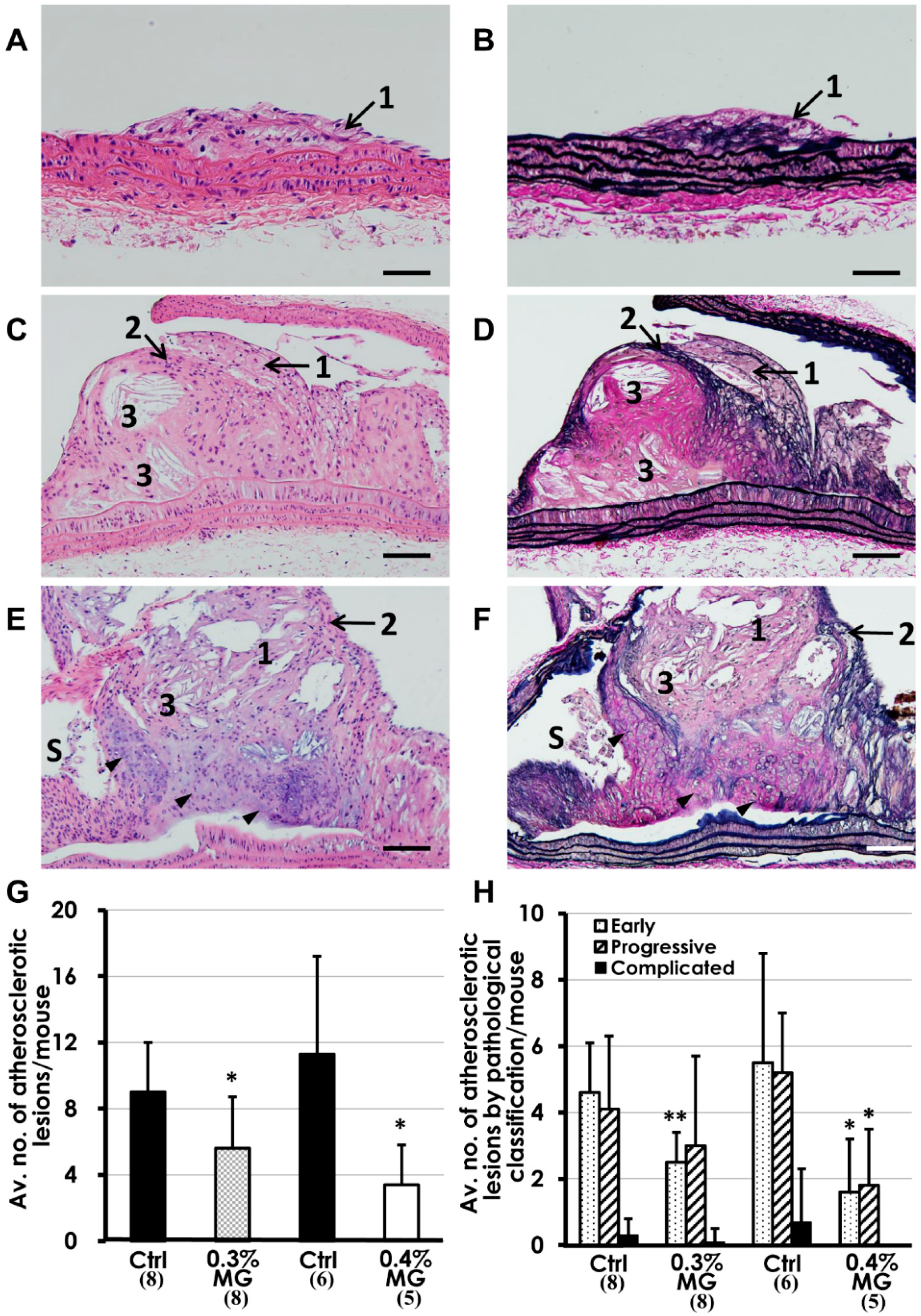
2.3. Oil Red O Staining of Atherosclerotic Lesions
2.4. Histopathological Analysis
2.5. Biochemical and Molecular Analyses
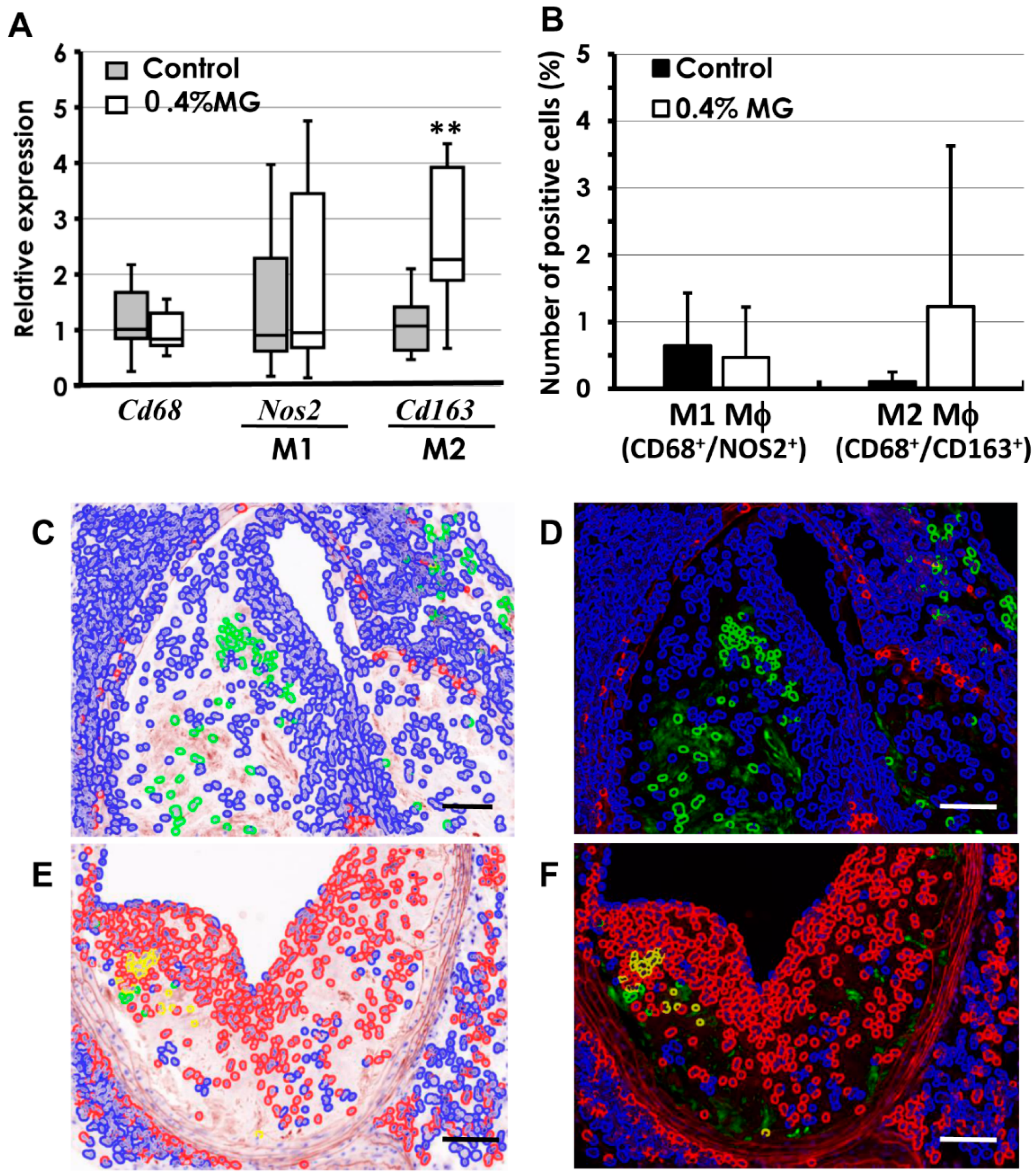
2.6. Quantitative Analysis of Macrophages
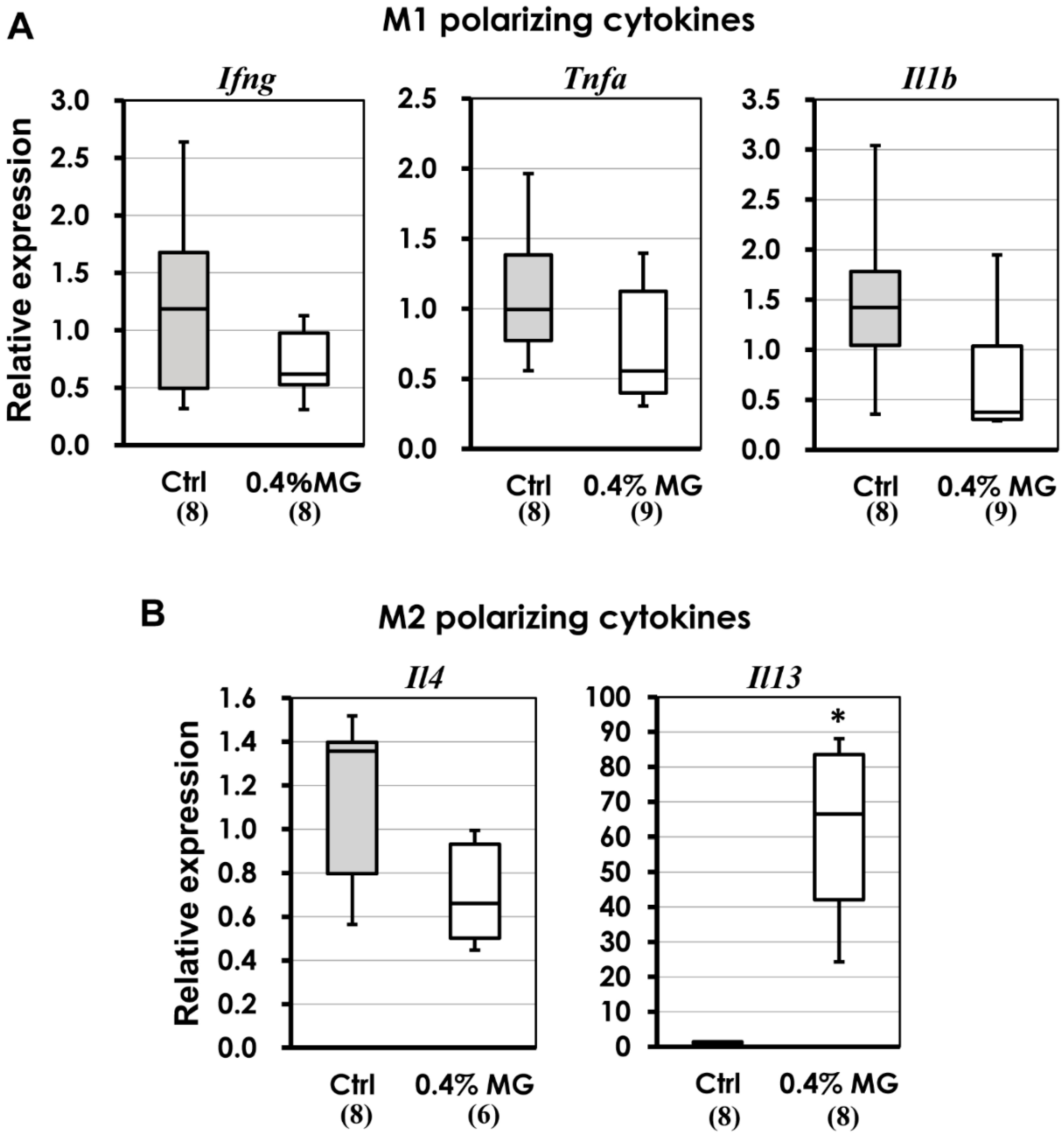
2.7. M1 and M2 Macrophage-Polarizing Cytokines
3. Discussion
4. Materials and Methods
4.1. MG Extraction and Purification
4.2. Animals
4.3. Experimental Design
4.4. Blood Chemistry and Oil Red O Staining (Experiments 1 and 2)
4.5. Histopathological Evaluation of Atherosclerotic Lesions (Experiments 1 and 2)
4.6. Cholesterol Lipoprotein Profiles, Blood Chemistry for LPL and sLOX-1, and Real-Time PCR Analysis of FFPE Tissues (Experiment 3)
4.7. Quantitative Immunofluorescence Analysis (Experiment 3)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | alanine aminotransferase |
| Apoe−/− | Apoe-deficient |
| AST | aspartate aminotransferase |
| BUN | blood urea nitrogen |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMSO | dimethyl sulfoxide |
| Fasn | Fatty acid synthase |
| Fatp | Fatty acid transporter |
| FFPE | formalin-fixed paraffin-embedded |
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase |
| H&E | hematoxylin/eosin |
| HDL | high-density lipoprotein |
| HMG-CoA synthase | 3-hydroxy-3-methylglutaryl-Coenzyme A synthase |
| HMG-CoA reductase | 3-hydroxy-3-methylglutaryl-Coenzyme A reductase |
| LDL | low-density lipoprotein |
| Ldlr−/− | low-density lipoprotein receptor-deficient |
| Ifng | Interferon-γ |
| Il1b | Interleukin-1β |
| Il4 | Interleukin-4 |
| Il13 | Interleukin-13 |
| LPL | lipoprotein lipase |
| LSAB | labeled streptavidin-biotin |
| MG | mangostin |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| Op | Osteopetrotic |
| PGE2 | prostaglandin E2 |
| sLOX-1 | soluble lectin-like oxidized LDL receptor-1 |
| Tnfa | Tumor necrosis factor-α |
| VLDL | very low-density lipoprotein |
References
- World Health Organization. Cancer in Fact Sheets. Fact Sheets Updated September 2018. Available online: http://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 18 February 2019).
- White, C.J. Carotid artery stenting. J. Am. Coll. Cardiol. 2014, 64, 722–731. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Iinuma, M.; Nozawa, Y. Anti-cancer effects of xanthones from pericarps of mangosteen. Int. J. Mol. Sci. 2008, 9, 355–370. [Google Scholar] [CrossRef]
- Shibata, M.A.; Matoba, Y.; Tosa, H.; Iinuma, M. Effects of mangosteen pericarp extracts against mammary cancer. Altern. Integr. Med. 2013, 2, e8. [Google Scholar]
- Matsumoto, K.; Akao, Y.; Kobayashi, E.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Induction of apoptosis by xanthones from mangosteen in human leukemia cell lines. J. Nat. Prod. 2003, 66, 1124–1127. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Akao, Y.; Ohguchi, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorg. Med. Chem. 2005, 13, 6064–6069. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Shibata, M.A.; Shibata, E.; Morimoto, J.; Akao, Y.; Iinuma, M.; Tanigawa, N.; Otsuki, Y. Panaxanthone isolated from pericarp of Garcinia mangostana L. suppresses tumor growth and metastasis of a mouse model of mammary cancer. Anticancer Res. 2009, 29, 2485–2495. [Google Scholar] [PubMed]
- Shibata, M.A.; Iinuma, M.; Morimoto, J.; Kurose, H.; Akamatsu, K.; Okuno, Y.; Akao, Y.; Otsuki, Y. α-Mangostin extracted from the pericarp of the mangosteen (Garcinia mangostana Linn) reduces tumor growth and lymph node metastasis in an immunocompetent model of metastatic mammary cancer carrying a p53 mutation. BMC Med. 2011, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.A.; Hamaoka, H.; Morimoto, J.; Kanayama, T.; Maemura, K.; Ito, Y.; Iinuma, M.; Kondo, Y. Synthetic α-mangostin dilaurate strongly suppresses wide-spectrum organ metastasis in a mouse model of mammary cancer. Cancer Sci. 2018, 109, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Nabandith, V.; Suzui, M.; Morioka, T.; Kaneshiro, T.; Kinjo, T.; Matsumoto, K.; Akao, Y.; Iinuma, M.; Yoshimi, N. Inhibitory effects of crude α -mangostin, a xanthone derivative, on two different categories of colon preneoplastic lesions induced by 1, 2-dimethylhydrazine in the rat. Asian Pac. J. Cancer Prev. 2004, 5, 433–438. [Google Scholar] [PubMed]
- Aisha, A.F.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complement. Altern. Med. 2012, 12, e104. [Google Scholar]
- Johnson, J.J.; Petiwala, S.M.; Syed, D.N.; Rasmussen, J.T.; Adhami, V.M.; Siddiqui, I.A.; Kohl, A.M.; Mukhtar, H. α-Mangostin, a xanthone from mangosteen fruit, promotes cell cycle arrest in prostate cancer and decreases xenograft tumor growth. Carcinogenesis 2012, 33, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Mahabusarakam, W.; Proudfoot, J.; Taylor, W.; Croft, K. Inhibition of lipoprotein oxidation by prenylated xanthones derived from mangostin. Free Radic. Res. 2000, 33, 643–659. [Google Scholar] [CrossRef]
- Sampath, P.D.; Vijayaragavan, K. Ameliorative prospective of α-mangostin, a xanthone derivative from Garcinia mangostana against β-adrenergic cathecolamine-induced myocardial toxicity and anomalous cardiac TNF-α and COX-2 expressions in rats. Exp. Toxicol. Pathol. 2008, 60, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.; Chen, R.; Wu, D.; Cai, N.; Shi, X.; Li, B.; Pan, J. α-Mangostin suppresses interleukin-1β-induced apoptosis in rat chondrocytes by inhibiting the NF-κB signaling pathway and delays the progression of osteoarthritis in a rat model. Int. Immunopharmacol. 2017, 52, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Yamakuni, T.; Kondo, N.; Arakawa, T.; Oosawa, K.; Shimura, S.; Inoue, H.; Ohizumi, Y. γ-Mangostin inhibits inhibitor-κB kinase activity and decreases lipopolysaccharide-induced cyclooxygenase-2 gene expression in C6 rat glioma cells. Mol. Pharmacol. 2004, 66, 667–674. [Google Scholar] [CrossRef]
- Chen, L.G.; Yang, L.L.; Wang, C.C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.A.; Shibata, E.; Maemura, K.; Kondo, Y.; Harada-Shiba, M. Pathological and molecular analyses of atherosclerotic lesions in ApoE-knockout mice. Med. Mol. Morphol. 2017, 50, 130–144. [Google Scholar] [CrossRef]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. LOX-1-Mediated Effects on Vascular Cells in Atherosclerosis. Cell Physiol. Biochem. 2016, 38, 1851–1859. [Google Scholar] [CrossRef]
- Pantano, F.; Berti, P.; Guida, F.M.; Perrone, G.; Vincenzi, B.; Amato, M.M.; Righi, D.; Dell’aquila, E.; Graziano, F.; Catalano, V.; et al. The role of macrophages polarization in predicting prognosis of radically resected gastric cancer patients. J. Cell Mol. Med. 2013, 17, 1415–1421. [Google Scholar] [CrossRef]
- van Dijk, R.A.; Rijs, K.; Wezel, A.; Hamming, J.F.; Kolodgie, F.D.; Virmani, R.; Schaapherder, A.F.; Lindeman, J.H. Systematic evaluation of the cellular innate immune response during the process of human atherosclerosis. J. Am. Heart Assoc. 2016, 5, e002860. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics—2015 Update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef]
- Veniant, M.M.; Withycombe, S.; Young, S.G. Lipoprotein size and atherosclerosis susceptibility in Apoe(−/−) and Ldlr(−/−) mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1567–1570. [Google Scholar] [CrossRef]
- Grundy, S. Risk Assessment and Guidelines for the Management of High Blood Cholesterol. In Endotext (Endocrinology Book); De Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershman, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText. com, Inc.: South Dartmouth MA, USA, 2000. [Google Scholar]
- Peng, J.; Luo, F.; Ruan, G.; Peng, R.; Li, X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017, 16, e233. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Mabuchi, H. Lipoprotein lipase and atherosclerosis. Ann. Clin. Biochem. 2015, 52, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, K.; Inoue, Y.; Shima, A.; Iwasaki, K.; Kawamura, M.; Murase, T. The novel compound NO-1886 increases lipoprotein lipase activity with resulting elevation of high density lipoprotein cholesterol, and long-term administration inhibits atherogenesis in the coronary arteries of rats with experimental atherosclerosis. J. Clin. Investig. 1993, 92, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Yagyu, H.; Ishibashi, S.; Chen, Z.; Osuga, J.; Okazaki, M.; Perrey, S.; Kitamine, T.; Shimada, M.; Ohashi, K.; Harada, K.; et al. Overexpressed lipoprotein lipase protects against atherosclerosis in apolipoprotein E knockout mice. J. Lipid Res. 1999, 40, 1677–1685. [Google Scholar] [PubMed]
- Shimada, M.; Ishibashi, S.; Inaba, T.; Yagyu, H.; Harada, K.; Osuga, J.I.; Ohashi, K.; Yazaki, Y.; Yamada, N. Suppression of diet-induced atherosclerosis in low density lipoprotein receptor knockout mice overexpressing lipoprotein lipase. Proc. Natl. Acad. Sci. USA 1996, 93, 7242–7246. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Unoki, H.; Kojima, N.; Sun, H.; Shimoyamada, H.; Deng, H.; Okazaki, M.; Shikama, H.; Yamada, N.; Watanabe, T. Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 2001, 276, 40071–40079. [Google Scholar] [CrossRef] [PubMed]
- Babaev, V.R.; Patel, M.B.; Semenkovich, C.F.; Fazio, S.; Linton, M.F. Macrophage lipoprotein lipase promotes foam cell formation and atherosclerosis in low density lipoprotein receptor-deficient mice. J. Biol. Chem. 2000, 275, 26293–26299. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.P.; Gong, D.; Zhao, Z.W.; He, P.P.; Yu, X.H.; Ye, Q.; Huang, C.; Zhang, X.; Chen, L.Y.; Xie, W.; et al. MicroRNA-182 promotes lipoprotein lipase expression and atherogenesis by targeting histone deacetylase 9 in apolipoprotein E-knockout mice. Circ. J. 2017, 25, 244–253. [Google Scholar]
- Khuchua, Z.; Glukhov, A.I.; Strauss, A.W.; Javadov, S. Elucidating the beneficial role of PPAR agonists in cardiac diseases. Int. J. Mol. Sci. 2018, 19, 3464. [Google Scholar] [CrossRef]
- Matsuura, N.; Gamo, K.; Miyachi, H.; Iinuma, M.; Kawada, T.; Takahashi, N.; Akao, Y.; Tosa, H. γ-Mangostin from Garcinia mangostana pericarps as a dual agonist that activates Both PPARα and PPARδ. Biosci. Biotechnol. Biochem. 2013, 77, 2430–2435. [Google Scholar] [CrossRef]
- Choi, Y.H.; Bae, J.K.; Chae, H.S.; Kim, Y.M.; Sreymom, Y.; Han, L.; Jang, H.Y.; Chin, Y.W. α-Mangostin regulates hepatic steatosis and obesity through SirT1-AMPK and PPARγ pathways in high-fat diet-Induced obese mice. J. Agric. Food Chem. 2015, 63, 8399–8406. [Google Scholar] [CrossRef] [PubMed]
- Kume, N.; Kita, T. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in atherogenesis. Trends Cardiovasc. Med. 2001, 11, 22–25. [Google Scholar] [CrossRef]
- Kataoka, H.; Kume, N.; Miyamoto, S.; Minami, M.; Moriwaki, H.; Murase, T.; Sawamura, T.; Masaki, T.; Hashimoto, N.; Kita, T. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation 1999, 99, 3110–3117. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Arai, Y.; Kurihara, H.; Kita, T.; Sawamura, T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ. Res. 2005, 97, 176–184. [Google Scholar] [CrossRef]
- Mehta, J.L.; Sanada, N.; Hu, C.P.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.; et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef]
- Smith, J.D.; Trogan, E.; Ginsberg, M.; Grigaux, C.; Tian, J.; Miyata, M. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl. Acad. Sci. USA 1995, 92, 8264–8268. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, R.; Liu, D.; Wu, C.; Guo, P.; Lin, W. Asperlin Inhibits LPS-Evoked Foam Cell Formation and Prevents Atherosclerosis in ApoE(−/−) Mice. Mar. Drugs 2017, 15, 358. [Google Scholar] [CrossRef] [PubMed]
- Tarasuk, M.; Songprakhon, P.; Chimma, P.; Sratongno, P.; Na-Bangchang, K.; Yenchitsomanus, P.T. α-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 2017, 240, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-Aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Anti-inflammatory effect of Garcinia mangostana Linn. pericarp extract in methicillin-resistant Staphylococcus aureus-induced superficial skin infection in mice. Biomed. Pharmacother. 2019, 111, 705–713. [Google Scholar] [CrossRef]
- Tao, M.; Jiang, J.; Wang, L.; Li, Y.; Mao, Q.; Dong, J.; Zuo, J. α-Mangostin alleviated lipopolysaccharide Induced acute lung Injury in rats by suppressing NAMPT/NAD controlled inflammatory reactions. Evid. Based Complement. Alternat. Med. 2018, 2018, e5470187. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Syam, S.; Abdelwahab, S.I.; Thangavel, N. An anti-inflammatory molecular mechanism of action of α-mangostin, the major xanthone from the pericarp of Garcinia mangostana: An in silico, in vitro and in vivo approach. Food Funct. 2018, 9, 3860–3871. [Google Scholar] [CrossRef]
- Zuo, J.; Yin, Q.; Wang, Y.W.; Li, Y.; Lu, L.M.; Xiao, Z.G.; Wang, G.D.; Luan, J.J. Inhibition of NF-κB pathway in fibroblast-like synoviocytes by α-mangostin implicated in protective effects on joints in rats suffering from adjuvant-induced arthritis. Int. Immunopharmacol. 2018, 56, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Lee, L.T.; Hu, N.Y.; Huange, K.K.; Shih, Y.C.; Munekazu, I.; Li, J.M.; Chou, T.Y.; Wang, W.H.; Chen, T.S. Effects of α-mangostin on the expression of anti-inflammatory genes in U937 cells. Chin. Med. 2012, 7, e19. [Google Scholar] [CrossRef] [PubMed]
- Stachowicz, A.; Olszanecki, R.; Suski, M.; Wisniewska, A.; Toton-Zuranska, J.; Madej, J.; Jawien, J.; Bialas, M.; Okon, K.; Gajda, M.; et al. Mitochondrial aldehyde dehydrogenase activation by α-1 inhibits atherosclerosis and attenuates hepatic steatosis in apolipoprotein E-knockout mice. J. Am. Heart Assoc. 2014, 3, e001329. [Google Scholar] [CrossRef]
- LaBrecque, D.R.; Abbas, Z.; Anania, F.; Ferenci, P.; Khan, A.G.; Goh, K.L.; Hamid, S.S.; Isakov, V.; Lizarzabal, M.; Penaranda, M.M.; et al. World gastroenterology organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2014, 48, 467–473. [Google Scholar] [CrossRef]
- Lavine, J.E.; Schwimmer, J.B. Nonalcoholic fatty liver disease in the pediatric population. Clin. Liver Dis. 2004, 8, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Horton, J.D.; Hobbs, H.H. Human fatty liver disease: Old questions and new insights. Science 2011, 332, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Targher, G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: The plot thickens. Diabet. Med. 2007, 24, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, R.W.; Holloway, G.P.; Luiken, J.J.; Bonen, A.; Glatz, J.F. Fatty acid transport across the cell membrane: Regulation by fatty acid transporters. Prostaglandins Leukot. Essent. Fatty Acids 2010, 82, 149–154. [Google Scholar] [CrossRef]
- Quan, X.; Wang, Y.; Ma, X.; Liang, Y.; Tian, W.; Ma, Q.; Jiang, H.; Zhao, Y. α-Mangostin induces apoptosis and suppresses differentiation of 3T3-L1 cells via inhibiting fatty acid synthase. PLoS ONE 2012, 7, e33376. [Google Scholar] [CrossRef]
- Piedrahita, J.A.; Zhang, S.H.; Hagaman, J.R.; Oliver, P.M.; Maeda, N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc. Natl. Acad. Sci. USA 1992, 89, 4471–4475. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, Y.M.; Huh, J.H.; Lee, E.S.; Kwon, M.H.; Lee, B.R.; Ko, H.J.; Chung, C.H. α-Mangostin ameliorates hepatic steatosis and insulin resistance by inhibition C-C chemokine receptor 2. PLoS ONE 2017, 12, e0179204. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, K.; Kume, N.; Murase, T.; Minami, M.; Nakagawa, D.; Inada, T.; Tanaka, M.; Ueda, A.; Kominami, G.; Kambara, H.; et al. Serum soluble lectin-like oxidized low-density lipoprotein receptor-1 levels are elevated in acute coronary syndrome: A novel marker for early diagnosis. Circulation 2005, 112, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer Sequence (5′-->3′) | Reverse Primer Sequence (5′-->3′) |
|---|---|---|
| Timp1 | CAGAACCGCAGTGAAGAGT | CAAGGGATAGATAAACAGGGAAAC |
| Mmp9 | ATTCGCGTGGATAAGGAGTTC | GGCAGAAATAGGCTTTGTCTTG |
| Lox1 | CTCTGGCATAAAGAAAACTGTTACC | GCTTCTTCCGATGCAATCC |
| Cxcl16 | CCGCAGGGTACTTTGGATCAC | GGACTGCAACTGGAACCTGA |
| Klf5 | CTGTCAGATACAACAGAAGGAGT | GTGAGCTTTTAAGTGAGACGAC |
| Cd68 | GTCTCTCTCATTTCCTTATGGACAG | GCTCTGATGTAGGTCCTGTTTG |
| Nos2 | GGGCAGTGGAGAGATTTTG | TGCAAGTGAAATCCGATGTG |
| Cd163 | AGTCCTGGATCATCTGTGACAAC | ACACGTCCAGAACAGTCTGTATG |
| Infg | CATGAGTATTGCCAAGTTTGAGGTC | ATTGAATGCTTGGCGCTGGA |
| Tnfa | CACGCTCTTCTGTCTACTGAACT | GAGGCCATTTGGGAACTTCTCAT |
| Il1b | GAGAATGACCTGTTCTTTGAAGTTG | GTTTGGAAGCAGCCCTTCAT |
| Il4 | GATGCCTGGATTCATCGATAAG | GTCTTTCAGTGATGTGGACTTG |
| Il13 | GCTGAGCAACATCACACAAG | CATACCATGCTGCCGTTG |
| Gapdh | TGGCCTTCCGTGTTCCTACC | AGCCCAAGATGCCCTTCAGT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibata, M.-A.; Harada-Shiba, M.; Shibata, E.; Tosa, H.; Matoba, Y.; Hamaoka, H.; Iinuma, M.; Kondo, Y. Crude α-Mangostin Suppresses the Development of Atherosclerotic Lesions in Apoe-Deficient Mice by a Possible M2 Macrophage-Mediated Mechanism. Int. J. Mol. Sci. 2019, 20, 1722. https://doi.org/10.3390/ijms20071722
Shibata M-A, Harada-Shiba M, Shibata E, Tosa H, Matoba Y, Hamaoka H, Iinuma M, Kondo Y. Crude α-Mangostin Suppresses the Development of Atherosclerotic Lesions in Apoe-Deficient Mice by a Possible M2 Macrophage-Mediated Mechanism. International Journal of Molecular Sciences. 2019; 20(7):1722. https://doi.org/10.3390/ijms20071722
Chicago/Turabian StyleShibata, Masa-Aki, Mariko Harada-Shiba, Eiko Shibata, Hideki Tosa, Yoshinobu Matoba, Hitomi Hamaoka, Munekazu Iinuma, and Yoichi Kondo. 2019. "Crude α-Mangostin Suppresses the Development of Atherosclerotic Lesions in Apoe-Deficient Mice by a Possible M2 Macrophage-Mediated Mechanism" International Journal of Molecular Sciences 20, no. 7: 1722. https://doi.org/10.3390/ijms20071722
APA StyleShibata, M.-A., Harada-Shiba, M., Shibata, E., Tosa, H., Matoba, Y., Hamaoka, H., Iinuma, M., & Kondo, Y. (2019). Crude α-Mangostin Suppresses the Development of Atherosclerotic Lesions in Apoe-Deficient Mice by a Possible M2 Macrophage-Mediated Mechanism. International Journal of Molecular Sciences, 20(7), 1722. https://doi.org/10.3390/ijms20071722