High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus
Abstract
1. Introduction
2. Results
2.1. Effects of High Protein Diet on Body Weight and Plasma Metabolic Parameters
2.2. Non-Enzymatic and Enzymatic Antioxidants, Total Antioxidant/Oxidant Status, and Oxidative Damage Product Level in Plasma/Serum
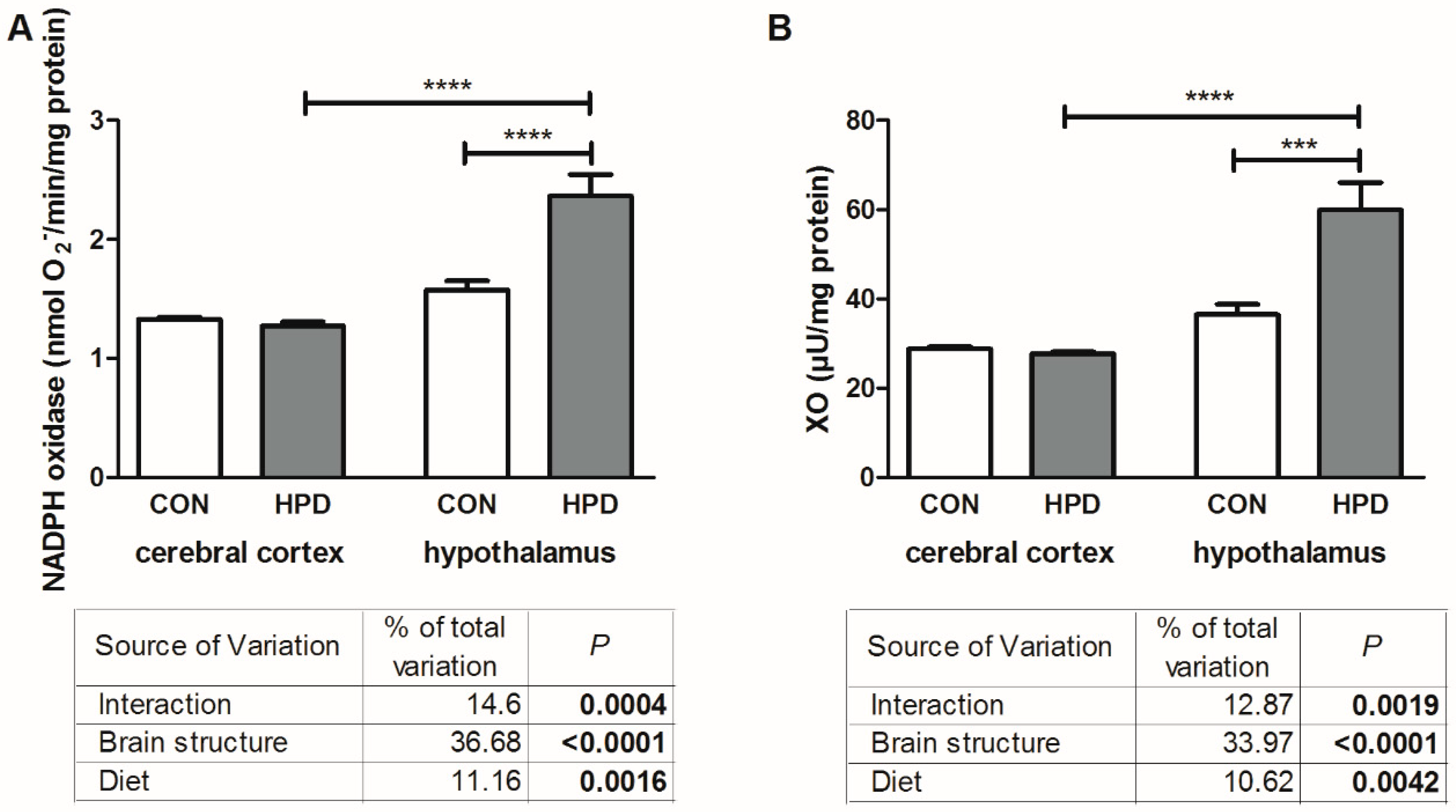
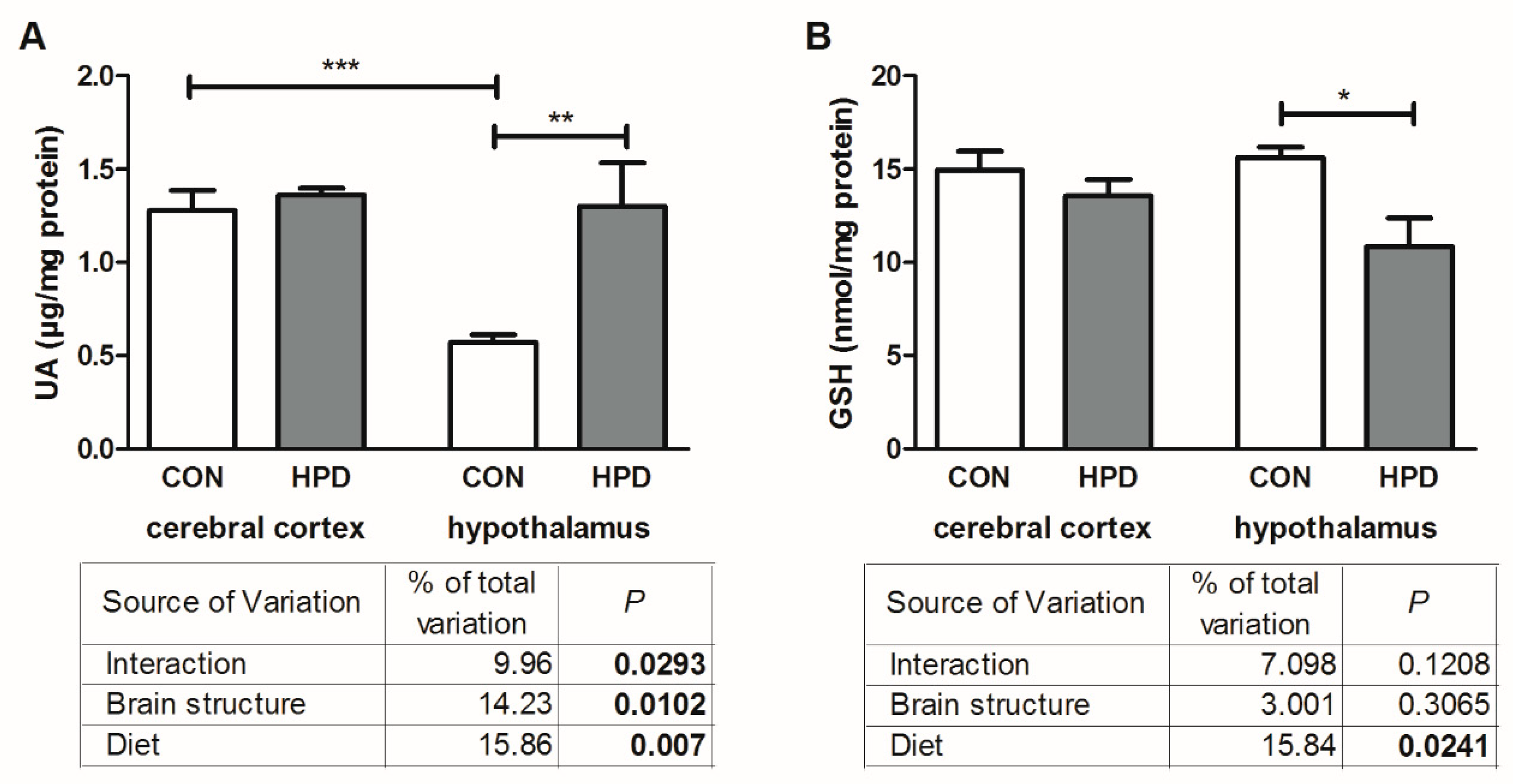
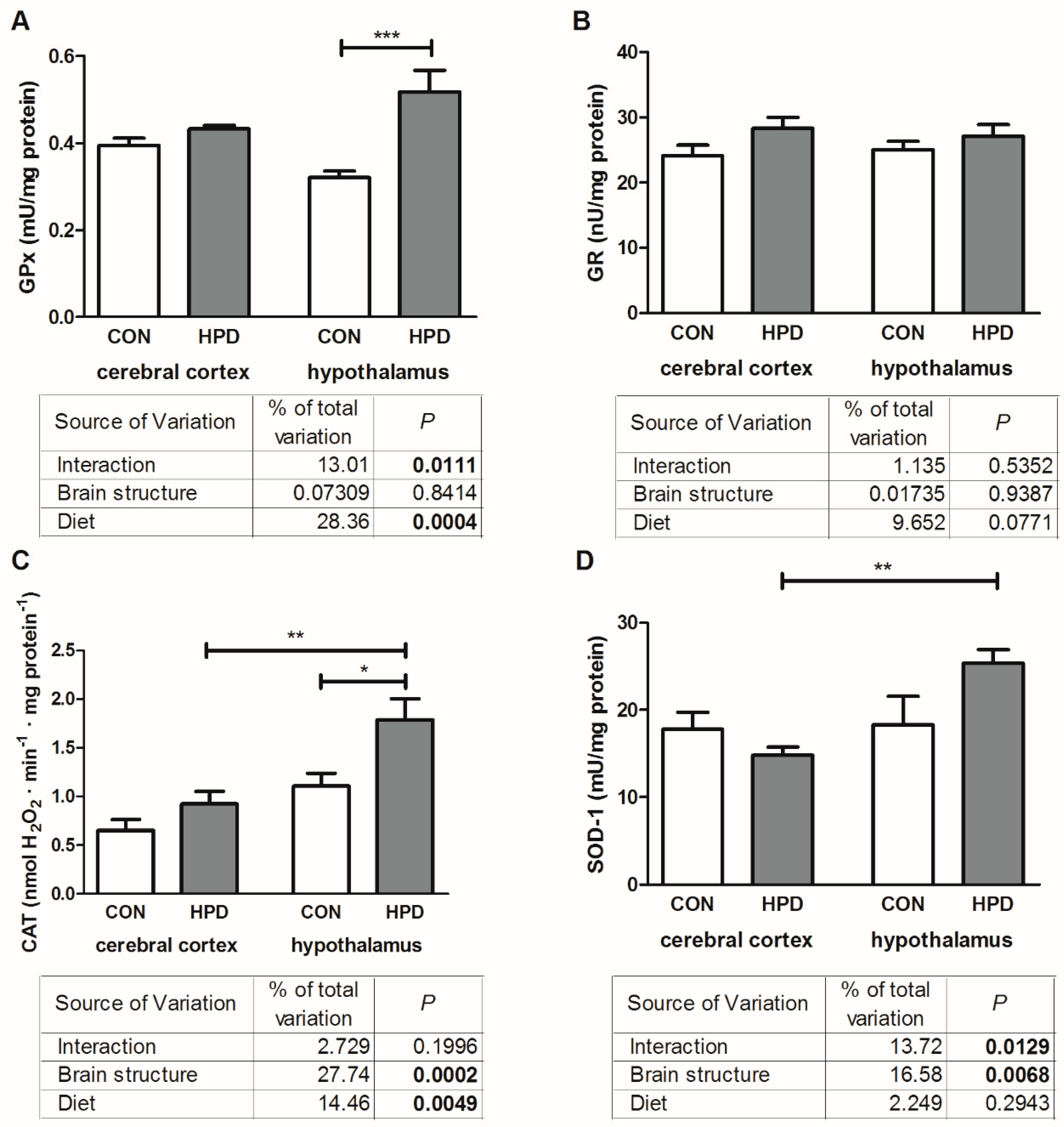
2.3. Pro-Oxidant Enzymes and Antioxidants in Cerebral Cortex and Hypothalamus
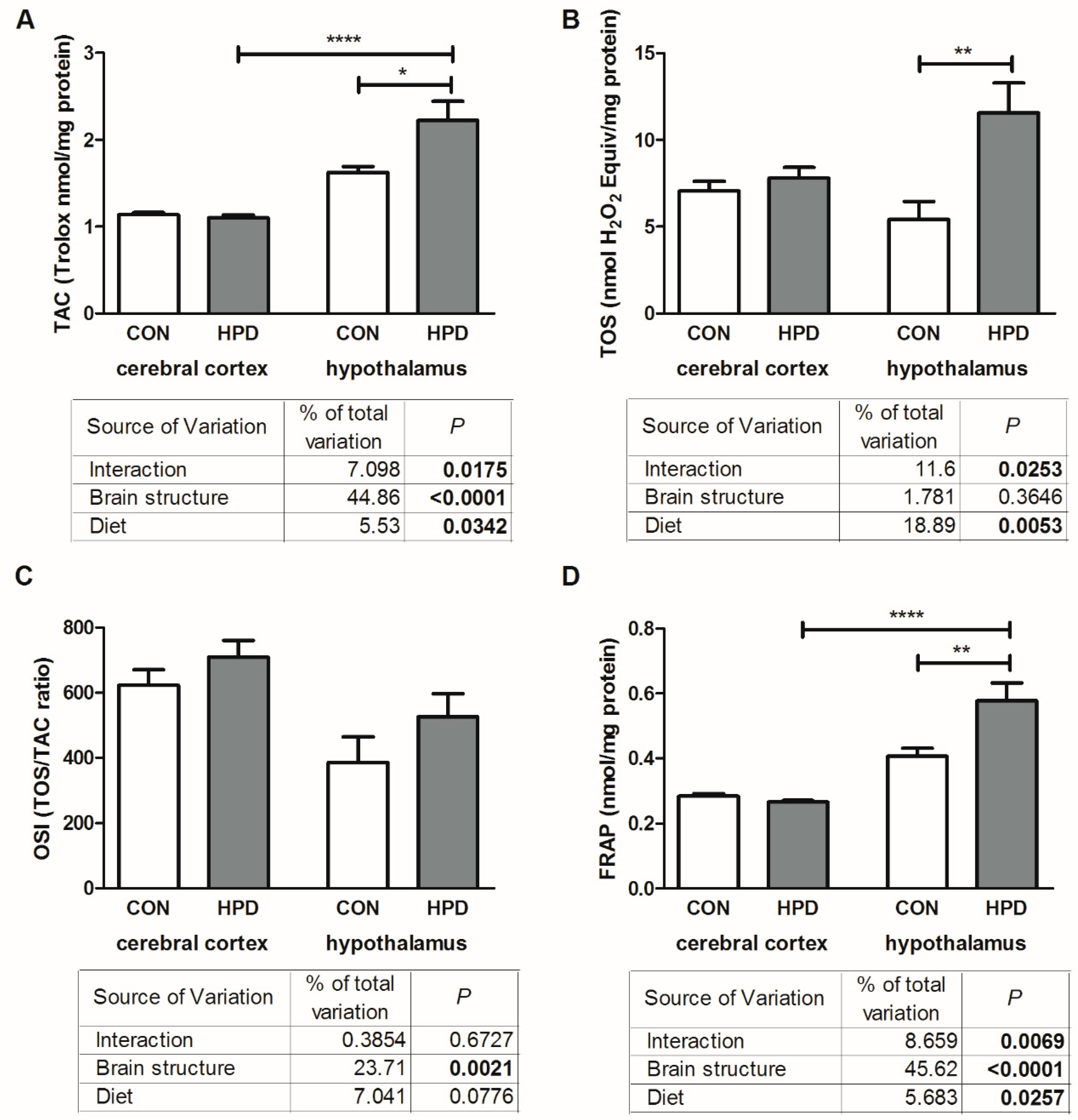
2.4. Total Antioxidant/Oxidant Status in Cerebral Cortex and Hypothalamus
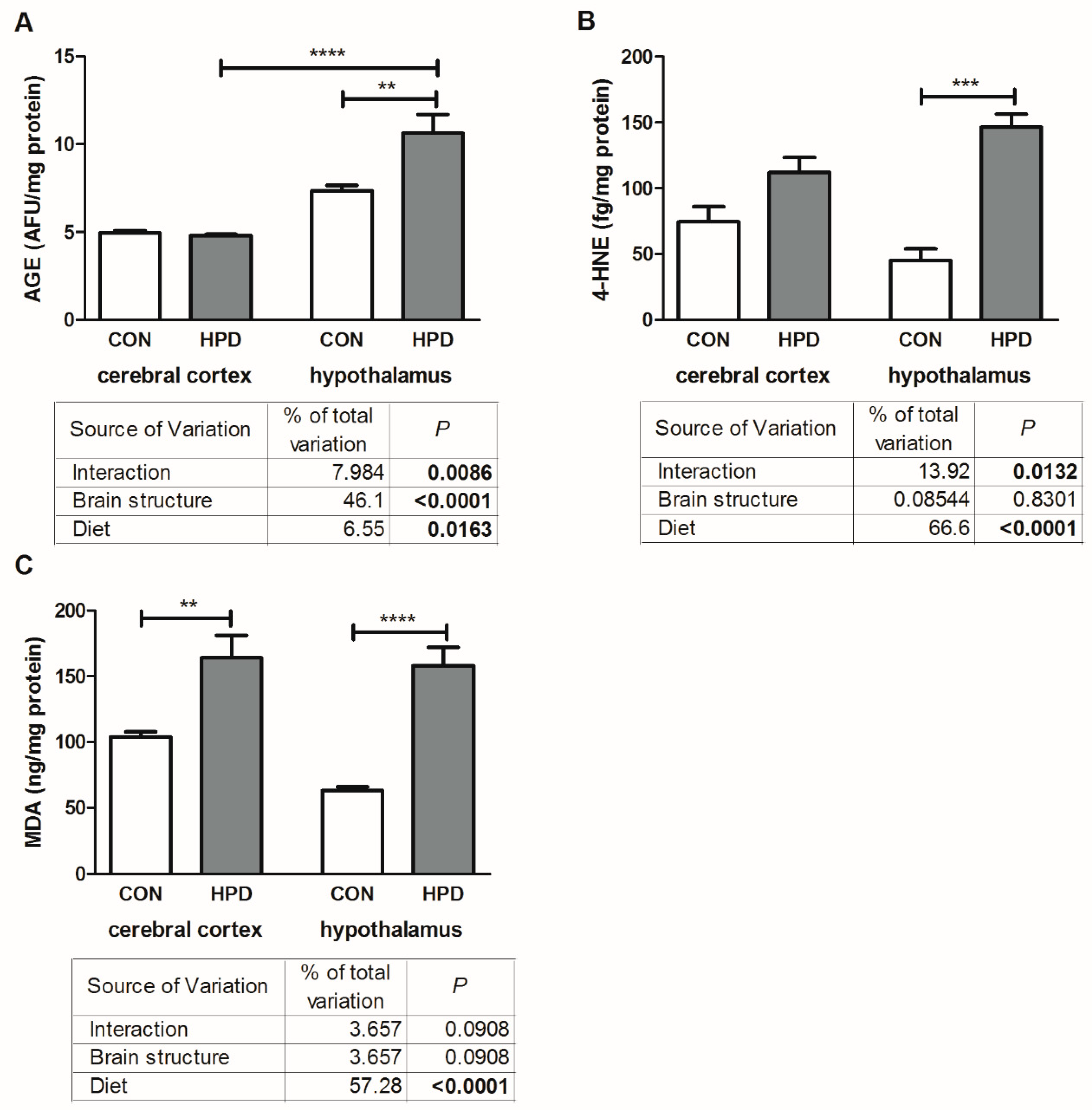
2.5. Oxidative Damage Products in Cerebral Cortex and Hypothalamus
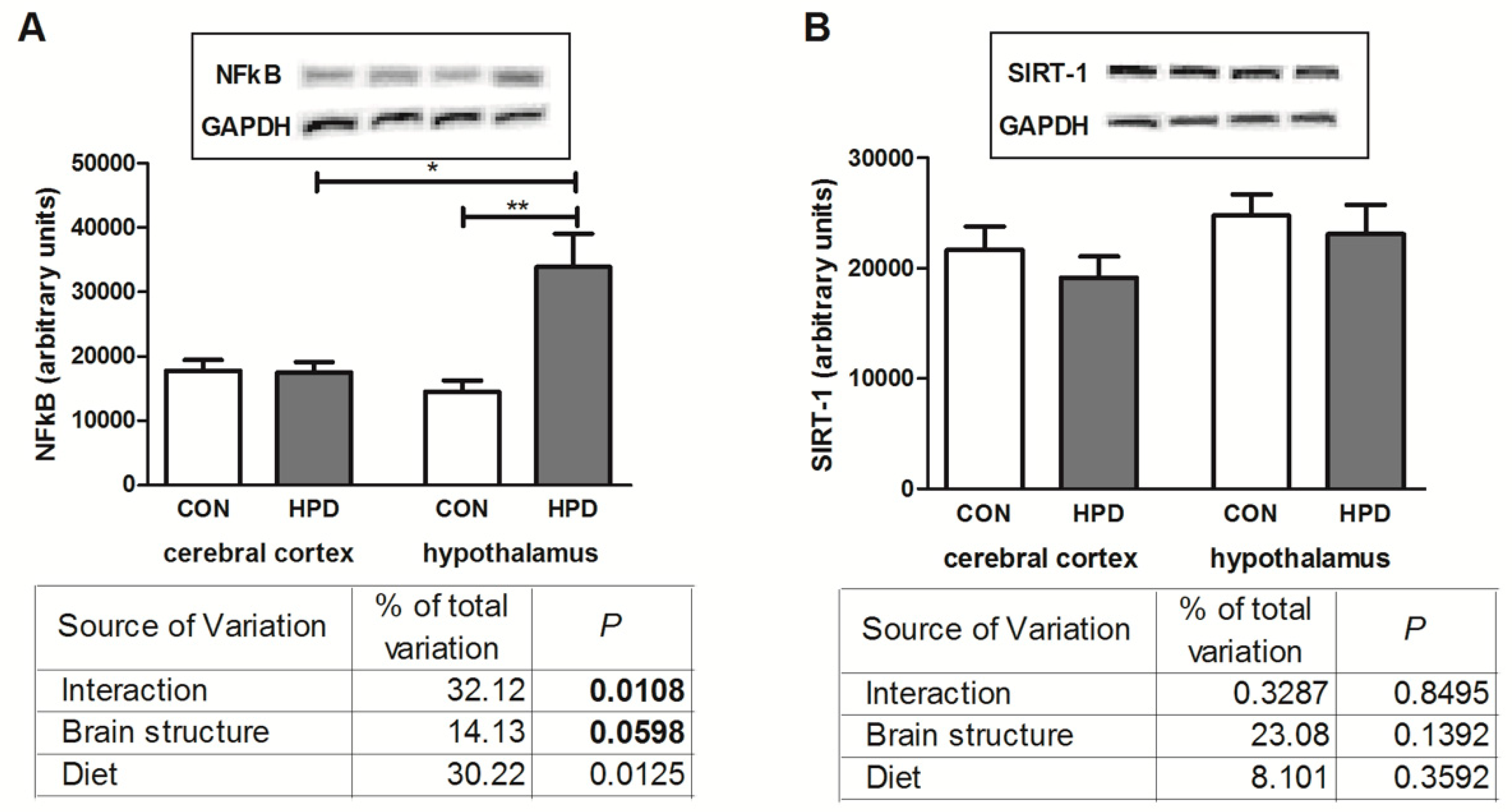
2.6. NFκB Expression and SIRT-1
3. Discussion
4. Materials and Methods
4.1. Plasma Insulin, Glucose, Adiponectin, and Leptin Concentrations
4.2. Pro-Oxidant Enzymes and Antioxidants in Cerebral Cortex and Hypothalamus
4.3. Total Antioxidant/Oxidant Status
4.4. Oxidative Modification Products
4.5. Western Blot Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynonenal |
| AGE | advanced glycation end products |
| BBB | blood–brain barrier |
| BCA | bicinchoninic acid |
| BCAA | branched-chain amino acids |
| BHT | butylated hydroxytoluene |
| CAT | catalase |
| CNS | central nervous system |
| FRAP | ferric reducing ability of plasma |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GPx | glutathione peroxidase |
| GR | glutathione reductase |
| GSH | reduced glutathione |
| HOMA-IR | homeostatic model assessment of β-cell function and insulin resistance |
| HPD | high protein diet |
| MDA | malondialdehyde |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NFκB | nuclear factor-κB |
| OS | oxidative stress |
| OSI | oxidative stress index |
| PBS | phosphate buffered saline |
| PUFAs | polyunsaturated fatty acids |
| RAGE | receptor for advanced glycation end products |
| ROS | reactive oxygen species |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| SIRT-1 | sirtuin 1 |
| SOD-1 | superoxide dismutase-1 |
| TAC | total antioxidant capacity |
| TBARS | thiobarbituric acid reactive substances |
| TNF-alpha | tumor necrosis factor alpha |
| TOS | total oxidant status |
| UA | uric acid |
| WHO | World Health Organization |
References
- Keller, U. Dietary proteins in obesity and in diabetes. Int. J. Vitam. Nutr. Res. 2011, 81, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Raben, A.; Geiker, N. The role of higher protein diets in weight control and obesity-related comorbidities. Int. J. Obes. 2015, 39, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Halton, T.L.; Hu, F.B. The effects of high protein diets on thermogenesis, satiety and weight loss: A critical review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- WHO. Energy and Protein Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation (724); WHO: Geneva, Switzerland, 1985. [Google Scholar]
- Metges, C.C.; Barth, C.A. Metabolic consequences of a high dietary-protein intake in adulthood: Assessment of the available evidence. J. Nutr. 2000, 130, 886–889. [Google Scholar] [CrossRef]
- Delimaris, I. Adverse effects associated with protein intake above the recommended dietary allowance for adults. ISRN Nutr. 2013, 2013, 126929. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Petzke, K.J.; Proll, J.; Brückner, J.; Metges, C.C. Plasma protein carbonyl concentration is not enhanced by chronic intake of high-protein diets in adult rats. J. Nutr. Biochem. 1999, 10, 268–273. [Google Scholar] [CrossRef]
- Gu, C.; Shi, Y.; Le, G. Effect of dietary protein level and origin on the redox status in the digestive tract of mice. Int. J. Mol. Sci. 2008, 9, 464–475. [Google Scholar] [CrossRef]
- Gu, C.; Xu, H. Effect of oxidative damage due to excessive protein ingestion on pancreas function in mice. Int. J. Mol. Sci. 2010, 11, 4591–4600. [Google Scholar] [CrossRef]
- Kołodziej, U.; Maciejczyk, M.; Niklińska, W.; Waszkiel, D.; Żendzian-Piotrowska, M.; Żukowski, P.; Zalewska, A. Chronic high-protein diet induces oxidative stress and alters the salivary gland function in rats. Arch. Oral Biol. 2017, 84, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Journel, M.; Chaumontet, C.; Darcel, N.; Fromentin, G.; Tomé, D. Brain responses to high-protein diets. Adv. Nutr. 2012, 3, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lovekamp-Swan, T.; Glendenning, M.L.; Schreihofer, D.A. A high soy diet enhances neurotropin receptor and Bcl-XLgene expression in the brains of ovariectomized female rats. Brain Res. 2007, 1159, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Méndez-López, M.; Méndez, M.; Arias, J.; Arias, J.L. Effects of a high protein diet on cognition and brain metabolism in cirrhotic rats. Physiol. Behav. 2015, 149, 220–228. [Google Scholar] [CrossRef]
- Pedrini, S.; Thomas, C.; Brautigam, H.; Schmeidler, J.; Ho, L.; Fraser, P.; Westaway, D.; Hyslop, P.S.G.; Martins, R.N.; Buxbaum, J.D.; et al. Dietary composition modulates brain mass and solubilizable A levels in a mouse model of aggressive Alzheimer’s amyloid pathology. Mol. Neurodegener. 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Michaelis, E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Camiletti-Moirón, D.; Aparicio, V.A.; Aranda, P.; Radak, Z. Does exercise reduce brain oxidative stress? A systematic review. Scand. J. Med. Sci. Sport. 2013, 23, 202–212. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Orlacchio, A.; Maccarrone, M. Is modulation of oxidative stress an answer? the state of the art of redox therapeutic actions in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Maciejczyk, M.; Żebrowska, E.; Zalewska, A.; Chabowski, A. Redox Balance, Antioxidant Defense, and Oxidative Damage in the Hypothalamus and Cerebral Cortex of Rats with High Fat Diet-Induced Insulin Resistance. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Mandal, P.K.; Tripathi, M.; Sugunan, S. Brain oxidative stress: Detection and mapping of anti-oxidant marker “Glutathione” in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem. Biophys. Res. Commun. 2012, 417, 43–48. [Google Scholar] [CrossRef]
- Choromańska, M.; Klimiuk, A.; Kostecka-Sochoń, P.; Wilczyńska, K.; Kwiatkowski, M.; Okuniewska, N.; Waszkiewicz, N.; Zalewska, A.; Maciejczyk, M. Antioxidant defence, oxidative stress and oxidative damage in saliva, plasma and erythrocytes of dementia patients. Can salivary AGE be a marker of dementia? Int. J. Mol. Sci. 2017, 18, 2205. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Mikoluc, B.; Pietrucha, B.; Heropolitanska-Pliszka, E.; Pac, M.; Motkowski, R.; Car, H. Oxidative stress, mitochondrial abnormalities and antioxidant defense in Ataxia-telangiectasia, Bloom syndrome and Nijmegen breakage syndrome. Redox Biol. 2017, 11, 375–383. [Google Scholar] [CrossRef]
- Falkowski, M.; Maciejczyk, M.; Koprowicz, T.; Mikołuć, B.; Milewska, A.; Zalewska, A.; Car, H. Whey Protein Concentrate WPC-80 Improves Antioxidant Defense Systems in the Salivary Glands of 14-Month Wistar Rats. Nutrients 2018, 10, 782. [Google Scholar] [CrossRef]
- Camiletti-Móiron, D.; Arianna Aparicio, V.; Nebot, E.; Medina, G.; Martínez, R.; Kapravelou, G.; Andrade, A.; Porres, J.M.; López-Jurado, M.; Aranda, P. High-protein diet induces oxidative stress in rat brain: Protective action of high-intensity exercise against lipid peroxidation. Nutr. Hosp. 2015, 31, 866–874. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Kretowski, A.J.; Antonowicz, B.; Ratajczak-Wrona, W.; Jablonska, E.; Zaleski, P.; Waszkiel, D.; Ladny, J.R.; Zukowski, P.; et al. The redox balance in erythrocytes, plasma, and periosteum of patients with titanium fixation of the jaw. Front. Physiol. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Lubitz, I.; Ricny, J.; Atrakchi-Baranes, D.; Shemesh, C.; Kravitz, E.; Liraz-Zaltsman, S.; Maksin-Matveev, A.; Cooper, I.; Leibowitz, A.; Uribarri, J.; et al. High dietary advanced glycation end products are associated with poorer spatial learning and accelerated Aβ deposition in an Alzheimer mouse model. Aging Cell 2016, 15, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef]
- Uribarri, J.; Tuttle, K.R. Advanced glycation end products and nephrotoxicity of high-protein diets. Clin. J. Am. Soc. Nephrol. 2006, 1, 1293–1299. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- De Assis, D.R.; Maria, R.C.; Ferreira, G.C.; Schuck, P.F.; Latini, A.; Dutra-Filho, C.S.; Wannmacher, C.M.D.; Wyse, A.T.S.; Wajner, M. Na+,K+ ATPase activity is markedly reduced by cis-decenoic acid in synaptic plasma membranes from cerebral cortex of rats. Exp. Neurol. 2005, 197, 143–149. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Wang, Z.; Pu, X.; Zhou, S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol. Appl. Pharmacol. 2011, 254, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Enciu, A.M.; Gherghiceanu, M.; Popescu, B.O. Triggers and effectors of oxidative stress at blood-brain barrier level: Relevance for brain ageing and neurodegeneration. Oxid. Med. Cell. Longev. 2013, 2013, 297512. [Google Scholar] [CrossRef] [PubMed]
- Snelson, M.; Mamo, J.C.L.; Lam, V.; Giles, C.; Takechi, R. Differential effects of high-protein diets derived from soy and casein on blood–brain barrier integrity in mild-type mice. Front. Nutr. 2017, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.J.; Yamada, K.; Sugisawa, A.; Saito, K.; Miyajima, T.; Umegaki, K. Enhanced oxidative damage induced by total body irradiation in mice fed a low protein diet. Indian J. Radiat. Biol. 2002, 78, 425–432. [Google Scholar] [CrossRef]
- Shin, S.J. Does a high protein diet induce oxidative damage? SM J. Food Nutr. Disord. 2015, 1, 1001. [Google Scholar]
- Soulsby, M.E.; Phillips, B.; Chowdhury, P. Effects of soy-protein diet on elevated brain lipid peroxide levels induced by simulated weightlessness. Ann. Clin. Lab. Sci. 2004, 34, 103–106. [Google Scholar]
- Jean, C.; Rome, S.; Aattouri, N.; Fromentin, G.; Achagiotis, C.L.; Tome, D. Metabolic evidence for adaptation to a high protein diet in rats. J. Nutr. 2001, 131, 91–98. [Google Scholar] [CrossRef]
- Petzke, K.J.; Elsner, A.; Proll, J.; Thielecke, F.; Metges, C.C. Long-term high protein intake does not increase oxidative stress in rats. J. Nutr. 2000, 130, 2889–2896. [Google Scholar] [CrossRef]
- Villa, R.F.; Ferrari, F.; Gorini, A. Energy metabolism of rat cerebral cortex, hypothalamus and hypophysis during ageing. Neuroscience 2012, 227, 55–66. [Google Scholar] [CrossRef]
- Morrison, C.D.; Pistell, P.J.; Ingram, D.K.; Johnson, W.D.; Liu, Y.; Fernandez-Kim, S.O.; White, C.L.; Purpera, M.N.; Uranga, R.M.; Bruce-Keller, A.J.; et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: Implications for decreased Nrf2 signaling. J. Neurochem. 2010, 114, 1581–1589. [Google Scholar] [CrossRef]
- Borys, J.; Maciejczyk, M.; Antonowicz, B.; Krętowski, A.; Waszkiel, D.; Bortnik, P.; Czarniecka-Bargłowska, K.; Kocisz, M.; Szulimowska, J.; Czajkowski, M.; et al. Exposure to Ti4Al4V Titanium Alloy Leads to Redox Abnormalities, Oxidative Stress, and Oxidative Damage in Patients Treated for Mandible Fractures. Oxid. Med. Cell. Longev. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Matczuk, J.; Kurek, K.; Waszkiel, D.; Żendzian-Piotrowska, M.; Zalewska, A. Effect of N-acetylcysteine on antioxidant defense, oxidative modification, and salivary gland function in a rat model of insulin resistance. Oxid. Med. Cell. Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Minieri, C.A.; Ollerenshaw, J.D.; Alexander, R.W. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 1994, 74, 1141–1148. [Google Scholar] [CrossRef]
- Prajda, N.; Weber, G. Malignant transformation-linked imbalance: Decreased xanthine oxidase activity in hepatomas. FEBS Lett. 1975, 59, 245–249. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Mize, C.E.; Langdon, R.G. Hepatic glutathione reductase. I. Purification and general kinetic properties. J. Biol. Chem. 1962, 237, 1589–1595. [Google Scholar]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1984; Volume 105, pp. 121–126. ISBN 9780121820053. [Google Scholar]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [PubMed]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim. Biophys. Acta - Gen. Subj. 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. In Basic Protein and Peptide Protocols; Humana Press: Totowa, NJ, USA, 1994; Volume 32, pp. 5–8. [Google Scholar]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Mikłosz, A.; Łukaszuk, B.; Żendzian-Piotrowska, M.; Brańska-Januszewska, J.; Ostrowska, H.; Chabowski, A. Challenging of AS160/TBC1D4 Alters Intracellular Lipid milieu in L6 Myotubes Incubated with Palmitate. J. Cell. Physiol. 2017, 232, 2373–2386. [Google Scholar] [CrossRef] [PubMed]
| Parameter | CON | HPD |
|---|---|---|
| Body weight (g) | 341 ± 2.49 | 350 ± 7.57 |
| Glucose concentration (mg/dL) | 99.8 ± 2.49 | 101 ± 5.12 |
| Insulin concentration (µU/mL) | 4.75 ± 0.02 | 4.95 ± 0.14 |
| HOMA-IR | 3.08 ± 0.17 | 3.56 ± 0.14 |
| Adiponectin (µg/mL) | 23.4 ± 0.57 | 22.4 ± 0.55 |
| Leptin (ng/mL) | 26.4 ± 0.63 | 24.8 ± 0.61 |
| Food intake (g/day) | 21.2 ± 0.84 | 16.2 ± 0.63 * |
| Energy intake (mJ/day) | 0.28 ± 0.01 | 0.27 ± 0.03 |
| Protein energy intake (mJ/day) | 0.06 ± 0.05 | 0.13 ± 0.04 * |
| Cerebral cortex total protein concentration (µg/mL) | 2742 ± 83.1 | 2796 ± 43.4 |
| Hypothalamus total protein concentration (µg/mL) | 1710 ± 66.1 | 1529 ± 53.3 |
| Parameter | CON | HPD |
|---|---|---|
| GPx (mU/mg protein) | 0.44 ± 0.02 | 0.64 ± 0.06 * |
| GR (nU/mg protein) | 11.8 ± 0.77 | 9.55 ± 0.32 |
| CAT (nmol H2O2·min−1·mg protein−1) | 6.02 ± 0.49 | 25.6 ± 1.52 * |
| SOD-1 (mU/mg protein) | 54.3 ± 2.02 | 42.8 ± 0.65 * |
| UA (µg/mg protein) | 2.71 ± 0.27 | 4.89 ± 0.58 * |
| GSH (nmol/mg protein) | 7.30 ± 0.71 | 3.06 ± 0.59 * |
| TAC (Trolox nmol/mg protein) | 3.94 ± 0.13 | 5.14 ± 0.51 |
| TOS (nmol H2O2 Equiv/mg protein) | 13.5 ± 2.87 | 27.5 ± 2.22 * |
| OSI (TOS/TAC ratio) | 344 ± 78.5 | 542 ± 38.9 |
| FRAP (nmol/mg protein) | 1.14 ± 0.09 | 1.51 ± 0.09 * |
| AGE (AFU/mg protein) | 3.26 ± 0.21 | 4.06 ± 0.21 * |
| 4-HNE (fg/mg protein) | 279 ± 62.6 | 1028 ± 85.7 * |
| MDA (ng/mg protein) | 471 ± 28.7 | 607 ± 44 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żebrowska, E.; Maciejczyk, M.; Żendzian-Piotrowska, M.; Zalewska, A.; Chabowski, A. High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus. Int. J. Mol. Sci. 2019, 20, 1547. https://doi.org/10.3390/ijms20071547
Żebrowska E, Maciejczyk M, Żendzian-Piotrowska M, Zalewska A, Chabowski A. High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus. International Journal of Molecular Sciences. 2019; 20(7):1547. https://doi.org/10.3390/ijms20071547
Chicago/Turabian StyleŻebrowska, Ewa, Mateusz Maciejczyk, Małgorzata Żendzian-Piotrowska, Anna Zalewska, and Adrian Chabowski. 2019. "High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus" International Journal of Molecular Sciences 20, no. 7: 1547. https://doi.org/10.3390/ijms20071547
APA StyleŻebrowska, E., Maciejczyk, M., Żendzian-Piotrowska, M., Zalewska, A., & Chabowski, A. (2019). High Protein Diet Induces Oxidative Stress in Rat Cerebral Cortex and Hypothalamus. International Journal of Molecular Sciences, 20(7), 1547. https://doi.org/10.3390/ijms20071547







