Cross-Talk between Neurohormonal Pathways and the Immune System in Heart Failure: A Review of the Literature
Abstract
1. Introduction
2. Mechanisms of Immune-Modulation in HFrEF
2.1. Renin-Angiotensin Aldosterone System (RAAS)
2.2. Nervous Sympathetic System
2.2.1. Adrenergic Receptors (AR)
2.2.2. Effects of AR Activation on the Innate Immune System
2.3. Natriuretic Peptides System and Immunomodulation
3. Mechanism of Immune Modulation in Heart Failure with Preserved Ejection Fraction
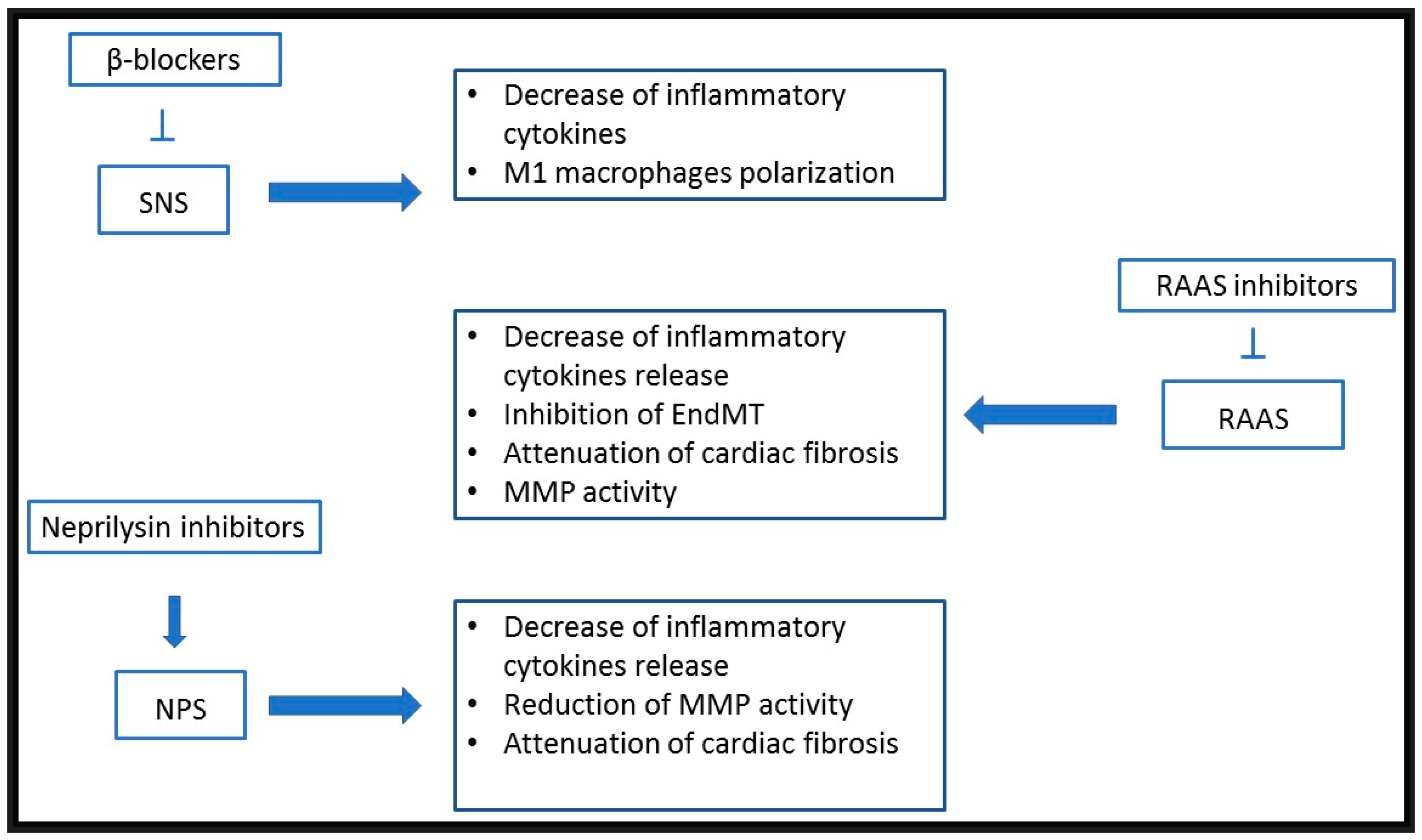
4. Therapeutic Translation
5. Conclusions
Funding
Conflicts of Interest
References
- Braunwald, E. Heart failure. JACC Heart Fail. 2013, 1, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, E.; Prota, C.; Matturro, R.; Citro, R. Amniotic fluid embolism in a grown-up congenital heart disease patient. J. Cardiovasc. Echogr. 2019, 29, 20–22. [Google Scholar] [CrossRef]
- Kraigher-Krainer, E.; Shah, A.M.; Gupta, D.K.; Santos, A.; Claggett, B.; Pieske, B.; Zile, M.R.; Voors, A.A.; Lefkowitz, M.P.; Packer, M.; et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 63, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, S.; Kaye, D.M. Management of heart failure with preserved ejection fraction: A review. Clin. Ther. 2015, 37, 2186–2198. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur. Heart. J. 2018, 39, 2780–2792. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, J.; Sugiyama, S.; Nozaki, T.; Sugamura, K.; Konishi, M.; Ohba, K.; Matsuzawa, Y.; Akiyama, E.; Yamamoto, E.; Sakamoto, K.; et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J. Am. Coll. Cardiol. 2011, 57, 861–869. [Google Scholar] [CrossRef]
- Tobian, L.; Tomboulian, A.; Janecek, J. The effect of high perfusion pressures on the granulation of juxtaglomerular cells in an isolated kidney. J. Clin. Investig. 1959, 38, 605–610. [Google Scholar] [CrossRef]
- Jhund, P.S.; McMurray, J.J. The neprilysin pathway in heart failure: A review and guide on the use of sacubitril/valsartan. Heart 2016, 102, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M. Cardiac remodelling and RAS inhibition. Ther. Adv. Cardiovasc. Dis. 2016, 10, 162–171. [Google Scholar] [CrossRef]
- Schirone, L.; Forte, M.; Palmerio, S.; Yee, D.; Nocella, C.; Angelini, F.; Pagano, F.; Schiavon, S.; Bordin, A.; Carrizzo, A.; et al. A Review of the Molecular Mechanisms Underlying the Development and Progression of Cardiac Remodeling. Oxid. Med. Cell. Longev. 2017, 2017, 3920195. [Google Scholar] [CrossRef] [PubMed]
- Combs, M.D.; Yutzey, K.E. Heart valve development: Regulatory networks in development and disease. Circ. Res. 2009, 105, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Pimentel, M.E.; Runyan, R.B. Multiple transforming growth factor-beta isoforms and receptors function during epithelial-mesenchymal cell transformation in the embryonic heart. Cells Tissues Organs 2007, 185, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lu, M.F.; Schwartz, R.J.; Martin, J.F. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 2005, 132, 5601–5611. [Google Scholar] [CrossRef] [PubMed]
- Garside, V.C.; Chang, A.C.; Karsan, A.; Hoodless, P.A. Co-ordinating Notch, BMP, and TGF-beta signaling during heart valve development. Cell. Mol. Life Sci. 2013, 70, 2899–2917. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Lyu, X.; Wang, Q.; Hu, M.; Zhang, X. Endothelial to mesenchymal transition in the cardiovascular system. Life Sci. 2017, 184, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Aisagbonhi, O.; Rai, M.; Ryzhov, S.; Atria, N.; Feoktistov, I.; Hatzopoulos, A.K. Experimental myocardial infarction triggers canonical Wnt signaling and endothelial-to-mesenchymal transition. Dis. Model. Mech. 2011, 4, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Moonen, J.R.; Lee, E.S.; Schmidt, M.; Maleszewska, M.; Koerts, J.A.; Brouwer, L.A.; van Kooten, T.G.; van Luyn, M.J.; Zeebregts, C.J.; Krenning, G.; et al. Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc. Res. 2015, 108, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; von Gise, A.; Liu, Q.; Hu, T.; Tian, X.; He, L.; Pu, W.; Huang, X.; He, L.; Cai, C.L.; et al. Yap1 is required for endothelial to mesenchymal transition of the atrioventricular cushion. J. Biol. Chem. 2014, 289, 18681–18692. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Peng, Z.; Zu, C.; Ma, J.; Lu, S.; Zhong, J.; Zhang, S. Losartan Attenuates Myocardial Endothelial-To-Mesenchymal Transition in Spontaneous Hypertensive Rats via Inhibiting TGF-beta/Smad Signaling. PLoS ONE 2016, 11, e0155730. [Google Scholar] [CrossRef]
- Crowley, S.D.; Rudemiller, N.P. Immunologic Effects of the Renin-Angiotensin System. J. Am. Soc. Nephrol. 2017, 28, 1350–1361. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R.; Jamaluddin, M.; Han, Y.; Patterson, C.; Runge, M.S. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol. Cell. Biochem. 2000, 212, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Esther, C.R.; Marino, E.M.; Howard, T.E.; Machaud, A.; Corvol, P.; Capecchi, M.R.; Bernstein, K.E. The critical role of tissue angiotensin-converting enzyme as revealed by gene targeting in mice. J. Clin. Investig. 1997, 99, 2375–2385. [Google Scholar] [CrossRef] [PubMed]
- Moura Santos, D.; Ribeiro Marins, F.; Limborco-Filho, M.; de Oliveira, M.L.; Hamamoto, D.; Xavier, C.H.; Moreira, F.A.; Santos, R.A.; Campagnole-Santos, M.J.; Peliky Fontes, M.A. Chronic overexpression of angiotensin-(1-7) in rats reduces cardiac reactivity to acute stress and dampens anxious behavior. Stress 2017, 20, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, S.; Sparkenbaugh, E.; Pawlinski, R. Tissue factor, protease activated receptors and pathologic heart remodelling. Thromb. Haemost. 2014, 112, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Ushio-Fukai, M.; Lassegue, B.; Alexander, R.W. Angiotensin II signaling in vascular smooth muscle. New concepts. Hypertension 1997, 29, 366–373. [Google Scholar] [CrossRef]
- Suzuki, H.; Motley, E.D.; Frank, G.D.; Utsunomiya, H.; Eguchi, S. Recent progress in signal transduction research of the angiotensin II type-1 receptor: Protein kinases, vascular dysfunction and structural requirement. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2005, 3, 305–322. [Google Scholar] [CrossRef]
- Yin, G.; Yan, C.; Berk, B.C. Angiotensin II signaling pathways mediated by tyrosine kinases. Int. J. Biochem. Cell Biol. 2003, 35, 780–783. [Google Scholar] [CrossRef]
- Higuchi, S.; Ohtsu, H.; Suzuki, H.; Shirai, H.; Frank, G.D.; Eguchi, S. Angiotensin II signal transduction through the AT1 receptor: Novel insights into mechanisms and pathophysiology. Clin. Sci. 2007, 112, 417–428. [Google Scholar] [CrossRef]
- Kawai, T.; Forrester, S.J.; O’Brien, S.; Baggett, A.; Rizzo, V.; Eguchi, S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017, 125, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Burchfield, J.S.; Hill, J.A. Pathological ventricular remodeling: Therapies: Part 2 of 2. Circulation 2013, 128, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.; Stegbauer, J.; Linker, R.A. Macrophages in neuroinflammation: Role of the renin-angiotensin-system. Pflugers Arch. 2017, 469, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Li, Y.; Xiao, C.; Du, J. Angiotensin II induces inflammation leading to cardiac remodeling. Front. Biosci. 2012, 17, 221–231. [Google Scholar] [CrossRef]
- Maning, J.; Negussie, S.; Clark, M.A.; Lymperopoulos, A. Biased agonism/antagonism at the AngII-AT1 receptor: Implications for adrenal aldosterone production and cardiovascular therapy. Pharmacol. Res. 2017, 125, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hoch, N.E.; Guzik, T.J.; Chen, W.; Deans, T.; Maalouf, S.A.; Gratze, P.; Weyand, C.; Harrison, D.G. Regulation of T-cell function by endogenously produced angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R208–R216. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, M.; McDermott, D.H.; Sechler, J.M.; Tinckam, K.; Takakura, A.; Carpenter, C.B.; Milford, E.; Abdi, R. Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II-induced inflammation. J. Am. Soc. Nephrol. 2007, 18, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004, 63, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Sriramula, S.; Francis, J. Tumor Necrosis Factor—Alpha Is Essential for Angiotensin II-Induced Ventricular Remodeling: Role for Oxidative Stress. PLoS ONE 2015, 10, e0138372. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Guan, J. Antifibrotic therapies to control cardiac fibrosis. Biomater. Res. 2016, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Gurantz, D.; Cowling, R.T.; Varki, N.; Frikovsky, E.; Moore, C.D.; Greenberg, B.H. IL-1beta and TNF-alpha upregulate angiotensin II type 1 (AT1) receptors on cardiac fibroblasts and are associated with increased AT1 density in the post-MI heart. J. Mol. Cell. Cardiol. 2005, 38, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Nangaku, M.; Miyata, T.; Inagi, R.; Yamada, K.; Kurokawa, K.; Fujita, T. Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 2003, 42, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, M.; Santulli, G.; Pascale, V.; Trimarco, B.; Iaccarino, G. Adrenergic receptors and metabolism: Role in development of cardiovascular disease. Front. Physiol. 2013, 4, 265. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Naga Prasad, S.V.; Lefkowitz, R.J.; Koch, W.J.; Rockman, H.A. Level of beta-adrenergic receptor kinase 1 inhibition determines degree of cardiac dysfunction after chronic pressure overload-induced heart failure. Circulation 2005, 111, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Michelotti, G.A.; Price, D.T.; Schwinn, D.A. Alpha 1-adrenergic receptor regulation: Basic science and clinical implications. Pharmacol. Ther. 2000, 88, 281–309. [Google Scholar] [CrossRef]
- Capote, L.A.; Mendez Perez, R.; Lymperopoulos, A. GPCR signaling and cardiac function. Eur. J. Pharmacol. 2015, 763, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, R.J.; Shenoy, S.K. Transduction of receptor signals by beta-arrestins. Science 2005, 308, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Lymperopoulos, A. Arrestins in the Cardiovascular System: An Update. Prog. Mol. Biol. Transl. Sci. 2018, 159, 27–57. [Google Scholar] [CrossRef] [PubMed]
- Desimine, V.L.; McCrink, K.A.; Parker, B.M.; Wertz, S.L.; Maning, J.; Lymperopoulos, A. Biased Agonism/Antagonism of Cardiovascular GPCRs for Heart Failure Therapy. Int. Rev. Cell Mol. Biol. 2018, 339, 41–61. [Google Scholar] [CrossRef]
- Nobles, K.N.; Xiao, K.; Ahn, S.; Shukla, A.K.; Lam, C.M.; Rajagopal, S.; Strachan, R.T.; Huang, T.Y.; Bressler, E.A.; Hara, M.R.; et al. Distinct phosphorylation sites on the beta(2)-adrenergic receptor establish a barcode that encodes differential functions of beta-arrestin. Sci. Signal. 2011, 4, ra51. [Google Scholar] [CrossRef]
- Franco, A.; Sorriento, D.; Gambardella, J.; Pacelli, R.; Prevete, N.; Procaccini, C.; Matarese, G.; Trimarco, B.; Iaccarino, G.; Ciccarelli, M. GRK2 moderates the acute mitochondrial damage to ionizing radiation exposure by promoting mitochondrial fission/fusion. Cell Death Discov. 2018, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Krupnick, J.G.; Benovic, J.L. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 289–319. [Google Scholar] [CrossRef] [PubMed]
- Siryk-Bathgate, A.; Dabul, S.; Lymperopoulos, A. Current and future G protein-coupled receptor signaling targets for heart failure therapy. Drug Des. Dev. Ther. 2013, 7, 1209–1222. [Google Scholar] [CrossRef]
- Baillie, G.S.; Sood, A.; McPhee, I.; Gall, I.; Perry, S.J.; Lefkowitz, R.J.; Houslay, M.D. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc. Natl. Acad. Sci. USA 2003, 100, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Lorton, D.; Bellinger, D.L.; Schaller, J.A.; Shewmaker, E.; Osredkar, T.; Lubahn, C. Altered sympathetic-to-immune cell signaling via beta(2)-adrenergic receptors in adjuvant arthritis. Clin. Dev. Immunol. 2013, 2013, 764395. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, C.L.; Lorton, D.; Schaller, J.A.; Sweeney, S.J.; Bellinger, D.L. Targeting alpha- and beta-Adrenergic Receptors Differentially Shifts Th1, Th2, and Inflammatory Cytokine Profiles in Immune Organs to Attenuate Adjuvant Arthritis. Front. Immunol. 2014, 5, 346. [Google Scholar] [CrossRef] [PubMed]
- Badou, A.; Savignac, M.; Moreau, M.; Leclerc, C.; Foucras, G.; Cassar, G.; Paulet, P.; Lagrange, D.; Druet, P.; Guery, J.C.; et al. Weak TCR stimulation induces a calcium signal that triggers IL-4 synthesis, stronger TCR stimulation induces MAP kinases that control IFN-gamma production. Eur. J. Immunol. 2001, 31, 2487–2496. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, J.; Xin, J.; Feng, Y.; Hu, G.; Shen, J.; Li, M.; Zhang, Y.; Xiao, H.; Wang, L. beta3-adrenergic receptor activation induces TGFbeta1 expression in cardiomyocytes via the PKG/JNK/c-Jun pathway. Biochem. Biophys. Res. Commun. 2018, 503, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bylund, D.B.; Eikenberg, D.C.; Hieble, J.P.; Langer, S.Z.; Lefkowitz, R.J.; Minneman, K.P.; Molinoff, P.B.; Ruffolo, R.R., Jr.; Trendelenburg, U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994, 46, 121–136. [Google Scholar] [PubMed]
- Monaco, S.; Rusciano, M.R.; Maione, A.S.; Soprano, M.; Gomathinayagam, R.; Todd, L.R.; Campiglia, P.; Salzano, S.; Pastore, L.; Leggiero, E.; et al. A novel cross-talk between calcium/calmodulin kinases II and IV regulates cell proliferation in myeloid leukemia cells. Cell. Signal. 2015, 27, 204–214. [Google Scholar] [CrossRef]
- Maione, A.S.; Cipolletta, E.; Sorriento, D.; Borriello, F.; Soprano, M.; Rusciano, M.R.; D’Esposito, V.; Markabaoui, A.K.; De Palma, G.D.; Martino, G.; et al. Cellular subtype expression and activation of CaMKII regulate the fate of atherosclerotic plaque. Atherosclerosis 2017, 256, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Faure, C.; Gouhier, C.; Langer, S.Z.; Graham, D. Quantification of α1-Adrenoceptor Subtypes in Human Tissues by Competitive RT-PCR Analysis. Biochem. Biophys. Res. Commun. 1995, 213, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Price, D.T.; Lefkowitz, R.J.; Caron, M.G.; Berkowitz, D.; Schwinn, D.A. Localization of mRNA for three distinct alpha 1-adrenergic receptor subtypes in human tissues: Implications for human alpha-adrenergic physiology. Mol. Pharmacol. 1994, 45, 171–175. [Google Scholar] [PubMed]
- Kavelaars, A. Regulated expression of alpha-1 adrenergic receptors in the immune system. Brain Behav. Immun. 2002, 16, 799–807. [Google Scholar] [CrossRef]
- Rouppe van der Voort, C.; Kavelaars, A.; van de Pol, M.; Heijnen, C.J. Noradrenaline induces phosphorylation of ERK-2 in human peripheral blood mononuclear cells after induction of alpha(1)-adrenergic receptors. J. Neuroimmunol. 2000, 108, 82–91. [Google Scholar] [CrossRef]
- Maestroni, G.J. Dendritic cell migration controlled by alpha 1b-adrenergic receptors. J. Immunol. 2000, 165, 6743–6747. [Google Scholar] [CrossRef] [PubMed]
- Nahrendorf, M.; Swirski, F.K. Monocyte and macrophage heterogeneity in the heart. Circ. Res. 2013, 112, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Ratge, D.; Wiedemann, A.; Kohse, K.P.; Wisser, H. Alterations of beta-adrenoceptors on human leukocyte subsets induced by dynamic exercise: Effect of prednisone. Clin. Exp. Pharmacol. Physiol. 1988, 15, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Kraemer, W.J.; Mastro, A.M.; Denegar, C.R.; Volek, J.S.; Hakkinen, K.; Anderson, J.M.; Lee, E.C.; Maresh, C.M. Leukocyte beta2-adrenergic receptor expression in response to resistance exercise. Med. Sci. Sports Exerc. 2011, 43, 1422–1432. [Google Scholar] [CrossRef] [PubMed]
- Schopf, R.E.; Lemmel, E.M. Control of the production of oxygen intermediates of human polymorphonuclear leukocytes and monocytes by beta-adrenergic receptors. J. Immunopharmacol. 1983, 5, 203–216. [Google Scholar] [CrossRef]
- Guirao, X.; Kumar, A.; Katz, J.; Smith, M.; Lin, E.; Keogh, C.; Calvano, S.E.; Lowry, S.F. Catecholamines increase monocyte TNF receptors and inhibit TNF through beta 2-adrenoreceptor activation. Am. J. Physiol. 1997, 273, E1203–E1208. [Google Scholar] [PubMed]
- Kobayashi, M.; Jeschke, M.G.; Asai, A.; Kogiso, M.; Yoshida, S.; Herndon, D.N.; Suzuki, F. Propranolol as a modulator of M2b monocytes in severely burned patients. J. Leukoc. Biol. 2011, 89, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Spengler, R.N.; Allen, R.M.; Remick, D.G.; Strieter, R.M.; Kunkel, S.L. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J. Immunol. 1990, 145, 1430–1434. [Google Scholar] [PubMed]
- Engler, H.; Engler, A.; Bailey, M.T.; Sheridan, J.F. Tissue-specific alterations in the glucocorticoid sensitivity of immune cells following repeated social defeat in mice. J. Neuroimmunol. 2005, 163, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Rittirsch, D.; Nadeau, B.A.; Chen, A.J.; Sarma, J.V.; Zetoune, F.S.; McGuire, S.R.; List, R.P.; Day, D.E.; Hoesel, L.M.; et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007, 449, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Sharma, R. The syndrome of cardiac cachexia. Int. J. Cardiol. 2002, 85, 51–66. [Google Scholar] [CrossRef]
- Adamopoulos, S.; Parissis, J.T.; Kremastinos, D.T. A glossary of circulating cytokines in chronic heart failure. Eur. J. Heart Fail. 2001, 3, 517–526. [Google Scholar] [CrossRef]
- Sun, M.; Dawood, F.; Wen, W.H.; Chen, M.; Dixon, I.; Kirshenbaum, L.A.; Liu, P.P. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation 2004, 110, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; McTiernan, C.F.; Frye, C.S.; Slawson, S.E.; Lemster, B.H.; Koretsky, A.P.; Demetris, A.J.; Feldman, A.M. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ. Res. 1997, 81, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Kribbs, S.B.; Clubb, F.J., Jr.; Michael, L.H.; Didenko, V.V.; Hornsby, P.J.; Seta, Y.; Oral, H.; Spinale, F.G.; Mann, D.L. Pathophysiologically relevant concentrations of tumor necrosis factor-alpha promote progressive left ventricular dysfunction and remodeling in rats. Circulation 1998, 97, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Valen, G.; Yan, Z.Q.; Hansson, G.K. Nuclear factor kappa-B and the heart. J. Am. Coll. Cardiol. 2001, 38, 307–314. [Google Scholar] [CrossRef]
- Mellman, I.; Steinman, R.M. Dendritic cells: Specialized and regulated antigen processing machines. Cell 2001, 106, 255–258. [Google Scholar] [CrossRef]
- Panina-Bordignon, P.; Mazzeo, D.; Lucia, P.D.; D’Ambrosio, D.; Lang, R.; Fabbri, L.; Self, C.; Sinigaglia, F. Beta2-agonists prevent Th1 development by selective inhibition of interleukin 12. J. Clin. Investig. 1997, 100, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Du, R.Z.; Qiu, J.P.; Tang, Y.Q.; Chen, S.C. Bisoprolol reverses epinephrine-mediated inhibition of cell emigration through increases in the expression of beta-arrestin 2 and CCR7 and PI3K phosphorylation, in dendritic cells loaded with cholesterol. Thromb. Res. 2013, 131, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Wiedermann, F.J.; Herold, M.; Tilg, H.; Moes, N.; Muhlberger, V.; Beimpold, H.; Knapp, E. Plasma levels of atrial natriuretic peptide and tumor necrosis factor-alpha during transient myocardial ischemia in patients with stable angina. Klin. Wochenschr. 1991, 69, 944. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J.; Niedermühlbichler, M.; Braunsteiner, H.; Widermann, C.J. Priming of polymorphonuclear neutrophils by atrial natriuretic peptide in vitro. J. Clin. Investig. 1992, 89, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Vollmar, A.M.; Forster, R.; Schulz, R. Effects of atrial natriuretic peptide on phagocytosis and respiratory burst in murine macrophages. Eur. J. Pharmacol. 1997, 319, 279–285. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Hartung, T.; Vollmar, A.M. cGMP-mediated inhibition of TNF-alpha production by the atrial natriuretic peptide in murine macrophages. J. Immunol. 2000, 165, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A.K.; Weber, N.C.; Furst, R.; Bildner, N.; Kulhanek-Heinze, S.; Vollmar, A.M. Inhibition of p38 MAPK activation via induction of MKP-1: Atrial natriuretic peptide reduces TNF-alpha-induced actin polymerization and endothelial permeability. Circ. Res. 2002, 90, 874–881. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Weber, N.C.; Vollmar, A.M. Induction of IκB: Atrial natriuretic peptide as a regulator of the NF-κB pathway. Biochem. Biophys. Res. Commun. 2002, 295, 1068–1076. [Google Scholar] [CrossRef]
- Moss, R.B.; Golightly, M.G. In vitro enhancement of natural cytotoxicity by atrial natriuretic peptide fragment 4-28. Peptides 1991, 12, 851–854. [Google Scholar] [CrossRef]
- Morita, R.; Ukyo, N.; Furuya, M.; Uchiyama, T.; Hori, T. Atrial natriuretic peptide polarizes human dendritic cells toward a Th2-promoting phenotype through its receptor guanylyl cyclase-coupled receptor A. J. Immunol. 2003, 170, 5869–5875. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Izzi, V.; D’Aquilio, F.; Carotenuto, F.; Di Nardo, P.; Baldini, P.M. Brain Natriuretic Peptide (BNP) regulates the production of inflammatory mediators in human THP-1 macrophages. Regul. Pept. 2008, 148, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, A.; Foster, P.; Prescott, C.; Scotland, R.; Ahluwalia, A. Natriuretic peptide receptor-C regulates coronary blood flow and prevents myocardial ischemia/reperfusion injury: Novel cardioprotective role for endothelium-derived C-type natriuretic peptide. Circulation 2004, 110, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Scotland, R.S.; Cohen, M.; Foster, P.; Lovell, M.; Mathur, A.; Ahluwalia, A.; Hobbs, A.J. C-type natriuretic peptide inhibits leukocyte recruitment and platelet-leukocyte interactions via suppression of P-selectin expression. Proc. Natl. Acad. Sci. USA 2005, 102, 14452–14457. [Google Scholar] [CrossRef] [PubMed]
- Norman, H.S.; Oujiri, J.; LaRue, S.J.; Chapman, C.B.; Margulies, K.B.; Sweitzer, N.K. Decreased Cardiac Functional Reserve in Heart Failure with Preserved Systolic Function. J. Card. Fail. 2011, 17, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kass, D.A. Heart failure with preserved ejection fraction: Mechanisms, clinical features, and therapies. Circ. Res. 2014, 115, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Loffredo, F.S.; Nikolova, A.P.; Pancoast, J.R.; Lee, R.T. Heart failure with preserved ejection fraction: Molecular pathways of the aging myocardium. Circ. Res. 2014, 115, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Mottram, P.M.; Haluska, B.; Leano, R.; Cowley, D.; Stowasser, M.; Marwick, T.H. Effect of aldosterone antagonism on myocardial dysfunction in hypertensive patients with diastolic heart failure. Circulation 2004, 110, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.G.; Oparil, S. Diastolic dysfunction and heart failure with preserved ejection fraction: rationale for RAAS antagonist/CCB combination therapy. J. Am. Soc. Hypertens. 2009, 3, 52–68. [Google Scholar]
- Nagata, K.; Obata, K.; Xu, J.; Ichihara, S.; Noda, A.; Kimata, H.; Kato, T.; Izawa, H.; Murohara, T.; Yokota, M. Mineralocorticoid receptor antagonism attenuates cardiac hypertrophy and failure in low-aldosterone hypertensive rats. Hypertension 2006, 47, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, E.J.; Domanski, M.J.; Krause-Steinrauf, H.; Bristow, M.R.; Lavori, P.W. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N. Engl. J. Med. 2001, 344, 1659–1667. [Google Scholar] [CrossRef]
- Poole-Wilson, P.A.; Swedberg, K.; Cleland, J.G.; Di Lenarda, A.; Hanrath, P.; Komajda, M.; Lubsen, J.; Lutiger, B.; Metra, M.; Remme, W.J.; et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol or Metoprolol European Trial (COMET): Randomised controlled trial. Lancet 2003, 362, 7–13. [Google Scholar] [CrossRef]
- Nishio, R.; Shioi, T.; Sasayama, S.; Matsumori, A. Carvedilol increases the production of interleukin-12 and interferon-gamma and improves the survival of mice infected with the encephalomyocarditis virus. J. Am. Coll. Cardiol. 2003, 41, 340–345. [Google Scholar] [CrossRef]
- Kurum, T.; Tatli, E.; Yuksel, M. Effects of carvedilol on plasma levels of pro-inflammatory cytokines in patients with ischemic and nonischemic dilated cardiomyopathy. Tex. Heart Inst. J. 2007, 34, 52–59. [Google Scholar]
- Sagiv, M.; Ben-Sira, D.; Goldhammer, E. Beta-blockers, exercise, and the immune system in men with coronary artery disease. Med. Sci. Sports Exerc. 2002, 34, 587–591. [Google Scholar]
- ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: A randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. Lancet 1995, 345, 669–685. [Google Scholar] [CrossRef]
- Devita, C.; Fazzini, P.F.; Geraci, E.; Tavazzi, L.; Tognoni, G.; Lo Vecchio, C.; Boeri, R.; Damico, G.; Loi, U.; Marubini, E.; et al. GISSI-3: Effects of lisinopril and transdermal glyceryl trinitrate singly and together on 6-week mortality and ventricular function after acute myocardial infarction. Lancet 1994, 343, 1115–1122. [Google Scholar]
- Gao, X.M.; Tsai, A.; Al-Sharea, A.; Su, Y.; Moore, S.; Han, L.P.; Kiriazis, H.; Dart, A.M.; Murphy, A.J.; Du, X.J. Inhibition of the Renin-Angiotensin System Post Myocardial Infarction Prevents Inflammation-Associated Acute Cardiac Rupture. Cardiovasc. Drugs Ther. 2017, 31, 145–156. [Google Scholar] [CrossRef]
- Lymperopoulos, A.; Rengo, G.; Koch, W.J. Adrenergic nervous system in heart failure: Pathophysiology and therapy. Circ. Res. 2013, 113, 739–753. [Google Scholar] [CrossRef]
- Loan Le, T.Y.; Mardini, M.; Howell, V.M.; Funder, J.W.; Ashton, A.W.; Mihailidou, A.S. Low-dose spironolactone prevents apoptosis repressor with caspase recruitment domain degradation during myocardial infarction. Hypertension 2012, 59, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Zannad, F.; McMurray, J.J.; Krum, H.; van Veldhuisen, D.J.; Swedberg, K.; Shi, H.; Vincent, J.; Pocock, S.J.; Pitt, B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011, 364, 11–21. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Kaikita, K.; Sato, K.; Sueta, D.; Fujisue, K.; Arima, Y.; Oimatsu, Y.; Mitsuse, T.; Onoue, Y.; Araki, S.; et al. Cardioprotective Effects of LCZ696 (Sacubitril/Valsartan) After Experimental Acute Myocardial Infarction. JACC Basic Transl. Sci. 2017, 2, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Drosatos, K.; Lymperopoulos, A.; Kennel, P.J.; Pollak, N.; Schulze, P.C.; Goldberg, I.J. Pathophysiology of sepsis-related cardiac dysfunction: Driven by inflammation, energy mismanagement, or both? Curr. Heart. Fail. Rep. 2015, 12, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Kadokami, T.; McTiernan, C.F.; Kubota, T.; Frye, C.S.; Bounoutas, G.S.; Robbins, P.D.; Watkins, S.C.; Feldman, A.M. Effects of soluble TNF receptor treatment on lipopolysaccharide-induced myocardial cytokine expression. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H2281–H2291. [Google Scholar] [CrossRef] [PubMed]
- Louis, A.; Cleland, J.G.; Crabbe, S.; Ford, S.; Thackray, S.; Houghton, T.; Clark, A. Clinical Trials Update: CAPRICORN, COPERNICUS, MIRACLE, STAF, RITZ-2, RECOVER and RENAISSANCE and cachexia and cholesterol in heart failure. Highlights of the Scientific Sessions of the American College of Cardiology, 2001. Eur. J. Heart Fail. 2001, 3, 381–387. [Google Scholar] [CrossRef]
- Gullestad, L.; Aass, H.; Fjeld, J.G.; Wikeby, L.; Andreassen, A.K.; Ihlen, H.; Simonsen, S.; Kjekshus, J.; Nitter-Hauge, S.; Ueland, T.; et al. Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 2001, 103, 220–225. [Google Scholar] [CrossRef]
- Damas, J.K.; Gullestad, L.; Aukrust, P. Cytokines as new treatment targets in chronic heart failure. Curr. Control Trials Cardiovasc. Med. 2001, 2, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Takahashi, T.; Golstein, P.; Nagata, S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75, 1169–1178. [Google Scholar] [CrossRef]
- Skudicky, D.; Bergemann, A.; Sliwa, K.; Candy, G.; Sareli, P. Beneficial effects of pentoxifylline in patients with idiopathic dilated cardiomyopathy treated with angiotensin-converting enzyme inhibitors and carvedilol: Results of a randomized study. Circulation 2001, 103, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Champion, S.; Lapidus, N.; Cherie, G.; Spagnoli, V.; Oliary, J.; Solal, A.C. Pentoxifylline in heart failure: A meta-analysis of clinical trials. Cardiovasc. Ther. 2014, 32, 159–162. [Google Scholar] [CrossRef] [PubMed]
 stimulation;
stimulation;  inhibition; RAAS: renin-angiotensin-aldosterone system; AS: adrenergic system; NPS: natriuretic peptides system; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; AT1R: angiotensin receptor 1; AR: adrenergic receptor; M: monocyte; Ma: macrophage; M1: type 1 macrophage; M2: type 2 macrophage; DC: dendritic cell; Th0: non polarized lymphocyte; Th1: Th1 lymphocytes; Th2: Th2 lymphocytes; TNF: tumor necrosis factor; IL: interleukin; i-NOS: inducible nitric oxide synthase; ROS: radical oxygen species; LTB4: leukotriene B4; PGE2:prostaglandin E2; IFN: interferon.
inhibition; RAAS: renin-angiotensin-aldosterone system; AS: adrenergic system; NPS: natriuretic peptides system; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; AT1R: angiotensin receptor 1; AR: adrenergic receptor; M: monocyte; Ma: macrophage; M1: type 1 macrophage; M2: type 2 macrophage; DC: dendritic cell; Th0: non polarized lymphocyte; Th1: Th1 lymphocytes; Th2: Th2 lymphocytes; TNF: tumor necrosis factor; IL: interleukin; i-NOS: inducible nitric oxide synthase; ROS: radical oxygen species; LTB4: leukotriene B4; PGE2:prostaglandin E2; IFN: interferon.
 stimulation;
stimulation;  inhibition; RAAS: renin-angiotensin-aldosterone system; AS: adrenergic system; NPS: natriuretic peptides system; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; AT1R: angiotensin receptor 1; AR: adrenergic receptor; M: monocyte; Ma: macrophage; M1: type 1 macrophage; M2: type 2 macrophage; DC: dendritic cell; Th0: non polarized lymphocyte; Th1: Th1 lymphocytes; Th2: Th2 lymphocytes; TNF: tumor necrosis factor; IL: interleukin; i-NOS: inducible nitric oxide synthase; ROS: radical oxygen species; LTB4: leukotriene B4; PGE2:prostaglandin E2; IFN: interferon.
inhibition; RAAS: renin-angiotensin-aldosterone system; AS: adrenergic system; NPS: natriuretic peptides system; ANP: atrial natriuretic peptide; BNP: brain natriuretic peptide; AT1R: angiotensin receptor 1; AR: adrenergic receptor; M: monocyte; Ma: macrophage; M1: type 1 macrophage; M2: type 2 macrophage; DC: dendritic cell; Th0: non polarized lymphocyte; Th1: Th1 lymphocytes; Th2: Th2 lymphocytes; TNF: tumor necrosis factor; IL: interleukin; i-NOS: inducible nitric oxide synthase; ROS: radical oxygen species; LTB4: leukotriene B4; PGE2:prostaglandin E2; IFN: interferon.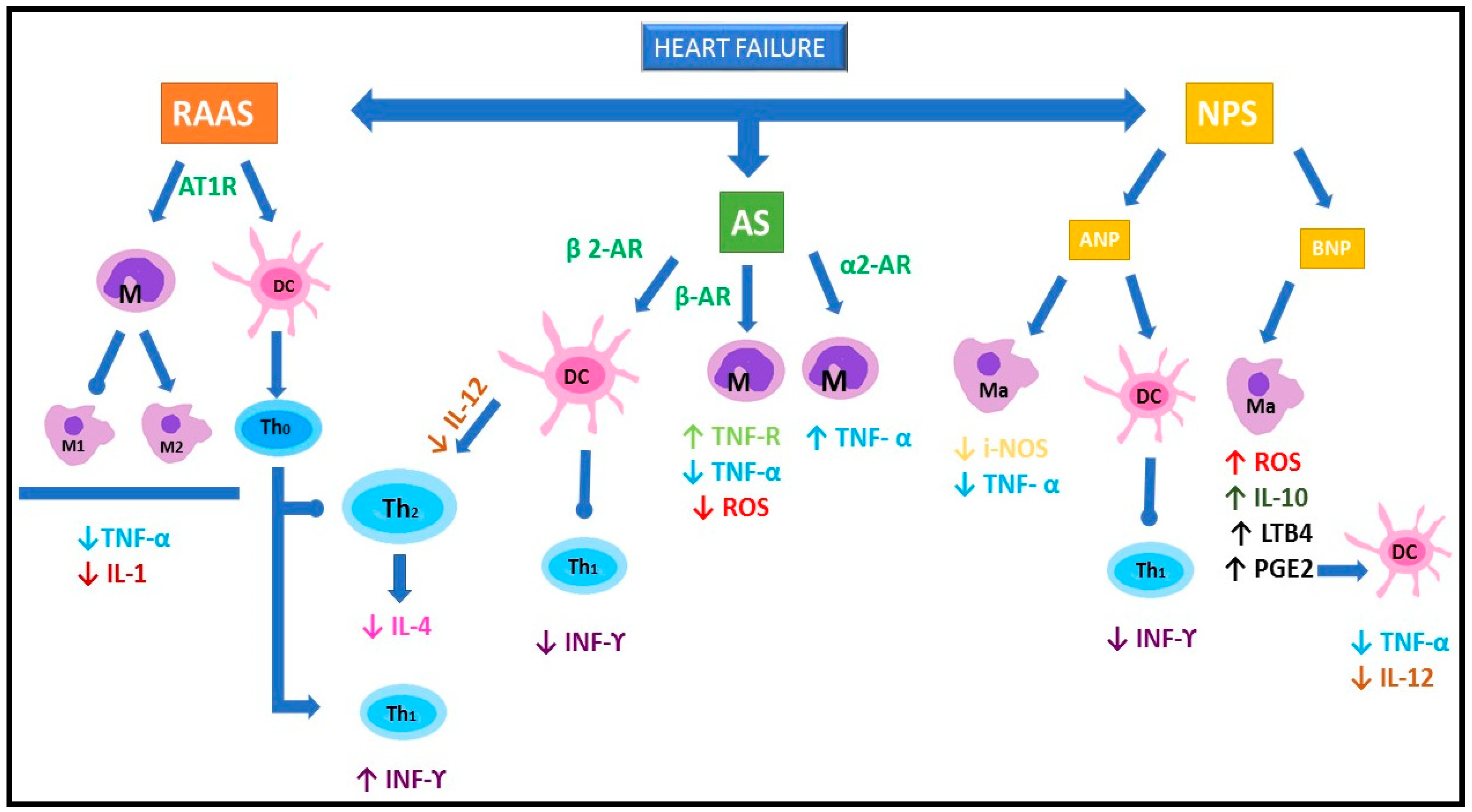
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Angelis, E.; Pecoraro, M.; Rusciano, M.R.; Ciccarelli, M.; Popolo, A. Cross-Talk between Neurohormonal Pathways and the Immune System in Heart Failure: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 1698. https://doi.org/10.3390/ijms20071698
De Angelis E, Pecoraro M, Rusciano MR, Ciccarelli M, Popolo A. Cross-Talk between Neurohormonal Pathways and the Immune System in Heart Failure: A Review of the Literature. International Journal of Molecular Sciences. 2019; 20(7):1698. https://doi.org/10.3390/ijms20071698
Chicago/Turabian StyleDe Angelis, Elena, Michela Pecoraro, Maria Rosaria Rusciano, Michele Ciccarelli, and Ada Popolo. 2019. "Cross-Talk between Neurohormonal Pathways and the Immune System in Heart Failure: A Review of the Literature" International Journal of Molecular Sciences 20, no. 7: 1698. https://doi.org/10.3390/ijms20071698
APA StyleDe Angelis, E., Pecoraro, M., Rusciano, M. R., Ciccarelli, M., & Popolo, A. (2019). Cross-Talk between Neurohormonal Pathways and the Immune System in Heart Failure: A Review of the Literature. International Journal of Molecular Sciences, 20(7), 1698. https://doi.org/10.3390/ijms20071698






