The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn
Abstract
1. Introduction
2. Results and Discussion
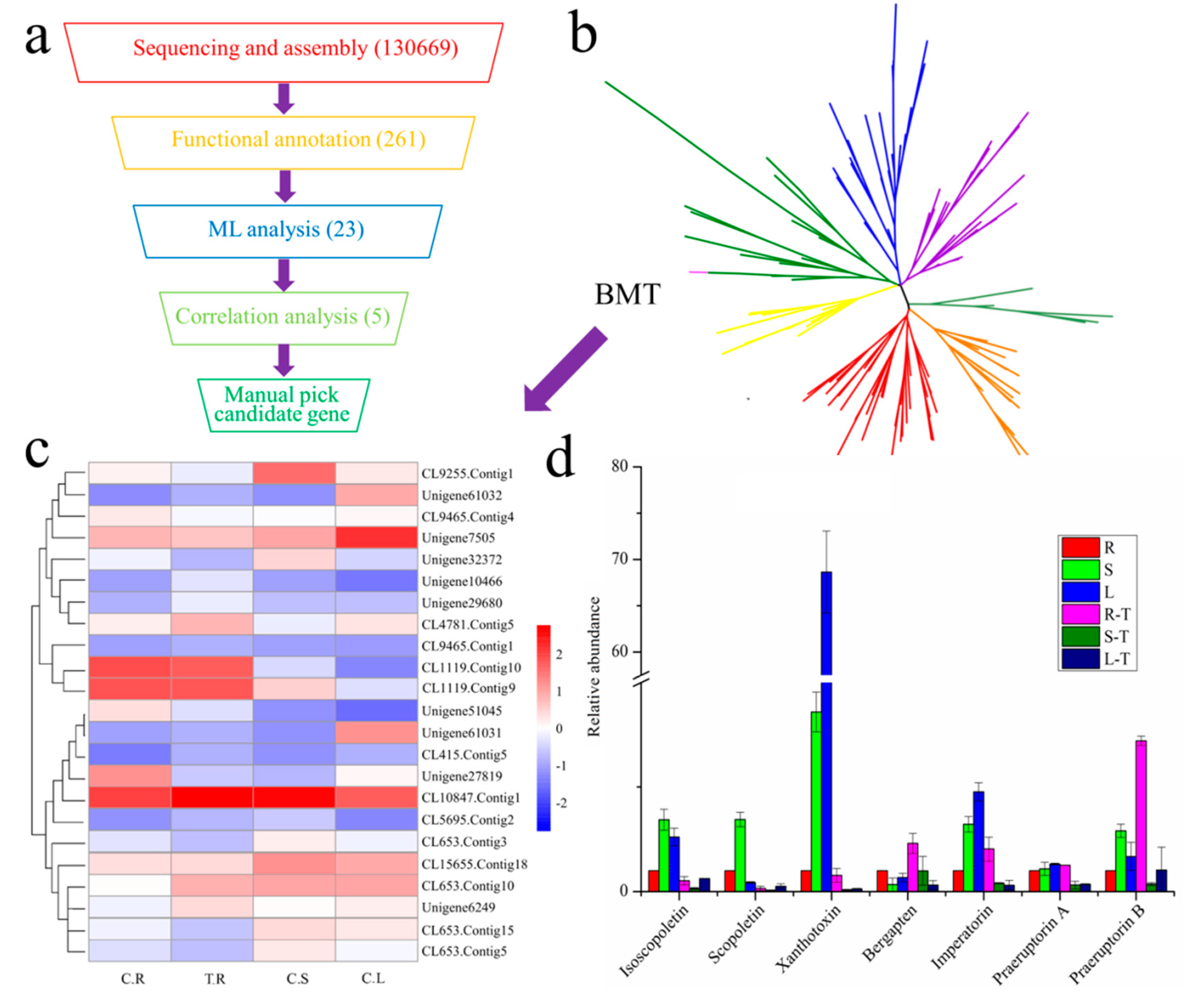
2.1. Candidate Gene Mining with Transcriptome Sequencing and Phytochemical Analysis
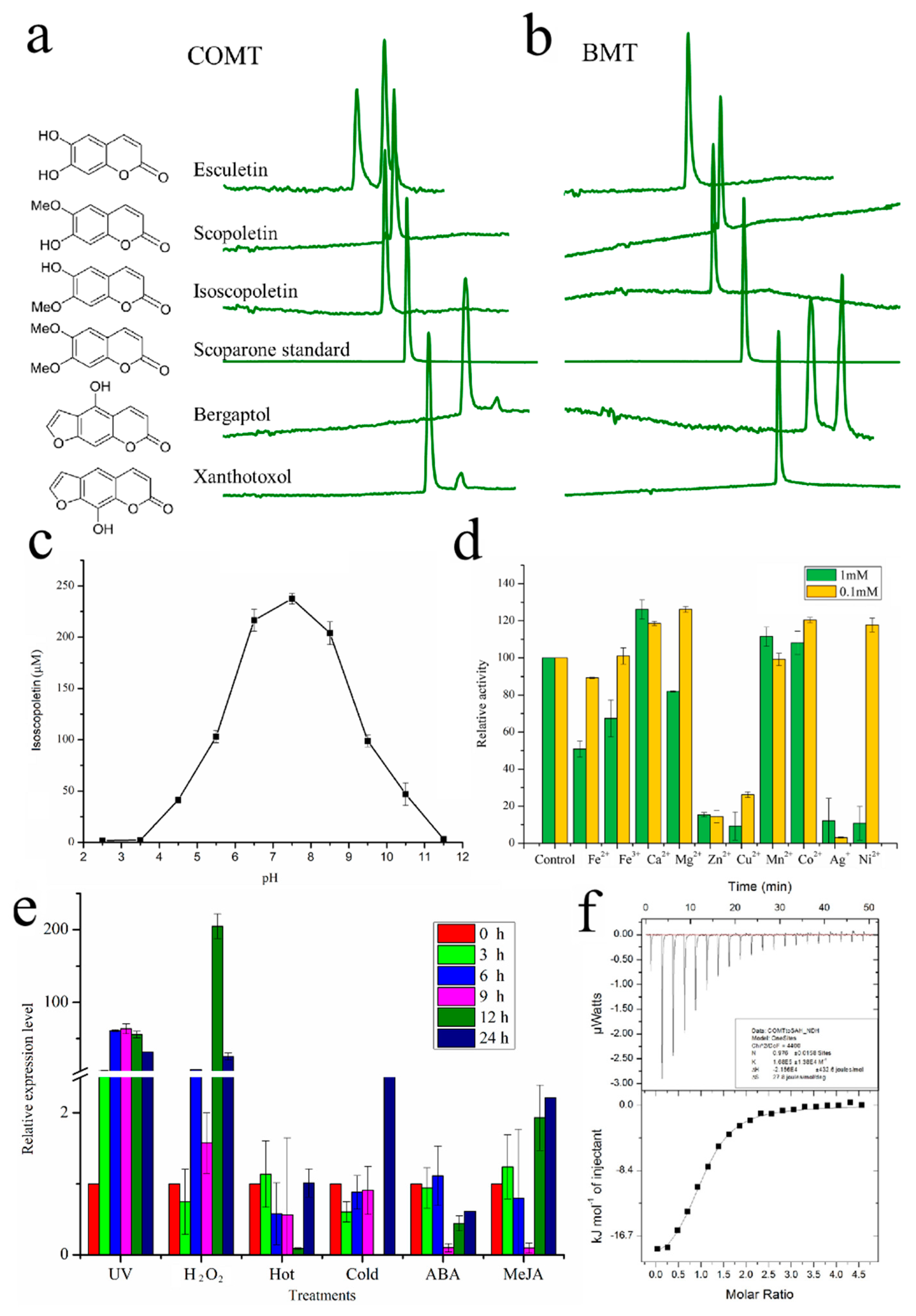
2.2. Functional Characterization of COMT-S
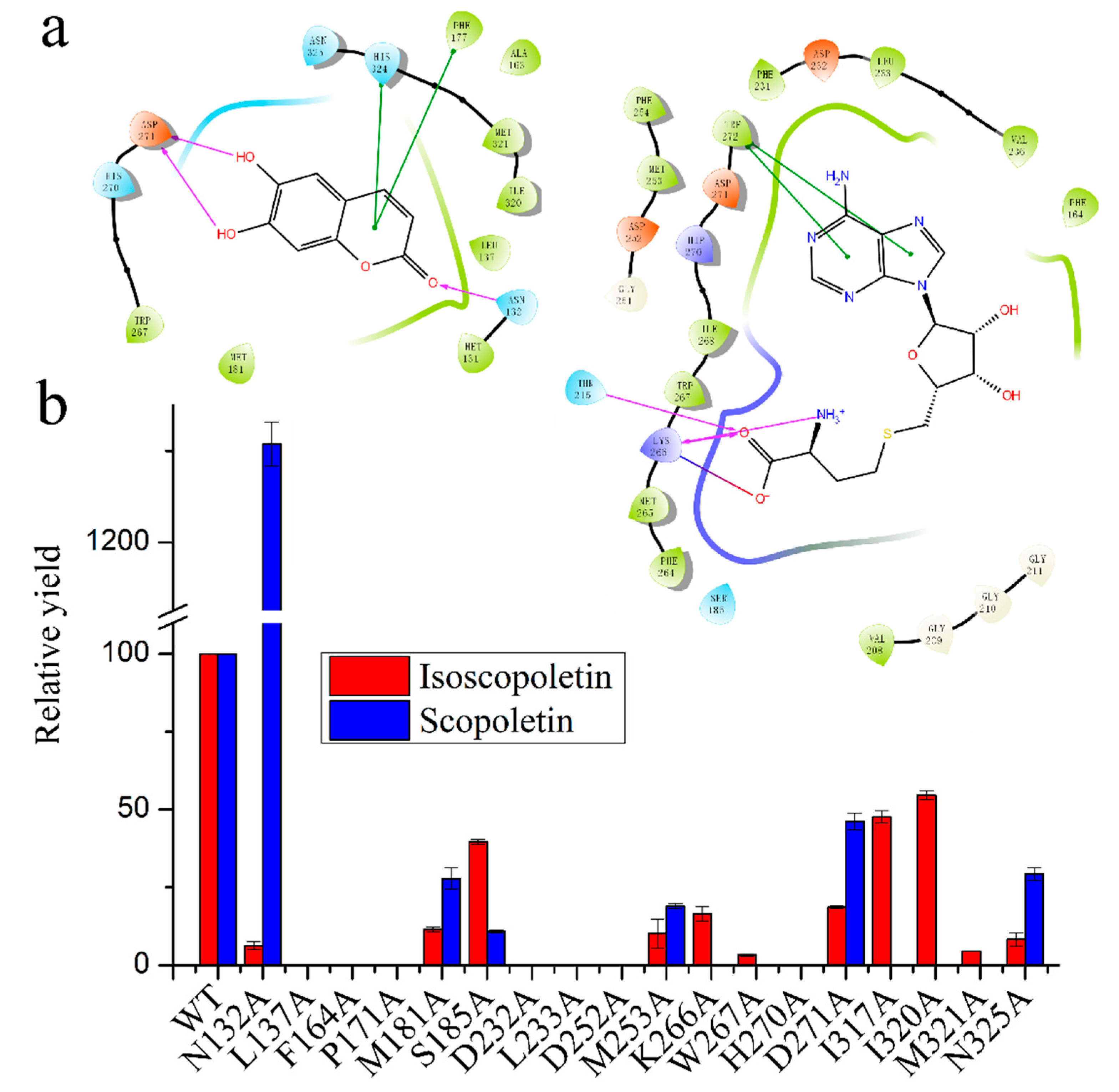
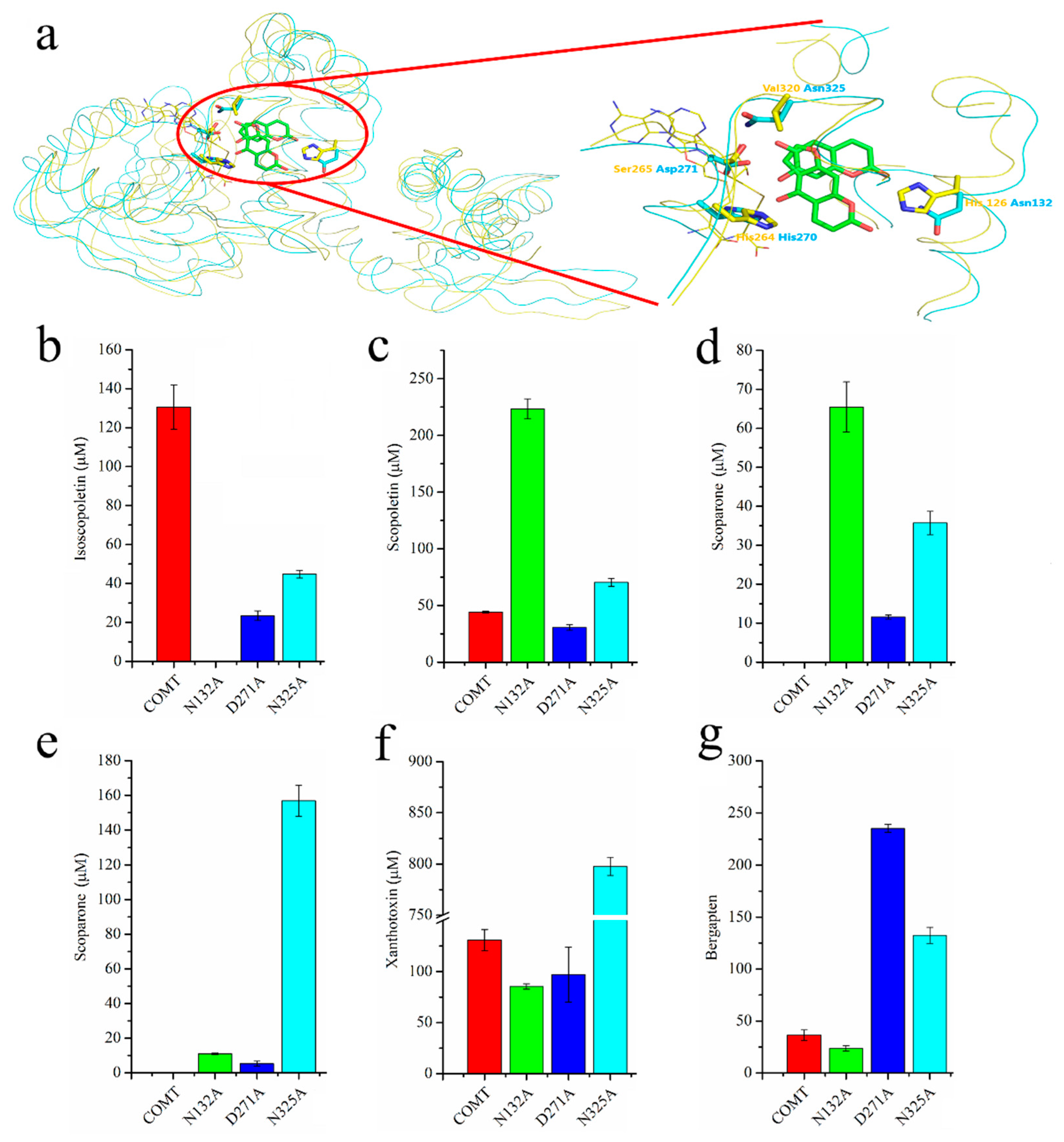
2.3. Docking of COMT-S to Identify the Key Residues Involved in SAH/Esculetin Binding
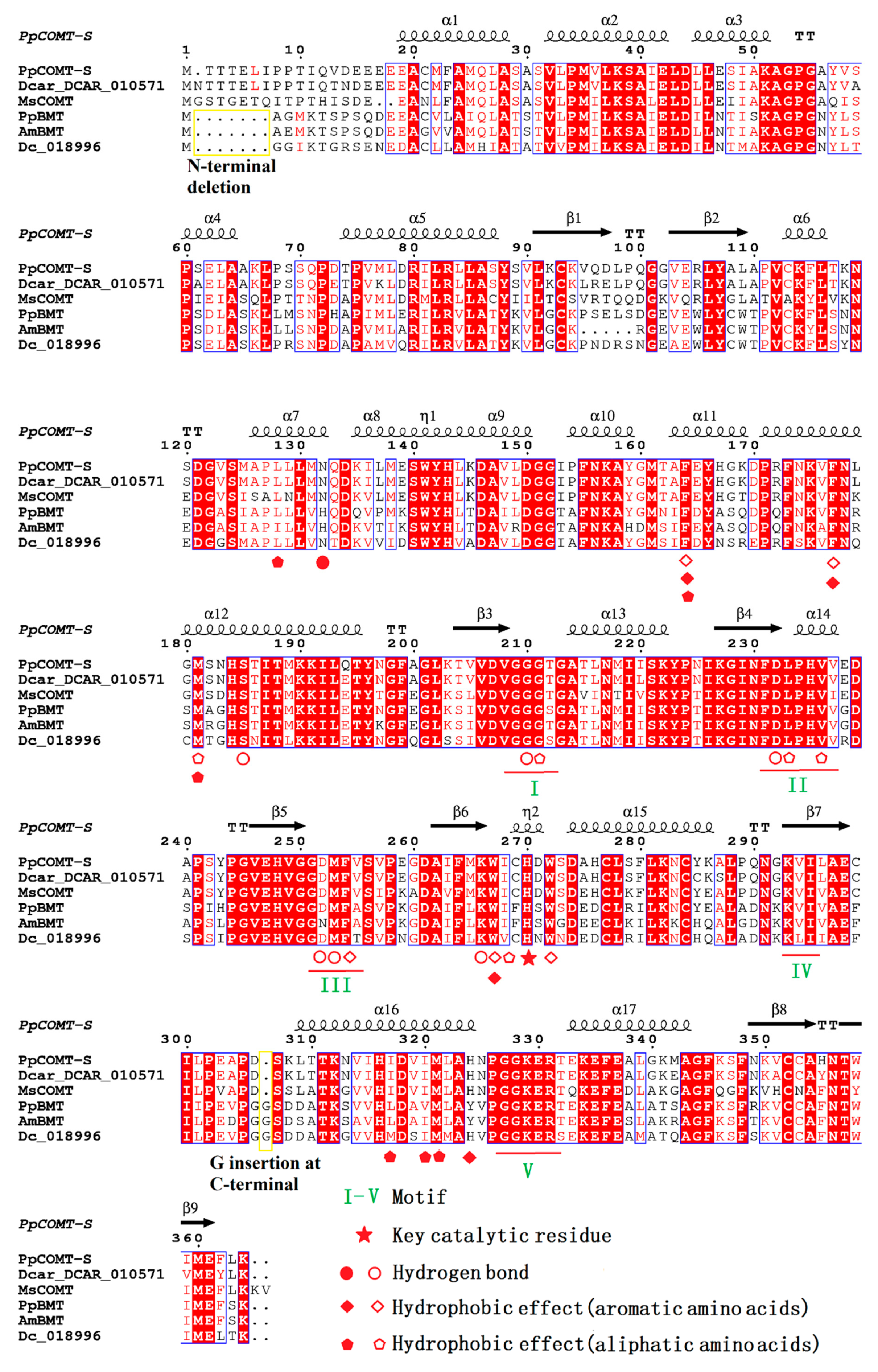
2.4. Sequence Alignment and Biochemical Analysis to Identify the Key Residues Involved in Substrate Heterozygosity
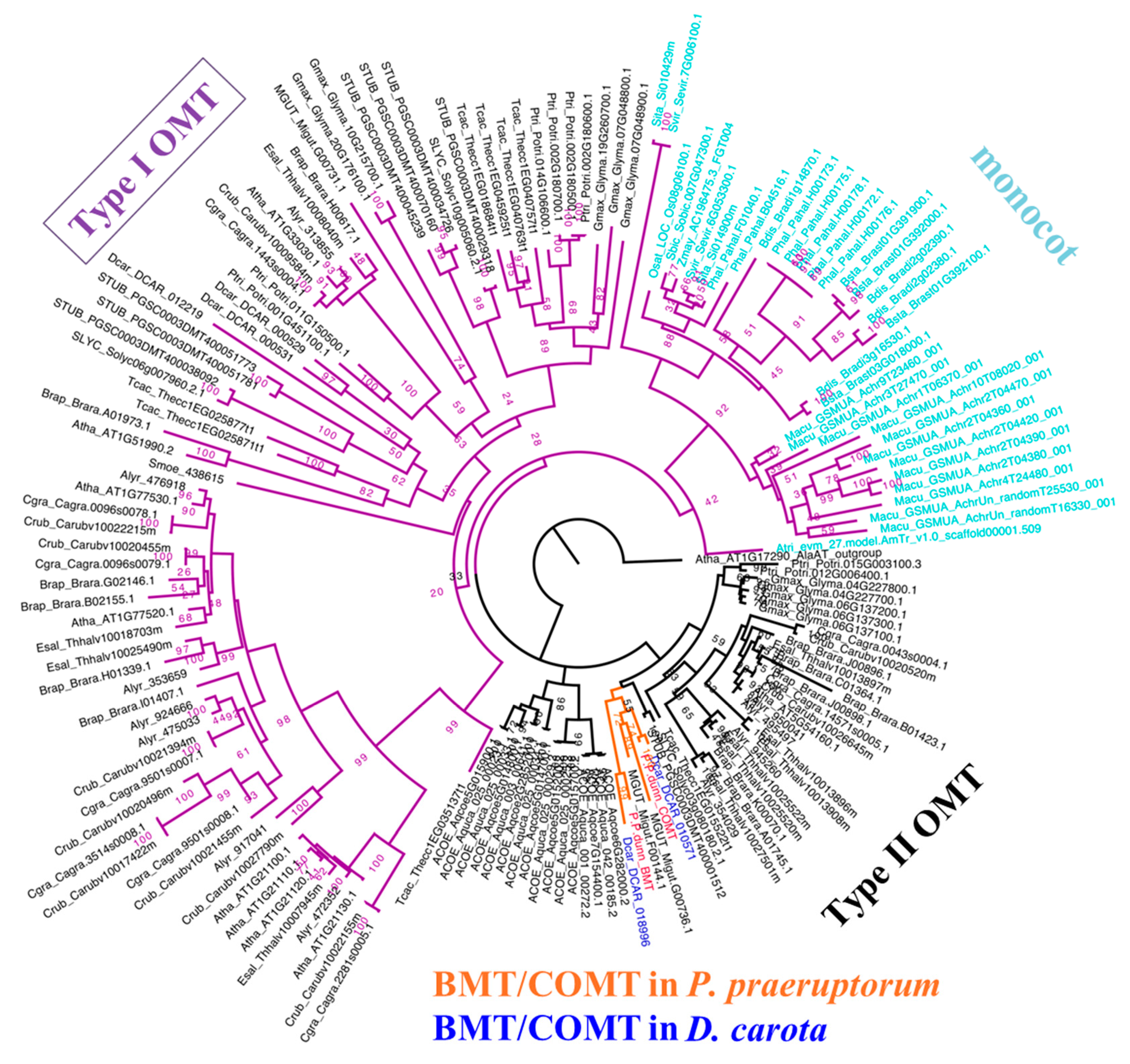
2.5. BMT Was Evolved as a Special Enzyme from COMT-S by Gene Duplication
3. Materials and Methods
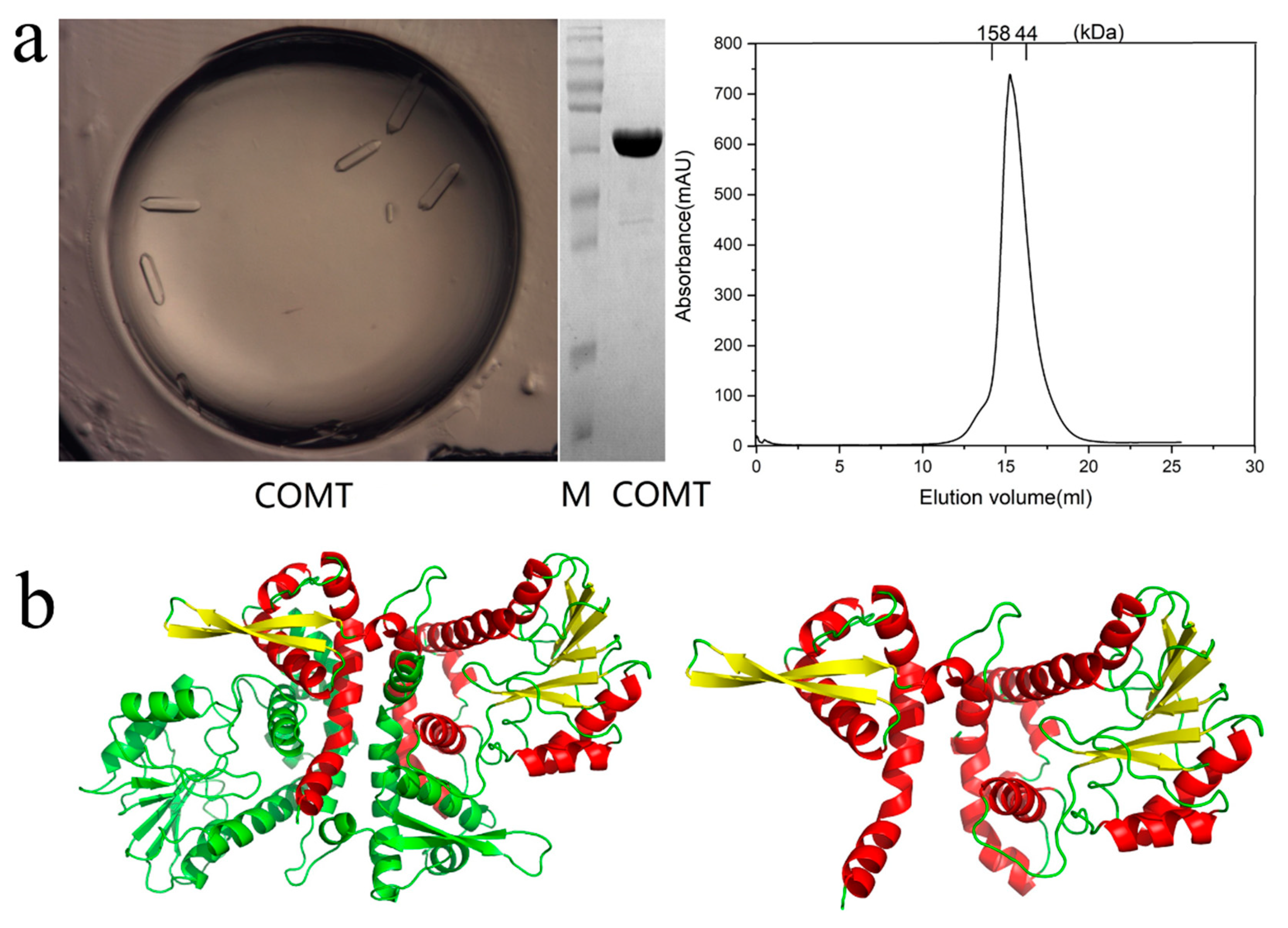
3.1. Protein Expression and Purification
3.2. Crystallization and Structure Determination
3.3. Enzymatic Activity Assays of PpBMT and COMT-S
3.4. HPLC/Electrospray-Ionization Quadrupole Time-of-Flight Mass Spectrometry (Q-TOF MS) Analysis of Coumarin Compounds Measurement
3.5. Q-PCR Analysis and Induction of COMT-S Expression by Elicitors
3.6. Transcriptome Sequence, Assembly, Bioinformatics Analysis, and Docking
3.7. Isothermal Titration Calorimetry
3.8. Evolutionary Analysis of BMT and COMT-S
3.9. Statistical Analysis and the Preparation of Graphs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OMTs | O-methyltransferases |
| COMT | caffeic acid O-methyltransferase |
| BMT | Bergaptol O-methyltransferase |
| HPLC | High Performance Liquid Chromatography |
| SD | Standard Deviation |
| PDB | Protein Data Bank |
| SAM | S-adenosyl-l-methionine |
| ESC | esculetin |
| SAH | S-adenosyl-l-homocysteine |
| NCBI | National Center for Biotechnology Information |
References
- Kumar, A.; Maurya, R.A.; Sharma, S.; Ahmad, P.; Singh, A.; Bhatia, G.; Srivastava, A.K. Pyranocoumarins: A new class of anti-hyperglycemic and anti-dyslipidemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 6447–6451. [Google Scholar] [CrossRef]
- Yu, P.J.; Jin, H.; Zhang, J.Y.; Wang, G.F.; Li, J.R.; Zhu, Z.G.; Tian, Y.X.; Wu, S.Y.; Xu, W.; Zhang, J.J. Pyranocoumarins isolated from Peucedanum praeruptorum Dunn suppress lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB and STAT3 activation. Inflammation 2012, 35, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Galanie, S.; Thodey, K.; Trenchard, I.J.; Interrante, M.F.; Smolke, C.D. Complete biosynthesis of opioids in yeast. Science 2015, 349, 1095–1100. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef]
- Qu, Y.; Easson, M.L.; Froese, J.; Simionescu, R.; Hudlicky, T.; de Luca, V. Completion of the seven-step pathway from tabersonine to the anticancer drug precursor vindoline and its assembly in yeast. Proc. Natl. Acad. Sci. USA 2015, 112, 6224–6229. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shen, X.; Yuan, Q.; Yan, Y. Microbial biosynthesis of the anticoagulant precursor 4-hydroxycoumarin. Nat. Commun. 2013, 4, 2603. [Google Scholar] [CrossRef]
- Sun, X.; Zhou, D.; Kandavelu, P.; Zhang, H.; Yuan, Q.; Wang, B.C.; Rose, J.; Yan, Y. Structural Insights into Substrate Specificity of Feruloyl-CoA 6’-Hydroxylase from Arabidopsis thaliana. Sci. Rep. 2015, 5, 10355. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, N.; Zeng, Z.; Xu, S.; Huang, C.; Wang, W.; Liu, T.; Luo, J.; Kong, L. Cloning, Functional Characterization, and Catalytic Mechanism of a Bergaptol O-Methyltransferase from Peucedanum praeruptorum Dunn. Front. Plant Sci. 2016, 7, 722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, T.; Luo, J.; Zhang, Q.; Xu, S.; Han, C.; Xu, J.; Chen, M.; Chen, Y.; Kong, L. Integration of a decrescent transcriptome and metabolomics dataset of Peucedanum praeruptorum to investigate the CYP450 and MDR genes involved in coumarins biosynthesis and transport. Front. Plant. Sci. 2015, 6, 996. [Google Scholar] [CrossRef] [PubMed]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytother. Res. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Zubieta, C.; Kota, P.; Ferrer, J.L.; Dixon, R.A.; Noel, J.P. Structural basis for the modulation of lignin monomer methylation by caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase. Plant Cell 2002, 14, 1265–1277. [Google Scholar] [CrossRef]
- Robin, A.Y.; Giustini, C.; Graindorge, M.; Matringe, M.; Dumas, R. Crystal structure of norcoclaurine-6-O-methyltransferase a key rate-limiting step in the synthesis of benzylisoquinoline alkaloids. Plant J. 2016, 87, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Hehmann, M.; Lukacin, R.; Ekiert, H.; Matern, U. Furanocoumarin biosynthesis in Ammi majus L. Cloning of bergaptol O-methyltransferase. Eur. J. Biochem. 2004, 271, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.X.; Liu, X.M.; Ge, C.H.; Gao, L.; Peng, X.M.; Zhao, P.P.; Yan, M. The Effects of Galangin on a Mouse Model of Vitiligo Induced by Hydroquinone. Phytother. Res. 2014, 28, 1533. [Google Scholar] [CrossRef] [PubMed]
- Parisa, S. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: A review. J. Ethnopharmacol. 2014, 156, 235–270. [Google Scholar]
- Hou, Z.; Luo, J.; Wang, J.; Kong, L. Separation of minor coumarins from Peucedanum praeruptorum using HSCCC and preparative HPLC guided by HPLC/MS. Sep. Purif. Technol. 2010, 75, 132–137. [Google Scholar] [CrossRef]
- Lv, H.; Luo, J.; Wang, X.; Kong, L. Application of UPLC-Quadrupole-TOF-MS Coupled with Recycling Preparative HPLC in Isolation and Preparation of Coumarin Isomers with Similar Polarity from Peucedanum praeruptorum. Chromatographia 2012, 76, 141–148. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Sharma, S.K.; Garrett, J.M.; Brown, S.A. Separation of the S-adenosylmethionine: 5-and 8-hydroxyfuranocoumarin O-methyltransferases of Ruta graveolens L. by general ligand affinity chromatography. Z. Naturforsch. C 1979, 34, 387–391. [Google Scholar] [CrossRef]
- Mcneely, W.; Kl, D.C.N. 5-Methoxypsoralen. A review of its effects in psoriasis and vitiligo. Drugs 1998, 56, 667. [Google Scholar] [CrossRef]
- Sun, H.; Wang, L.; Zhang, B.; Ma, J.; Hettenhausen, C.; Cao, G.; Sun, G.; Wu, J.; Wu, J. Scopoletin is a phytoalexin against Alternaria alternata in wild tobacco dependent on jasmonate signalling. J. Exp. Bot. 2014, 65, 4305–4315. [Google Scholar]
- Gnonlonfin, G.J.B.; Sanni, A.; Brimer, L. Review Scopoletin—A Coumarin Phytoalexin with Medicinal Properties. Crit. Rev. Plant Sci. 2012, 31, 47–56. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kuma, T.; Sasaki, H.; Sasaki, N.; Ozeki, Y.; Kobayashi, N.; Kitamura, Y. Constitutive expression of bergaptol O-methyltransferase in Glehnia littoralis cell cultures. Plant Cell Rep. 2009, 28, 257–265. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef]
- Yao, R.; Zhao, Y.; Liu, T.; Huang, C.; Xu, S.; Sui, Z.; Luo, J.; Kong, L. Identification and functional characterization of a p-coumaroyl CoA 2′-hydroxylase involved in the biosynthesis of coumarin skeleton from Peucedanum praeruptorum Dunn. Plant Mol. Biol. 2017, 95, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Minor, W.; Cymborowski, M.; Otwinowski, Z.; Chruszcz, M. HKL-3000: The integration of data reduction and structure solution–from diffraction images to an initial model in minutes. Acta Crystallogr. D 2006, 62, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.; McCoy, A. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 2011, 67, 235–242. [Google Scholar] [PubMed]
- Adams, P.D.; Grosse-Kunstleve, R.W.; Hung, L.W.; Ioerger, T.R.; McCoy, A.J.; Moriarty, N.W.; Read, R.J.; Sacchettini, J.C.; Sauter, N.K.; Terwilliger, T.C. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr. D 2002, 58, 1948–1954. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, J.; Xu, S.; Wang, W.; Liu, T.; Han, C.; Chen, Y.; Kong, L. Selection of Reference Genes for Gene Expression Normalization in Peucedanum praeruptorum Dunn under Abiotic Stresses, Hormone Treatments and Different Tissues. PLoS ONE 2016, 11, e0152356. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Sherman, W.; Beard, H.S.; Farid, R. Use of an induced fit receptor structure in virtual screening. Chem. Biol. Drug Des. 2006, 67, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinf. 2009, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006, 22, 2688. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, N.; Sui, Z.; Huang, C.; Zeng, Z.; Kong, L. The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. Int. J. Mol. Sci. 2019, 20, 1533. https://doi.org/10.3390/ijms20071533
Zhao Y, Wang N, Sui Z, Huang C, Zeng Z, Kong L. The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. International Journal of Molecular Sciences. 2019; 20(7):1533. https://doi.org/10.3390/ijms20071533
Chicago/Turabian StyleZhao, Yucheng, Nana Wang, Ziwei Sui, Chuanlong Huang, Zhixiong Zeng, and Lingyi Kong. 2019. "The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn" International Journal of Molecular Sciences 20, no. 7: 1533. https://doi.org/10.3390/ijms20071533
APA StyleZhao, Y., Wang, N., Sui, Z., Huang, C., Zeng, Z., & Kong, L. (2019). The Molecular and Structural Basis of O-methylation Reaction in Coumarin Biosynthesis in Peucedanum praeruptorum Dunn. International Journal of Molecular Sciences, 20(7), 1533. https://doi.org/10.3390/ijms20071533




