Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation
Abstract
1. Introduction
2. Results
2.1. Characterization of Mesenchymal STEM/Stromal Cells
2.2. Development of MSC-CM Bioprocessing Protocol
2.3. Evaluation of MSC-CM Functional Activity in Vitro
2.4. Development of the Prediction Model for MSC-CM Potency Using Regression Analysis
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Isolation of Adipose-Derived MSC
4.3. Collection of MSC conditioned medium
4.4. Analysis of Cell Viability
4.5. MSCs Immunophenotyping and Differentiation Assays
4.6. Analysis of Concentrations of Growth Factors in MSC-Conditioned Medium by ELISA
4.7. Migration of Fibroblasts in the Scratch Assay
4.8. Endothelial Cell Migration Analysis in the xCELLigence RTCA DP System
4.9. Regression Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MSC | mesenchymal stem/stromal cells |
| CM | conditioned medium |
| DMEMLG | Dulbecco’s Modified Eagle Medium with low glucose content |
| VEGF | vascular endothelial growth factor |
| HGF | hepatocyte growth factor |
| FGF2 | basic fibroblast growth factor |
| Angpt-1 | angiopoietin-1 |
| PEDF | pigment epithelial cells derived factor |
| FBS | fetal bovine serum |
References
- Robb, K.P.; Fitzgerald, J.C.; Barry, F.; Viswanathan, S. Mesenchymal stromal cell therapy: Progress in manufacturing and assessments of potency. Cytotherapy 2018, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- McLeod, C.M.; Mauck, R.L. On the origin and impact of mesenchymal stem cell heterogeneity: New insights and emerging tools for single cell analysis. Eur. Cells Mater. 2017, 34, 217–231. [Google Scholar] [CrossRef]
- Schwalie, P.C.; Dong, H.; Zachara, M.; Russeil, J.; Alpern, D.; Akchiche, N.; Caprara, C.; Sun, W.; Schlaudraff, K.-U.; Soldati, G.; et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 2018, 559, 103–108. [Google Scholar] [CrossRef]
- Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Kanareikina, M.A.; Kalinina, N.I.; Sysoeva, V.Y.; Dyikanov, D.T.; Stambolsky, D.V.; Tkachuk, V.A. Activation of β-adrenergic receptors is required for elevated α1A-adrenoreceptors expression and signaling in mesenchymal stromal cells. Sci. Rep. 2016, 6, 32835. [Google Scholar] [CrossRef]
- Sysoeva, V.Y.; Ageeva, L.V.; Tyurin-Kuzmin, P.A.; Sharonov, G.V.; Dyikanov, D.T.; Kalinina, N.I.; Tkachuk, V.A. Local angiotensin II promotes adipogenic differentiation of human adipose tissue mesenchymal stem cells through type 2 angiotensin receptor. Stem Cell Res. 2017, 25, 115–122. [Google Scholar] [CrossRef]
- Lee, H.Y.; Hong, I.S. Double-edged sword of mesenchymal stem cells: Cancer-promoting versus therapeutic potential. Cancer Sci. 2017, 108, 1939–1946. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, J.; Zhang, X.; Liu, Y.; Chen, J.; Hu, B.; Song, J.; Zhang, Y. Strategies to optimize adult stem cell therapy for tissue regeneration. Int. J. Mol. Sci. 2016, 17, 982. [Google Scholar] [CrossRef]
- Tano, N.; Kaneko, M.; Ichihara, Y.; Ikebe, C.; Coppen, S.R.; Shiraishi, M.; Shintani, Y.; Yashiro, K.; Warrens, A.; Suzuki, K. Allogeneic Mesenchymal Stromal Cells Transplanted Onto the Heart Surface Achieve Therapeutic Myocardial Repair Despite Immunologic Responses in Rats. J. Am. Heart Assoc. 2016, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, X.-T.; Yin, Y.; Wu, R.-X.; Tian, B.-M.; Chen, F.-M. Administration of signalling molecules dictates stem cell homing for in situ regeneration. J. Cell. Mol. Med. 2017, 21, 3162–3177. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef]
- Salazar, K.D.; Lankford, S.M.; Brody, A.R. Mesenchymal stem cells produce Wnt isoforms and TGF-β 1 that mediate proliferation and procollagen expression by lung fibroblasts. Am. J. Physiol. Cell. Mol. Physiol. 2009, 297, L1002–L1011. [Google Scholar] [CrossRef]
- Lozito, T.P.; Tuan, R.S. Mesenchymal stem cells inhibit both endogenous and exogenous MMPs via secreted TIMPs. J. Cell. Physiol. 2011, 226, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Andreeva, E.R.; Matveeva, D.K. Multipotent Mesenchymal Stromal Cells and Extracellular Matrix: Regulation under Hypoxia. Hum. Physiol. 2018, 44, 696–705. [Google Scholar] [CrossRef]
- Bhang, S.H.; Lee, S.; Shin, J.-Y.; Lee, T.-J.; Jang, H.-K.; Kim, B.-S. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol. Ther. 2014, 22, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; Kharlampieva, D.; Loguinova, M.; Butenko, I.; Pobeguts, O.; Efimenko, A.; Ageeva, L.; Sharonov, G.; Ischenko, D.; Alekseev, D.; et al. Characterization of secretomes provides evidence for adipose-derived mesenchymal stromal cells subtypes. Stem Cell Res. Ther. 2015, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Pawitan, J.A. Prospect of stem cell conditioned medium in regenerative medicine. Biomed Res. Int. 2014, 2014, 965849. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.; Chen, J.; Chang, F.; Wang, J.; Ding, J.; Chen, X. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater. 2018, 73, 103–111. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Wang, S.-J.; Zhang, J.-Y.; Jiang, W.-B.; Huang, A.-B.; Qi, Y.-S.; Ding, J.-X.; Chen, X.-S.; Jiang, D.; Yu, J.-K. 3D-Printed Poly(ε-caprolactone) Scaffold Augmented with Mesenchymal Stem Cells for Total Meniscal Substitution: A 12- and 24-Week Animal Study in a Rabbit Model. Am. J. Sports Med. 2017, 45, 1497–1511. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Chang, F.; Xu, W.; Ding, J. Repair of full-thickness articular cartilage defect using stem cell-encapsulated thermogel. Mater. Sci. Eng. C 2018, 88, 79–87. [Google Scholar] [CrossRef]
- Sagaradze, G.D.; Basalova, N.A.; Kirpatovsky, V.I.; Ohobotov, D.A.; Grigorieva, O.A.; Balabanyan, V.Y.; Kamalov, A.A.; Efimenko, A.Y. Application of rat cryptorchidism model for the evaluation of mesenchymal stromal cell secretome regenerative potential. Biomed. Pharmacother. 2019, 109, 1428–1436. [Google Scholar] [CrossRef]
- Kirpatovckii, V.I.; Kamalov, D.M.; Efimenko, A.Y.; Makarevich, P.I.; Sagaradze, G.D.; Makarevich, O.A.; Nimiritskii, P.P.; Osidak, E.O.; Domogatskii, S.P.; Karpov, V.K.; et al. Urinary bladder substitution using combined membrane based on secretions of human mesenchymal stem cells and type I collagen. Urologiia 2016, 6, 34–42. [Google Scholar]
- Fukuoka, H.; Suga, H.; Narita, K.; Watanabe, R.; Shintani, S. The latest advance in hair regeneration therapy using proteins secreted by adipose-derived stem cells. Am. J. Cosmet. Surg. 2012, 29, 273–282. [Google Scholar]
- Zhou, B.R.; Xu, Y.; Xu, Y.; Guo, S.L.; Wang, Y.; Zhu, F.; Permatasari, F.; Wu, D.; Yin, Z.Q.; Luo, D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.G.; Long, G.; Tyler, G.; Stefan, A.; Broadfoot, S.J.; Piccinini, A.M.; Middleton, J.; Kehoe, O. Mesenchymal Stem Cell-Conditioned Medium Reduces Disease Severity and Immune Responses in Inflammatory Arthritis. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al-Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M.; et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef]
- Fujita, Y.; Kadota, T.; Araya, J.; Ochiya, T.; Kuwano, K. Clinical Application of Mesenchymal Stem Cell-Derived Extracellular Vesicle-Based Therapeutics for Inflammatory Lung Diseases. J. Clin. Med. 2018, 7, 355. [Google Scholar] [CrossRef]
- Gimona, M.; Pachler, K.; Laner-Plamberger, S.; Schallmoser, K.; Rohde, E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int. J. Mol. Sci. 2017, 18, 1190. [Google Scholar] [CrossRef]
- Qiu, G.; Zheng, G.; Ge, M.; Wang, J.; Huang, R.; Shu, Q.; Xu, J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res. Ther. 2018, 9, 320. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, T.N.A.; Mohammad, T.R.; Abdullah, B.J.J.; Abu Kasim, N.H. Conditioned media serived from mesenchymal stem cell cultures: The next generation for regenerative medicine. J. Tissue Eng. Regen. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sagaradze, G.D.; Nimiritsky, P.P.; Akopyan, Z.A.; Makarevich, P.I.; Efimenko, A.Y. “Cell-Free Therapeutics” from Components Secreted by Mesenchymal Stromal Cells as a Novel Class of Biopharmaceuticals. In Biopharmaceuticals; InTech: London, UK, 2018; Volume 2, p. 64. ISBN 9789537619992. [Google Scholar]
- Leuning, D.G.; Beijer, N.R.M.; du Fossé, N.A.; Vermeulen, S.; Lievers, E.; van Kooten, C.; Rabelink, T.J.; de Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef] [PubMed]
- Bravery, C.A.; Carmen, J.; Fong, T.; Oprea, W.; Hoogendoorn, K.H.; Woda, J.; Burger, S.R.; Rowley, J.A.; Bonyhadi, M.L.; Van’t Hof, W. Potency assay development for cellular therapy products: An ISCT∗ review of the requirements and experiences in the industry. Cytotherapy 2013, 15, 9–19.e9. [Google Scholar] [CrossRef]
- Mendicino, M.; Bailey, A.M.; Wonnacott, K.; Puri, R.K.; Bauer, S.R. MSC-based product characterization for clinical trials: An FDA perspective. Cell Stem Cell 2014, 14, 141–145. [Google Scholar] [CrossRef]
- Jin, H.; Bae, Y.; Kim, M.; Kwon, S.-J.; Jeon, H.; Choi, S.; Kim, S.; Yang, Y.; Oh, W.; Chang, J. Comparative Analysis of Human Mesenchymal Stem Cells from Bone Marrow, Adipose Tissue, and Umbilical Cord Blood as Sources of Cell Therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Turnley, T.; Wishart, A.; Rowe, A.; Kallmeyer, K.; van Vollenstee, F.; Thomford, N.; Dandara, C.; Chopera, D.; Pepper, M.; et al. Fibroblast-Derived Extracellular Matrix Induces Chondrogenic Differentiation in Human Adipose-Derived Mesenchymal Stromal/Stem Cells in Vitro. Int. J. Mol. Sci. 2016, 17, 1259. [Google Scholar] [CrossRef] [PubMed]
- Odabas, S.; Elçin, A.E.; Elçin, Y.M. Isolation and Characterization of Mesenchymal Stem Cells. Methods Mol. Biol. 2014, 1109, 47–63. [Google Scholar] [CrossRef]
- Efimenko, A.; Dzhoyashvili, N.; Starostina, E.; Kalinina, N.; Parfyonova, E. Angiogenic properties of human adipose-derived mesenchymal stem cells decline with donor age owing to the impairment of proangiogenic factors secretion. Regen. Med. 2011, 6, 322–323. [Google Scholar]
- Efimenko, A.Y.; Dzhoyashvili, N.; Kalinina, N.; Kochegura, T.; Akchurin, R.; Tkachuk, V.; Parfyonova, Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl. Med. 2014, 3, 32–41. [Google Scholar] [CrossRef]
- Method for Stimulating Regenerative Processes in Ischemic Tissues. Available online: https://patents.google.com/patent/RU2497529C2/en (accessed on 25 February 2019).
- Kim, H.-K.; Lee, S.-G.; Lee, S.-W.; Oh, B.J.; Kim, J.H.; Kim, J.A.; Lee, G.; Jang, J.-D.; Joe, Y.A. A Subset of Paracrine Factors as Efficient Biomarkers for Predicting Vascular Regenerative Efficacy of Mesenchymal Stromal/Stem Cells. Stem Cells 2019, 37, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Feng, X.; Wei, T.; Wang, Y.; Wang, Y.; Wang, Z.; Tang, D.; Luo, Y.; Xiong, Z. Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res. Ther. 2019, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Hu, G.; Su, J.; Li, W.; Chen, Q.; Shou, P.; Xu, C.; Chen, X.; Huang, Y.; Zhu, Z.; et al. Mesenchymal stem cells: A new strategy for immunosuppression and tissue repair. Cell Res. 2010, 20, 510–518. [Google Scholar] [CrossRef]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. 2011, 9, 12. [Google Scholar] [CrossRef]
- Valtieri, M.; Sorrentino, A. The mesenchymal stromal cell contribution to homeostasis. J. Cell. Physiol. 2008, 217, 296–300. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar]
- Nimiritsky, P.; Eremichev, R.; Alexandrushkina, N.; Efimenko, A.; Tkachuk, V.; Makarevich, P. Unveiling Mesenchymal Stromal Cells’ Organizing Function in Regeneration. Int. J. Mol. Sci. 2019, 20, 823. [Google Scholar] [CrossRef]
- Kichenbrand, C.; Velot, E.; Menu, P.; Moby, V. Dental Pulp Stem Cell-Derived Conditioned Medium: An Attractive Alternative for Regenerative Therapy. Tissue Eng. Part B Rev. 2018, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, C.; Suzuki, K. Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. Biomed Res. Int. 2014, 2014, 951512. [Google Scholar] [CrossRef] [PubMed]
- Oikonomopoulos, A.; Deen, W.K. Van; Manansala, A.; Lacey, P.N.; Tomakili, T.A.; Ziman, A.; Hommes, D.W. Optimization of human mesenchymal stem cell manufacturing: The effects of animal/xeno-free media. Nat. Publ. Gr. 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef] [PubMed]
- Belinsky, G.S.; Sreekumar, B.; Andrejecsk, J.W.; Saltzman, W.M.; Gong, J.; Herzog, R.I.; Lin, S.; Horsley, V.; Carpenter, T.O.; Chung, C. Pigment epithelium-derived factor restoration increases bone mass and improves bone plasticity in a model of osteogenesis imperfecta type VI via Wnt3a blockade. FASEB J. 2016, 30, 2837–2848. [Google Scholar] [CrossRef]
- Fan, W.; Crawford, R.; Xiao, Y. The ratio of VEGF/PEDF expression in bone marrow mesenchymal stem cells regulates neovascularization. Differentiation. 2011, 81, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, R.; Rong, W.; Han, M.; Cui, C.; Feng, Z.; Sun, X.; Jin, S. Therapeutic effect of hepatocyte growth factor-overexpressing bone marrow-derived mesenchymal stem cells on CCl4-induced hepatocirrhosis. Cell Death Dis. 2018, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Zhu, Y.T.; Ding, J.; Zhou, F.Y.; Xue, J.X.; Jung, J.H.; Li, Z.J.; Gao, W.Y. Distribution of fibroblast growth factors and their roles in skin fibroblast cell migration. Mol. Med. Rep. 2016, 14, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Cartland, S.P.; Genner, S.W.; Zahoor, A.; Kavurma, M.M. Comparative evaluation of trail, FGF-2 and VEGF-A-Induced angiogenesis in vitro and in vivo. Int. J. Mol. Sci. 2016, 17, 2025. [Google Scholar] [CrossRef]
- Lai, R.C.; Arslan, F.; Lee, M.M.; Sze, N.S.K.; Choo, A.; Chen, T.S.; Salto-Tellez, M.; Timmers, L.; Lee, C.N.; El Oakley, R.M.; et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010, 4, 214–222. [Google Scholar] [CrossRef]
- Lee, M.S.; Youn, C.; Kim, J.H.; Park, B.J.; Ahn, J.; Hong, S.; Kim, Y.D.; Shin, Y.K.; Park, S.G. Enhanced cell growth of adipocyte-derived mesenchymal stem cells using chemically-defined serum-free media. Int. J. Mol. Sci. 2017, 18, 1779. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Ding, J.; He, P.; Liu, Y.; Cheng, H.; Zhou, C.; Meng, X. Autoserum: An Optimal Supplement for Bone Marrow Mesenchymal Stem Cells of Liver-Injured Rats. Stem Cells Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
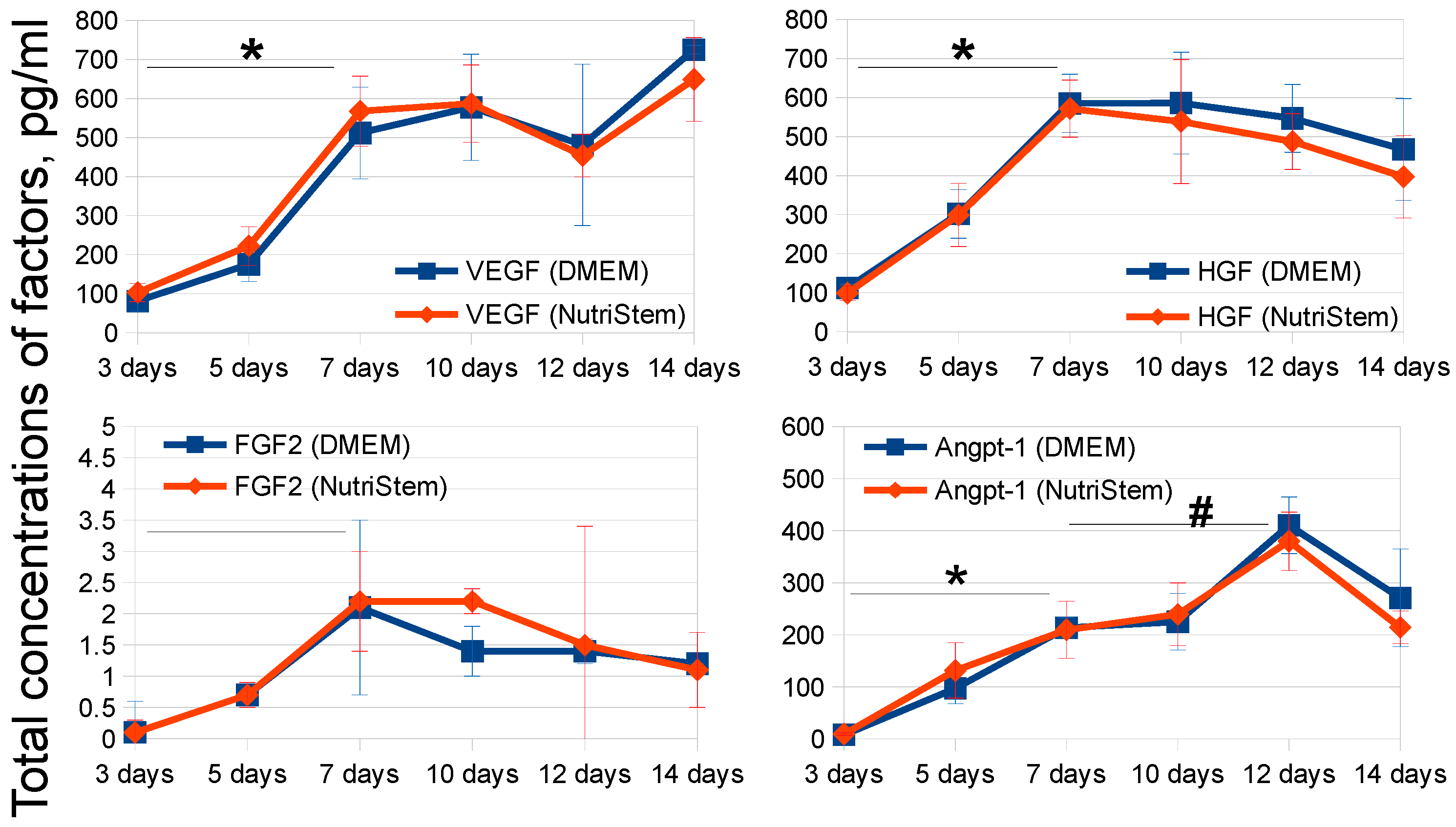
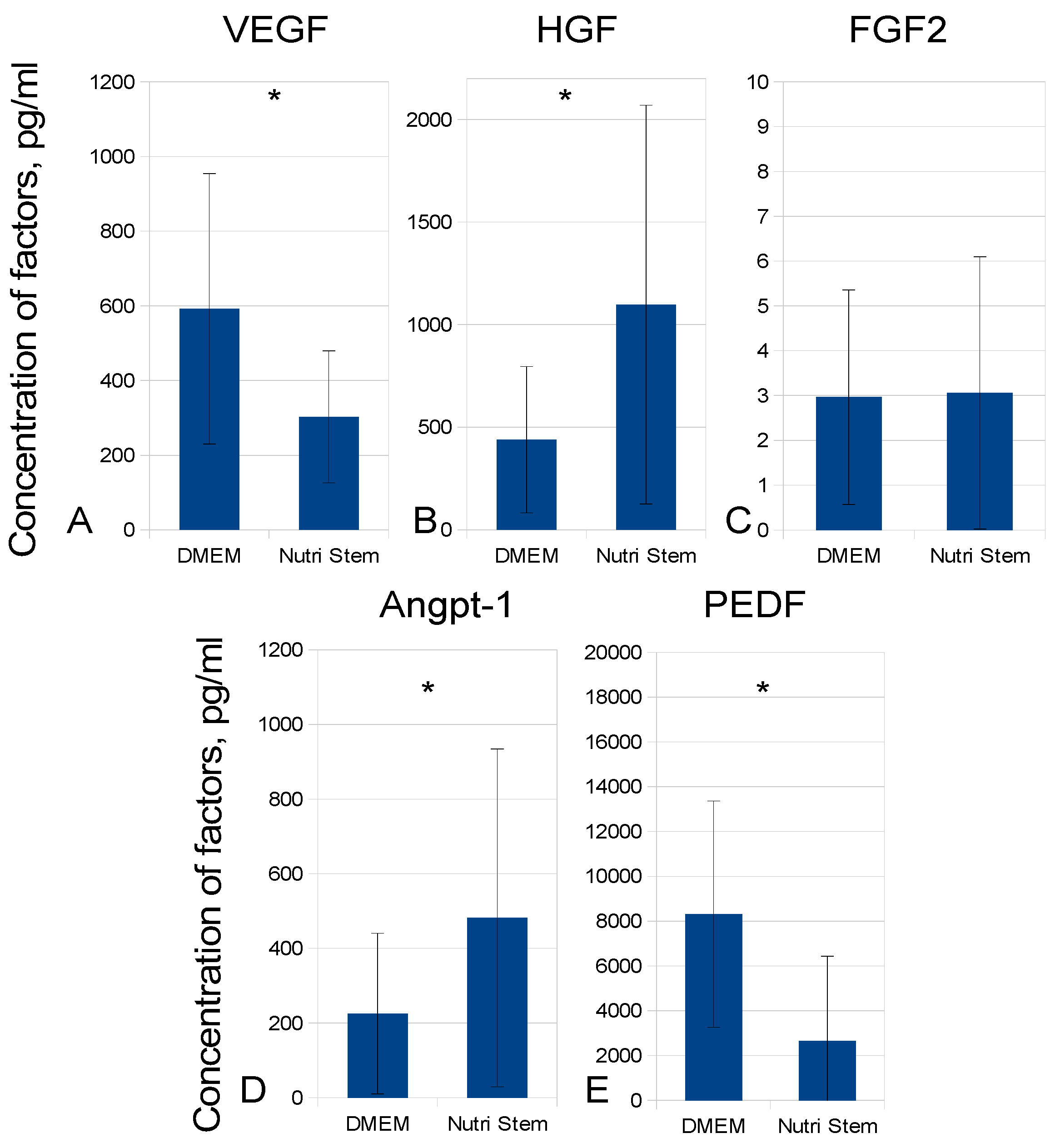
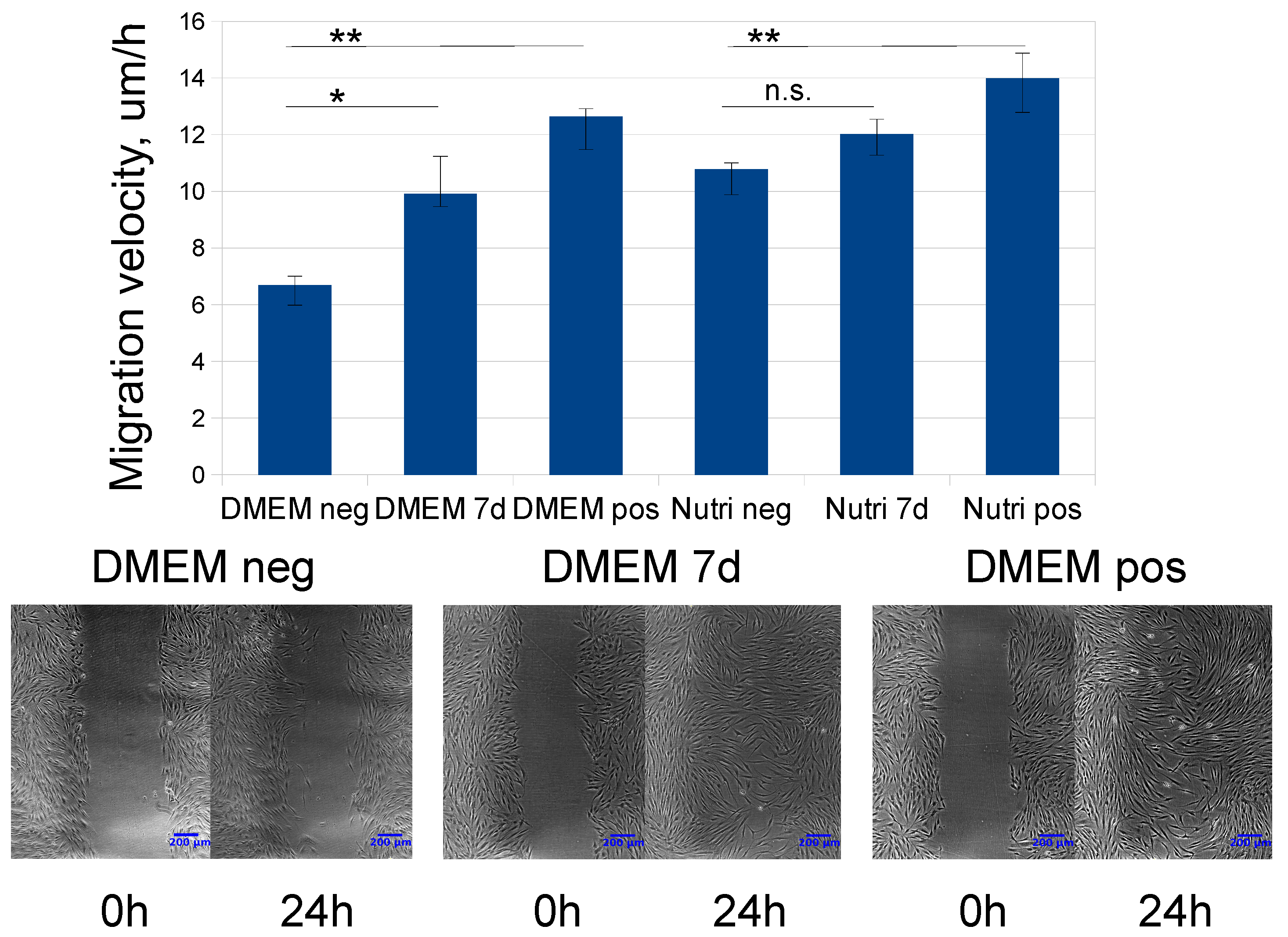
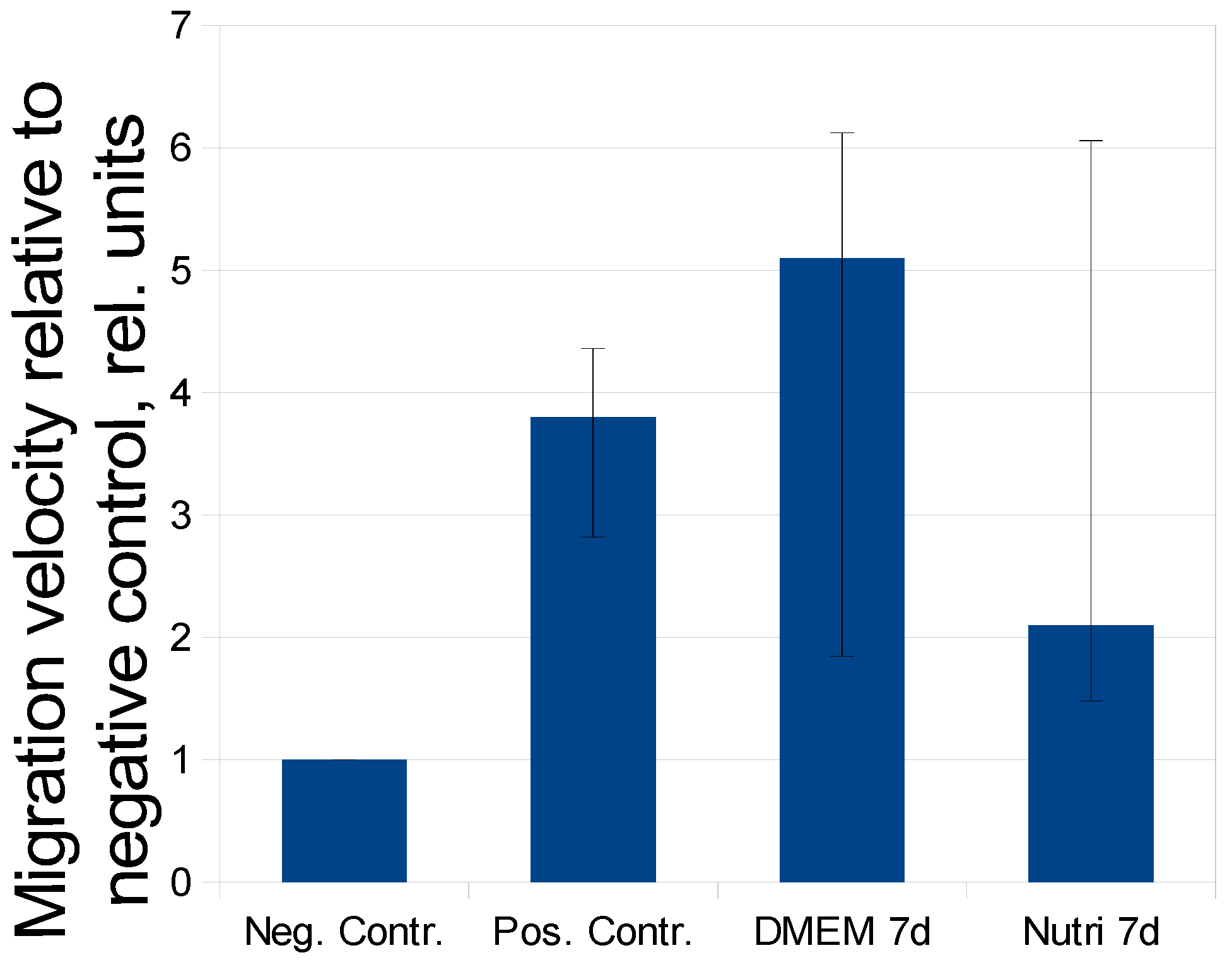
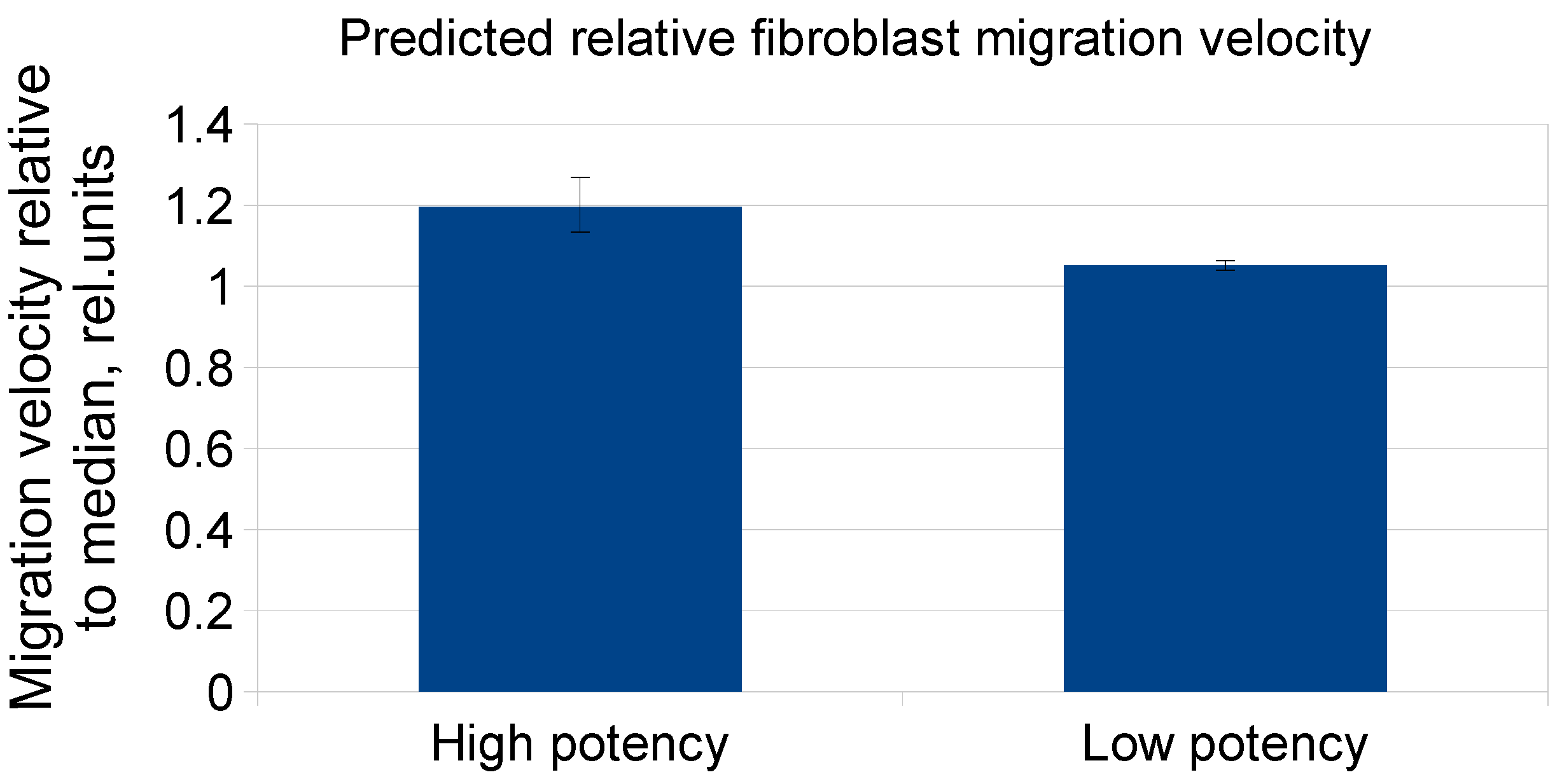
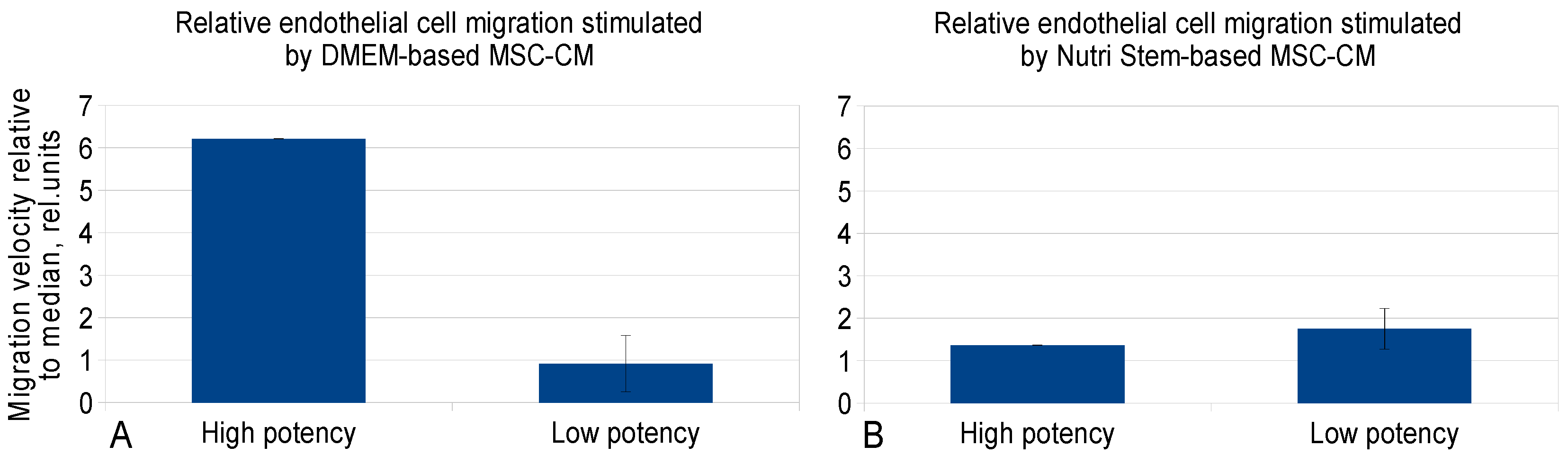
| Time, Days/Growth Medium | 3 Days | 5 Days | 7 Days | 10 Days | 12 Days | 14 Days |
|---|---|---|---|---|---|---|
| DMEM | 80.3 ± 1.5 | 77.0 ± 2.6 | 78.3 ± 3.2 | 71.7 ± 3.8 | 90.7 ± 6.7 | 59.7 ± 11.7 |
| Nutri Stem | 76.0 ± 4.0 | 77.3 ± 5.0 | 73.3 ± 11.2 | 72.0 ± 8.5 | 73.7 ± 13.1 | 76.0 ± 5.2 |
| Predictor. | Coefficient | P-Value | 95% Conf. Interval |
|---|---|---|---|
| Intercept | 0.58 | 0.53 | −1.9; 1.25 |
| VEGF | 0.67 | 0.46 | −1.35; 0.99 |
| HGF | 0.80 | 0.27 | −0.82; 1.62 |
| FGF2 | 0.67 | 0.44 | −0.74; 2.53 |
| Angpt-1 | −1.83 | 0.08 | −3.81; 0.78 |
| PEDF | 1.14 | 0.15 | −0.48; 1.64 |
| Medium type | −1.17 | 0.45 | −2.69; 2.62 |
| Single factor analysis | |||
| Predictor | Coefficient | P-value | 95% Conf. Interval |
| Intercept | 0.09 | 0.85 | −0.85; 1.03 |
| Angpt1 | −1.16 | 0.11 | −2.59; 0.27 |
| Predictor | Coefficient | P-Value | 95% Conf. Interval |
|---|---|---|---|
| Intercept | 2.75 | 0.61 | −7.7; 13.2 |
| VEGF | 2.76 | 0.31 | −2.52; 8.04 |
| HGF | 0.66 | 0.72 | −2.96; 4.29 |
| FGF2 | 8.78 | 0.69 | −34.11; 51.67 |
| Angpt1 | −1.46 | 0.70 | −9.01; 6.09 |
| PEDF | 0.89 | 0.54 | −1.99; 3.77 |
| Medium type | −4.10 | 0.70 | −24.8; 16.58 |
| Predictor | Coefficient | P-Value | 95% Conf. Interval |
|---|---|---|---|
| Intercept | 17.70 | 0.44 | −26.83;62.23 |
| VEGF | −1.12 | 0.78 | −8.93;6.69 |
| HGF | −1.18 | 0.68 | −6.81;4.46 |
| FGF2 | 60.19 | 0.44 | −92.44;212.81 |
| Angpt1 | 1.21 | 0.89 | −15.59;18 |
| PEDF | 0.81 | 0.66 | −2.82;4.44 |
| Single factor analysis | |||
| Predictor | Coefficient | P-value | 95% Conf. Interval |
| Intercept | 13.08 | 0.17 | −5.7;31.86 |
| FGF2 | 48.78 | 0.14 | −15.7;113.27 |
| Predictor | Coefficient | P-Value | 95% Conf. Interval |
|---|---|---|---|
| Intercept | −1.04 | 0.95 | −30.57;28.49 |
| VEGF | 4.81 | 0.76 | −25.56;35.17 |
| HGF | −0.72 | 0.96 | −28.07;26.63 |
| FGF2 | −1.73 | 0.91 | −32.6;29.13 |
| Angpt1 | 2.54 | 0.81 | −18.66;23.8 |
| PEDF | 0.45 | 0.98 | −37.8;38.7 |
| Single factor analysis | |||
| Predictor | Coefficient | P-value | 95% Conf. Interval |
| Intercept | −0.06 | 0.99 | −10.79;10.67 |
| VEGF | 7.68 | 0.42 | −10.84;26.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagaradze, G.; Grigorieva, O.; Nimiritsky, P.; Basalova, N.; Kalinina, N.; Akopyan, Z.; Efimenko, A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. Int. J. Mol. Sci. 2019, 20, 1656. https://doi.org/10.3390/ijms20071656
Sagaradze G, Grigorieva O, Nimiritsky P, Basalova N, Kalinina N, Akopyan Z, Efimenko A. Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. International Journal of Molecular Sciences. 2019; 20(7):1656. https://doi.org/10.3390/ijms20071656
Chicago/Turabian StyleSagaradze, Georgy, Olga Grigorieva, Peter Nimiritsky, Nataliya Basalova, Natalia Kalinina, Zhanna Akopyan, and Anastasia Efimenko. 2019. "Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation" International Journal of Molecular Sciences 20, no. 7: 1656. https://doi.org/10.3390/ijms20071656
APA StyleSagaradze, G., Grigorieva, O., Nimiritsky, P., Basalova, N., Kalinina, N., Akopyan, Z., & Efimenko, A. (2019). Conditioned Medium from Human Mesenchymal Stromal Cells: Towards the Clinical Translation. International Journal of Molecular Sciences, 20(7), 1656. https://doi.org/10.3390/ijms20071656





