Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine
Abstract
1. Introduction
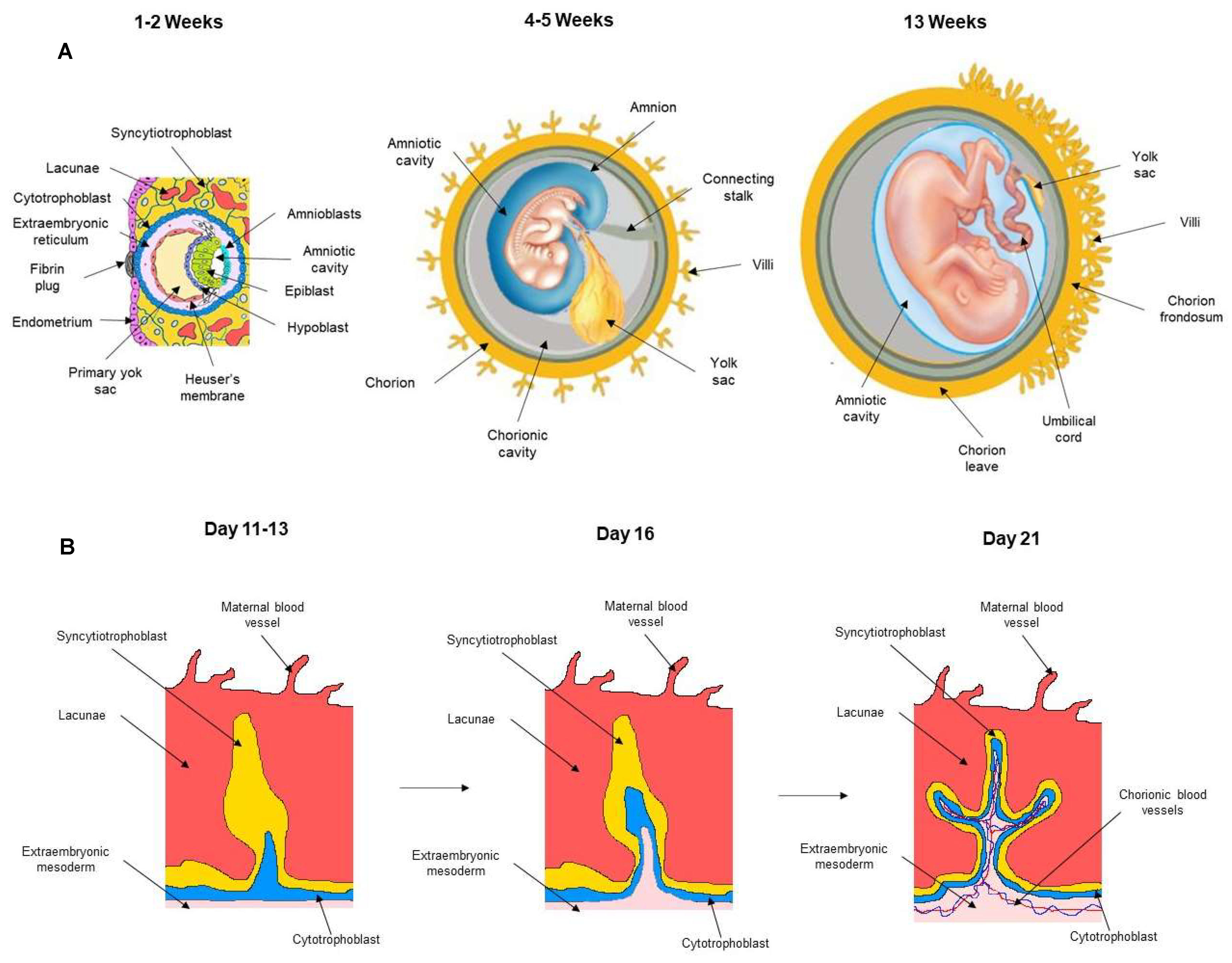
2. Development of Embryonic and Fetal Annexes
Immunological Properties of Fetal Cells
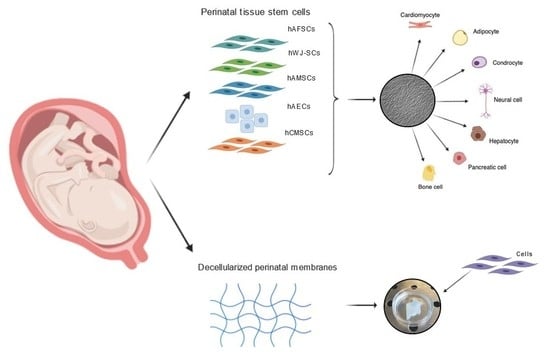
3. Stem Cells in Fetal Annexes
3.1. Stem Cells from Amnion/Chorion Membrane
3.2. Wharton Jelly-Derived Stem Cells
3.3. Amniotic Fluid-Derived Cells
4. Paracrine Effect of Fetal Annex-Derived Stem Cells
4.1. Paracrine Effect of hAECs
4.2. Paracrine Effects of Wharton’s Jelly Stem Cells
4.3. Paracrine Effects of Amniotic Fluid Cells
5. Fetal Annexes as a Scaffold in Regenerative Medicine
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuć, P.; Pancewicz-Wojtkiewicz, J.; Fil, D.; Karwowska, A.; Karczewski, J.; Mackiewicz, Z. Fetal membranes as a source of stem cells. Adv. Med. Sci. 2013, 58, 185–195. [Google Scholar]
- Cacciotti, I.; Ciocci, M.; Di Giovanni, E.; Nanni, F.; Melino, S. Hydrogen Sulfide-Releasing Fibrous Membranes: Potential Patches for Stimulating Human Stem Cells Proliferation and Viability under Oxidative Stress. Int. J. Mol. Sci. 2018, 19, 2368. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Polak, J.M. Stem cells in regenerative medicine: Introduction. Br. Med. Bull. 2011, 98, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Amabile, G. Induced Pluripotent Stem Cell-Derived Red Blood Cells and Platelet Concentrates: From Bench to Bedside. Cells 2017, 7, 2. [Google Scholar] [CrossRef]
- Antonucci, I.; Di Pietro, R.; Alfonsi, M.; Centurione, M.A.; Centurione, L.; Sancilio, S.; Pelagatti, F.; D’amico, M.A.; Di Baldassarre, A.; Piattelli, A.; et al. Human Second Trimester Amniotic Fluid Cells are Able to Create Embryoid Body-Like Structures in Vitro and to Show Typical Expression Profiles of Embryonic and Primordial Germ Cells. Cell Transplant. 2014, 23, 1501–1515. [Google Scholar] [CrossRef] [PubMed]
- Di Baldassarre, A.; Cimetta, E.; Bollini, S.; Gaggi, G.; Ghinassi, B. Human-Induced Pluripotent Stem Cell Technology and Cardiomyocyte Generation: Progress and Clinical Applications. Cells 2018, 7, 48. [Google Scholar] [CrossRef]
- Ilancheran, S.; Moodley, Y.; Manuelpillai, U. Human Fetal Membranes: A Source of Stem Cells for Tissue Regeneration and Repair? Placenta 2009, 30, 2–10. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, F.; Armentano, I.; Cacciotti, I.; Tiribuzi, R.; Quattrocelli, M.; Del Gaudio, C.; Fortunati, E.; Saino, E.; Caraffa, A.; Cerulli, G.G.; et al. Tuning Multi/Pluri-Potent Stem Cell Fate by Electrospun Poly(l-lactic acid)-Calcium-Deficient Hydroxyapatite Nanocomposite Mats. Biomacromolecules 2012, 13, 1350–1360. [Google Scholar] [CrossRef] [PubMed]
- Chatgilialoglu, A.; Rossi, M.; Alviano, F.; Poggi, P.; Zannini, C.; Marchionni, C.; Ricci, F.; Tazzari, P.L.; Taglioli, V.; Calder, P.C.; et al. Restored in vivo-like membrane lipidomics positively influence in vitro features of cultured mesenchymal stromal/stem cells derived from human placenta. Stem Cell Res. Ther. 2017, 8, 31. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, R.; Zou, Q.; Chen, Y.; Zhou, M.; Li, X.; Ran, R.; Chen, Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci. Rep. 2018, 8, 5014. [Google Scholar] [CrossRef] [PubMed]
- Spitzhorn, L.-S.; Rahman, M.S.; Schwindt, L.; Ho, H.-T.; Wruck, W.; Bohndorf, M.; Wehrmeyer, S.; Ncube, A.; Beyer, I.; Hagenbeck, C.; et al. Isolation and Molecular Characterization of Amniotic Fluid-Derived Mesenchymal Stem Cells Obtained from Caesarean Sections. Stem Cells Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Baban, B.; Chandler, P.; McCool, D.; Marshall, B.; Munn, D.H.; Mellor, A.L. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J. Reprod. Immunol. 2004, 61, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Cananzi, M.; De Coppi, P. CD117 + amniotic fluid stem cells: State of the art and future perspectives. Organogenesis 2012, 8, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Izzicupo, P.; D’Amico, M.A.; Bascelli, A.; Di Fonso, A.; D’Angelo, E.; Di Blasio, A.; Bucci, I.; Napolitano, G.; Gallina, S.; Di Baldassarre, A. Walking training affects dehydroepiandrosterone sulfate and inflammation independent of changes in spontaneous physical activity. Menopause 2012, 20, 455–463. [Google Scholar] [CrossRef]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.-J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise Review: Isolation and Characterization of Cells from Human Term Placenta: Outcome of the First International Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Karp, J.M.; Leng Teo, G.S. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef]
- Lim, R. Concise Review: Fetal Membranes in Regenerative Medicine: New Tricks from an Old Dog?: Fetal Membranes in Regenerative Medicine. STEM CELLS Transl. Med. 2017, 6, 1767–1776. [Google Scholar] [CrossRef]
- Trowsdale, J.; Betz, A.G. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nat. Immunol. 2006, 7, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Caruso, M.; Evangelista, M.; Parolini, O. Human term placental cells: Phenotype, properties and new avenues in regenerative medicine. Int. J. Mol. Cell Med. 2012, 1, 64–74. [Google Scholar]
- Migliaccio, A.R.; Martelli, F.; Verrucci, M.; Sanchez, M.; Valeri, M.; Migliaccio, G.; Vannucchi, A.M.; Zingariello, M.; Di Baldassarre, A.; Ghinassi, B.; et al. Gata1 expression driven by the alternative HS2 enhancer in the spleen rescues the hematopoietic failure induced by the hypomorphic Gata1low mutation. Blood 2009, 114, 2107–2120. [Google Scholar] [CrossRef]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef]
- Tamagawa, T.; Ishiwata, I.; Saito, S. Establishment and Characterization of a Pluripotent Stem Cell line Derived from Human Amniotic Membranes and Initiation of Germ Layers in vitro. Hum. Cell 2008, 17, 125–130. [Google Scholar] [CrossRef]
- Okere, B.; Alviano, F.; Costa, R.; Quaglino, D.; Ricci, F.; Dominici, M.; Paolucci, P.; Bonsi, L.; Iughetti, L. In vitro differentiation of human amniotic epithelial cells into insulin-producing 3D spheroids. Int. J. Immunopathol. Pharmacol. 2015, 28, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Balaji, S.; Zhou, Y.; Opara, E.C.; Soker, S. Combinations of Activin A or Nicotinamide with the Pancreatic Transcription Factor PDX1 Support Differentiation of Human Amnion Epithelial Cells Toward a Pancreatic Lineage. Cell. Reprogram. 2017, 19, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Maymó, J.L.; Riedel, R.; Pérez-Pérez, A.; Magatti, M.; Maskin, B.; Dueñas, J.L.; Parolini, O.; Sánchez-Margalet, V.; Varone, C.L. Proliferation and survival of human amniotic epithelial cells during their hepatic differentiation. PLoS ONE 2018, 13, e0191489. [Google Scholar] [CrossRef] [PubMed]
- Toda, A.; Okabe, M.; Yoshida, T.; Nikaido, T. The Potential of Amniotic Membrane/Amnion-Derived Cells for Regeneration of Various Tissues. J. Pharmacol. Sci. 2007, 105, 215–228. [Google Scholar] [CrossRef]
- Jiawen, S.; Jianjun, Z.; Jiewen, D.; Dedong, Y.; Hongbo, Y.; Jun, S.; Xudong, W.; Shen, S.G.F.; Lihe, G. Osteogenic Differentiation of Human Amniotic Epithelial Cells and Its Application in Alveolar Defect Restoration: Osteogenesis of hAECs In Vitro and In Vivo. STEM CELLS Transl. Med. 2014, 3, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Koike, C.; Zhou, K.; Takeda, Y.; Fathy, M.; Okabe, M.; Yoshida, T.; Nakamura, Y.; Kato, Y.; Nikaido, T. Characterization of Amniotic Stem Cells. Cell. Reprogram. 2014, 16, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.; Kim, Y.; Kim, M.; Kim, J.; Choi, H.; Jekarl, D.W.; Lee, S.; Kim, J.M.; Shin, J.-C.; Park, I.Y. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci. Rep. 2016, 6, 23544. [Google Scholar] [CrossRef]
- Magaña-Guerrero, F.S.; Domínguez-López, A.; Martínez-Aboytes, P.; Buentello-Volante, B.; Garfias, Y. Human Amniotic Membrane Mesenchymal Stem Cells inhibit Neutrophil Extracellular Traps through TSG-6. Sci. Rep. 2017, 7, 12426. [Google Scholar] [CrossRef]
- D’amico, M.A.; Ghinassi, B.; Izzicupo, P.; Di Ruscio, A.; Di Baldassarre, A. IL-6 Activates PI3K and PKCζ Signaling and Determines Cardiac Differentiation in Rat Embryonic H9c2 Cells: IL-6 and cardiac differentiation of H9c2 cells. J. Cell. Physiol. 2016, 231, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, G.; Spoldi, V.; Silini, A.; Parolini, O. Current View on Osteogenic Differentiation Potential of Mesenchymal Stromal Cells Derived from Placental Tissues. Stem Cell Rev. Rep. 2015, 11, 570–585. [Google Scholar] [CrossRef]
- Choi, Y.S.; Park, Y.-B.; Ha, C.-W.; Kim, J.A.; Heo, J.-C.; Han, W.-J.; Oh, S.-Y.; Choi, S.-J. Different characteristics of mesenchymal stem cells isolated from different layers of full term placenta. PLoS ONE 2017, 12, e0172642. [Google Scholar] [CrossRef]
- Witkowska-Zimny, M.; Wrobel, E. Perinatal sources of mesenchymal stem cells: Wharton’s jelly, amnion and chorion. Cell. Mol. Biol. Lett. 2011, 16, 493–514. [Google Scholar] [CrossRef]
- Ali, H.; Al-Yatama, M.K.; Abu-Farha, M.; Behbehani, K.; Al Madhoun, A. Multi-Lineage Differentiation of Human Umbilical Cord Wharton’s Jelly Mesenchymal Stromal Cells Mediates Changes in the Expression Profile of Stemness Markers. PLoS ONE 2015, 10, e0122465. [Google Scholar] [CrossRef]
- Rutigliano, L.; Corradetti, B.; Valentini, L.; Bizzaro, D.; Meucci, A.; Cremonesi, F.; Lange-Consiglio, A. Molecular characterization and in vitro differentiation of feline progenitor-like amniotic epithelial cells. Stem Cell Res. Ther. 2013, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.-H.; Jin, J.; Joe, J.-H.; Song, Y.-S.; So, B.-I.; Lim, S.M.; Cheon, G.J.; Woo, S.-K.; Ra, J.-C.; Lee, Y.-Y.; et al. In Vivo Differentiation of Human Amniotic Epithelial Cells into Cardiomyocyte-Like Cells and Cell Transplantation Effect on Myocardial Infarction in Rats: Comparison with Cord Blood and Adipose Tissue-Derived Mesenchymal Stem Cells. Cell Transplant. 2012, 21, 1687–1696. [Google Scholar] [CrossRef]
- Fanti, M.; Gramignoli, R.; Serra, M.; Cadoni, E.; Strom, S.C.; Marongiu, F. Differentiation of amniotic epithelial cells into various liver cell types and potential therapeutic applications. Placenta 2017, 59, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Nogami, M.; Tsuno, H.; Koike, C.; Okabe, M.; Yoshida, T.; Seki, S.; Matsui, Y.; Kimura, T.; Nikaido, T. Isolation and Characterization of Human Amniotic Mesenchymal Stem Cells and Their Chondrogenic Differentiation. Transplant. J. 2012, 93, 1221–1228. [Google Scholar] [CrossRef]
- Díaz-Prado, S.; Muiños-López, E.; Hermida-Gómez, T.; Rendal-Vázquez, M.E.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Multilineage differentiation potential of cells isolated from the human amniotic membrane. J. Cell. Biochem. 2010, 111, 846–857. [Google Scholar] [CrossRef]
- Raynaud, C.M.; Maleki, M.; Lis, R.; Ahmed, B.; Al-Azwani, I.; Malek, J.; Safadi, F.F.; Rafii, A. Comprehensive Characterization of Mesenchymal Stem Cells from Human Placenta and Fetal Membrane and Their Response to Osteoactivin Stimulation. Stem Cells Int. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Ventura, C.; Cantoni, S.; Bianchi, F.; Lionetti, V.; Cavallini, C.; Scarlata, I.; Foroni, L.; Maioli, M.; Bonsi, L.; Alviano, F.; et al. Hyaluronan Mixed Esters of Butyric and Retinoic Acid Drive Cardiac and Endothelial Fate in Term Placenta Human Mesenchymal Stem Cells and Enhance Cardiac Repair in Infarcted Rat Hearts. J. Biol. Chem. 2007, 282, 14243–14252. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Yao, R.; Tuan, R.S. Comparison of Proliferative and Multilineage Differentiation Potential of Human Mesenchymal Stem Cells Derived from Umbilical Cord and Bone Marrow. Stem Cells 2007, 25, 1384–1392. [Google Scholar] [CrossRef]
- Schneider, R.K.; Puellen, A.; Kramann, R.; Raupach, K.; Bornemann, J.; Knuechel, R.; Pérez-Bouza, A.; Neuss, S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials 2010, 31, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Nimsanor, N.; Phetfong, J.; Plabplueng, C.; Jangpatarapongsa, K.; Prachayasittikul, V.; Supokawej, A. Inhibitory effect of oxidative damage on cardiomyocyte differentiation from Wharton’s jelly-derived mesenchymal stem cells. Exp. Ther. Med. 2017, 14, 5329–5338. [Google Scholar] [CrossRef]
- Mitchell, K.E.; Weiss, M.L.; Mitchell, B.M.; Martin, P.; Davis, D.; Morales, L.; Helwig, B.; Beerenstrauch, M.; Abou-Easa, K.; Hildreth, T.; et al. Matrix Cells from Wharton’s Jelly Form Neurons and Glia. Stem Cells 2003, 21, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human Umbilical Cord Matrix Stem Cells: Preliminary Characterization and Effect of Transplantation in a Rodent Model of Parkinson’s Disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Raut, A.; Khanna, A. High-throughput sequencing to identify microRNA signatures during hepatic differentiation of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells: MicroRNA signatures during hepatic differentiation. Hepatol. Res. 2017, 47, 910–927. [Google Scholar] [CrossRef] [PubMed]
- Belame Shivakumar, S.; Bharti, D.; Baregundi Subbarao, R.; Park, J.-M.; Son, Y.-B.; Ullah, I.; Choe, Y.-H.; Lee, H.-J.; Park, B.-W.; Lee, S.-L.; et al. Pancreatic endocrine-like cells differentiated from human umbilical cords Wharton’s jelly mesenchymal stem cells using small molecules. J. Cell. Physiol. 2019, 234, 3933–3947. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, J.; Cui, H.; Wang, X.; Rong, H.; Shao, B.; Cui, H. Wharton’s jelly mesenchymal stem cells differentiate into retinal progenitor cells. Neural Regen. Res. 2013, 8, 1783–1792. [Google Scholar]
- Arnhold, S.; Glüer, S.; Hartmann, K.; Raabe, O.; Addicks, K.; Wenisch, S.; Hoopmann, M. Amniotic-Fluid Stem Cells: Growth Dynamics and Differentiation Potential after a CD-117-Based Selection Procedure. Stem Cells Int. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Glemžaitė, M.; Navakauskienė, R. Osteogenic Differentiation of Human Amniotic Fluid Mesenchymal Stem Cells Is Determined by Epigenetic Changes. Stem Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Di Baldassarre, A.; D’Amico, M.A.; Izzicupo, P.; Gaggi, G.; Guarnieri, S.; Mariggiò, M.A.; Antonucci, I.; Corneo, B.; Sirabella, D.; Stuppia, L.; et al. Cardiomyocytes Derived from Human CardiopoieticAmniotic Fluids. Sci. Rep. 2018, 8, 12028. [Google Scholar] [CrossRef]
- Maraldi, T.; Bertoni, L.; Riccio, M.; Zavatti, M.; Carnevale, G.; Resca, E.; Guida, M.; Beretti, F.; La Sala, G.B.; De Pol, A. Human amniotic fluid stem cells: Neural differentiation in vitro and in vivo. Cell Tissue Res. 2014, 357, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, D.; Li, B.; Guan, L.; Yan, Z.; Li, Y.; Pei, X.; Yue, W.; Wang, M.; Lu, Y.; et al. Human amniotic fluid-derived stem cells can differentiate into hepatocyte-like cells in vitro and in vivo. In Vitro Cell. Dev. Biol. Anim. 2011, 47, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, G.; Riccio, M.; Pisciotta, A.; Beretti, F.; Maraldi, T.; Zavatti, M.; Cavallini, G.M.; La Sala, G.B.; Ferrari, A.; De Pol, A. In vitro differentiation into insulin-producing β-cells of stem cells isolated from human amniotic fluid and dental pulp. Dig. Liver Dis. 2013, 45, 669–676. [Google Scholar] [CrossRef]
- Mu, X.-P.; Ren, L.-Q.; Yan, H.-W.; Zhang, X.-M.; Xu, T.-M.; Wei, A.-H.; Jiang, J.-L. Enhanced differentiation of human amniotic fluid-derived stem cells into insulin-producing cells in vitro. J. Diabetes Investig. 2017, 8, 34–43. [Google Scholar] [CrossRef]
- Troyer, D.L.; Weiss, M.L. Concise Review: Wharton’s Jelly-Derived Cells Are a Primitive Stromal Cell Population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Seyedjafari, E.; Zargar, S.J.; Birhanu, G.; Zandi-Karimi, A.; Beiki, B.; Tuzlakoglu, K. Osteogenic differentiation of mesenchymal stem cells cultured on PLLA scaffold coated with Wharton’s jelly. EXCLI J. 2017, 6, 785–794. [Google Scholar]
- Balbi, C.; Piccoli, M.; Barile, L.; Papait, A.; Armirotti, A.; Principi, E.; Reverberi, D.; Pascucci, L.; Becherini, P.; Varesio, L.; et al. First Characterization of Human Amniotic Fluid Stem Cell Extracellular Vesicles as a Powerful Paracrine Tool Endowed with Regenerative Potential: Amniotic Fluid Stem Cell Extracellular Vesicles. STEM CELLS Transl. Med. 2017, 6, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-S.; Joo, H.-W.; Park, I.-H.; Shen, G.-Y.; Lee, Y.; Shin, J.H.; Kim, H.; Shin, I.-S.; Kim, K.-S. Transplanted Human Amniotic Epithelial Cells Secrete Paracrine Proangiogenic Cytokines in Rat Model of Myocardial Infarctio. Cell Transplant. 2015, 24, 2055–2064. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, F.; Yang, D.; Wang, J.; Qiu, L.; Pang, X. Human amniotic epithelial cells regulate osteoblast differentiation through the secretion of TGFβ1 and microRNA-34a-5p. Int. J. Mol. Med. 2017, 41, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Lie, P.; Miao, T.; Yu, M.; Lu, Q.; Feng, T.; Li, J.; Zu, T.; Liu, X.; Li, H. Conditioned medium from umbilical cord mesenchymal stem cells induces migration and angiogenesis. Mol. Med. Rep. 2015, 12, 20–30. [Google Scholar] [CrossRef]
- Zhang, X.; Tu, H.; Yang, Y.; Fang, L.; Wu, Q.; Li, J. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Roles in Tumor Growth, Progression, and Drug Resistance. Stem Cells Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Meng, X.; Sun, B.; Xue, M.; Xu, P.; Hu, F.; Xiao, Z. Comparative analysis of microRNA expression in human mesenchymal stem cells from umbilical cord and cord blood. Genomics 2016, 107, 124–131. [Google Scholar] [CrossRef]
- Hauser, P.V.; Fazio, R.D.; Bruno, S.; Sdei, S.; Grange, C.; Bussolati, B.; Benedetto, C.; Camussi, G. Stem Cells Derived from Human Amniotic Fluid Contribute to Acute Kidney Injury Recovery. Am. J. Pathol. 2010, 177, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, X.; Shi, X.; Shi, X.; Zhang, W.; Wu, G.; Wang, X.; Su, L.; Hu, D. Exosomal MicroRNAs Derived from Human Amniotic Epithelial Cells Accelerate Wound Healing by Promoting the Proliferation and Migration of Fibroblasts. Stem Cells Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhang, G.; Cheng, Z.; Yin, D.; Du, T.; Ju, G.; Miao, S.; Liu, G.; Lu, M.; Zhu, Y. Microvesicles derived from human Wharton’s Jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res. Ther. 2014, 5, 40. [Google Scholar] [CrossRef]
- Dong, L.; Hao, H.; Liu, J.; Ti, D.; Tong, C.; Hou, Q.; Li, M.; Zheng, J.; Liu, G.; Fu, X.; et al. A Conditioned Medium of Umbilical Cord Mesenchymal Stem Cells Overexpressing Wnt7a Promotes Wound Repair and Regeneration of Hair Follicles in Mice. Stem Cells Int. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering: Decellularized scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Yesmin, S.; Paget, M.B.; Murray, H.E.; Downing, R. Bio-scaffolds in organ-regeneration: Clinical potential and current challenges. Curr. Res. Transl. Med. 2017, 65, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Jerman, U.D.; Veranič, P.; Kreft, M.E. Amniotic Membrane Scaffolds Enable the Development of Tissue-Engineered Urothelium with Molecular and Ultrastructural Properties Comparable to that of Native Urothelium. Tissue Eng. Part C Methods 2014, 20, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Dorazehi, F.; Nabiuni, M.; Jalali, H. Potential Use of Amniotic Membrane Derived Scaffold for Cerebrospinal Fluid Applications. Int. J. Mol. Cell. Med. 2018, 7, 91–101. [Google Scholar]
- Zelen, C.M.; Serena, T.E.; Fetterolf, D.E. Dehydrated human amnion/chorion membrane allografts in patients with chronic diabetic foot ulcers: A long-term follow-up study. Wound Med. 2014, 4, 1–4. [Google Scholar] [CrossRef]
- Lei, J.; Priddy, L.B.; Lim, J.J.; Massee, M.; Koob, T.J. Identification of Extracellular Matrix Components and Biological Factors in Micronized Dehydrated Human Amnion/Chorion Membrane. Adv. Wound Care 2017, 6, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Prado, S.; Rendal-Vázquez, M.E.; Muiños-López, E.; Hermida-Gómez, T.; Rodríguez-Cabarcos, M.; Fuentes-Boquete, I.; de Toro, F.J.; Blanco, F.J. Potential use of the human amniotic membrane as a scaffold in human articular cartilage repair. Cell Tissue Bank. 2010, 11, 183–195. [Google Scholar] [CrossRef]
- Francisco, J.C.; Correa Cunha, R.; Cardoso, M.A.; Baggio Simeoni, R.; Mogharbel, B.F.; Picharski, G.L.; Silva Moreira Dziedzic, D.; Guarita-Souza, L.C.; Carvalho, K.A.T. Decellularized Amniotic Membrane Scaffold as a Pericardial Substitute: An In Vivo Study. Transplant. Proceed. 2016, 48, 2845–2849. [Google Scholar] [CrossRef] [PubMed]
- Beiki, B.; Zeynali, B.; Seyedjafari, E. Fabrication of a three dimensional spongy scaffold using human Wharton’s jelly derived extra cellular matrix for wound healing. Mater. Sci. Eng. C 2017, 78, 627–638. [Google Scholar] [CrossRef]
| Cell Type | Phenotype | Morphology | ||
|---|---|---|---|---|
| Markers | References | |||
| hAECs | Embryonic cells | Nanog | [1,7] |  |
| OCT-4 | ||||
| SOX-2 | ||||
| Pluripotency | Tra1-60 | |||
| Tra1-81 | ||||
| SSEA3 | ||||
| SSEA4 | ||||
| Mesenchymal | CD24 | |||
| Endothelial cells | E-cadherin | |||
| Integrin α6 | ||||
| Integrin β1 | ||||
| Immune cells | CD9 | |||
| hAMSCs | Embryonic cells | OCT-3 | [28,29] |  |
| OCT-4 | ||||
| Klf4 | ||||
| c-myc | ||||
| Pluripotency | Tra1-60 | |||
| Tra1-81 | ||||
| SSEA3 | ||||
| SSEA4 | ||||
| Mesenchymal | CD90 | |||
| CD24 | ||||
| Vimentin | ||||
| CD73 | ||||
| Hematopoietic | CD44 | |||
| CD105 | ||||
| Muscle tissue | Desmin | |||
| hCMSCs | Embryonic cells | OCT-3 | [7] |  |
| Pluripotency | SSEA3/4 | |||
| REX1 | ||||
| Mesenchymal | CD90 | |||
| CD73 | ||||
| CD29 | ||||
| Hematopoietic | CD44 | |||
| CD105 | ||||
| CD19 | ||||
| hWJ-SCs | Embryonic cells | Nanog | [34,35] |  |
| OCT-3 | ||||
| OCT-4 | ||||
| SOX-2 | ||||
| Mesenchymal | CD90 | |||
| CD73 | ||||
| Hematopoietic | CD105 | |||
| hAFSCs | Embryonic cells | Nanog | [5,13] |  |
| OCT-4 | ||||
| Klf4 | ||||
| SOX-2 | ||||
| Pluripotency | Stella | |||
| Fragilis | ||||
| Ovol1 | ||||
| SSEA4 | ||||
| c-myc | ||||
| c-kit | ||||
| Mesenchymal | CD90 | |||
| CD73 | ||||
| Hematopoietic | CD105 | |||
| Cell Type | Differentiative Potential | |||||||
|---|---|---|---|---|---|---|---|---|
| Chondrogenic | Adipogenic | Osteogenic | Cardiac | Neuronal | Hepatic | Pancreatic | Retinal | |
| hAECs | [26,36] | [36] | [27] | [37] | [36] | [25,26,38] | [23,24] | |
| hAMSCs | [39] | [33,40] | [41] | [26,42] | ||||
| hCMSCs | [33] | [33] | [32] | |||||
| hWJ-SCs | [34,43] | [43] | [43,44] | [45] | [34,46,47] | [48] | [49] | [50] |
| hAFSCs | [11,51] | [11,51] | [11,51,52] | [53] | [54] | [55] | [56,57] | |
| Cell Type | Secretome | Ref. | |
|---|---|---|---|
| hAECs | Cytokines | ANG | [17,62] |
| EFG | |||
| IL-6 | |||
| MCP-1 | |||
| Dopamin | |||
| NGF | |||
| Neurotrophin-3 | |||
| BDNF | |||
| miRNAs | miR-34a-5p | [63] | |
| hWJ-SCs | Cytokines | EGF | [64,65] |
| IP-10 | |||
| ANG | |||
| BDNF | |||
| SDF-1 | |||
| IGF | |||
| TGF-α | |||
| TGF-β | |||
| HGF | |||
| VEGF | |||
| VEGF-R2 | |||
| FGF-b | |||
| PDGF-BB | |||
| IL-8 | |||
| IL-6 | |||
| IL-10 | |||
| VCAM-1 | |||
| MCP-1 | |||
| SCF | |||
| miRNAs | miR-7-5p | [66] | |
| miR-10a-5p | |||
| miR-99a-5p | |||
| miR-100-5p | |||
| miR142-3p | |||
| miR-144-3p | |||
| miR-196a-5p | |||
| miR-452-5p | |||
| hAFSCs | Cytokines | SFD-1a | [67] |
| PDGF-BB | |||
| VEGF | |||
| LIF | |||
| B-NGF | |||
| SCF | |||
| FGF-b | |||
| HGF | |||
| miRNA | miR-223 | [60] | |
| miR-146a | |||
| miR-let 7c | |||
| miR-21 | |||
| miR-126 | |||
| miR146b | |||
| miR-199a-3p | |||
| miR2-210 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaggi, G.; Izzicupo, P.; Di Credico, A.; Sancilio, S.; Di Baldassarre, A.; Ghinassi, B. Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1573. https://doi.org/10.3390/ijms20071573
Gaggi G, Izzicupo P, Di Credico A, Sancilio S, Di Baldassarre A, Ghinassi B. Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine. International Journal of Molecular Sciences. 2019; 20(7):1573. https://doi.org/10.3390/ijms20071573
Chicago/Turabian StyleGaggi, Giulia, Pascal Izzicupo, Andrea Di Credico, Silvia Sancilio, Angela Di Baldassarre, and Barbara Ghinassi. 2019. "Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine" International Journal of Molecular Sciences 20, no. 7: 1573. https://doi.org/10.3390/ijms20071573
APA StyleGaggi, G., Izzicupo, P., Di Credico, A., Sancilio, S., Di Baldassarre, A., & Ghinassi, B. (2019). Spare Parts from Discarded Materials: Fetal Annexes in Regenerative Medicine. International Journal of Molecular Sciences, 20(7), 1573. https://doi.org/10.3390/ijms20071573