A Novel Biomarker for Acute Kidney Injury, Vanin-1, for Obstructive Nephropathy: A Prospective Cohort Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
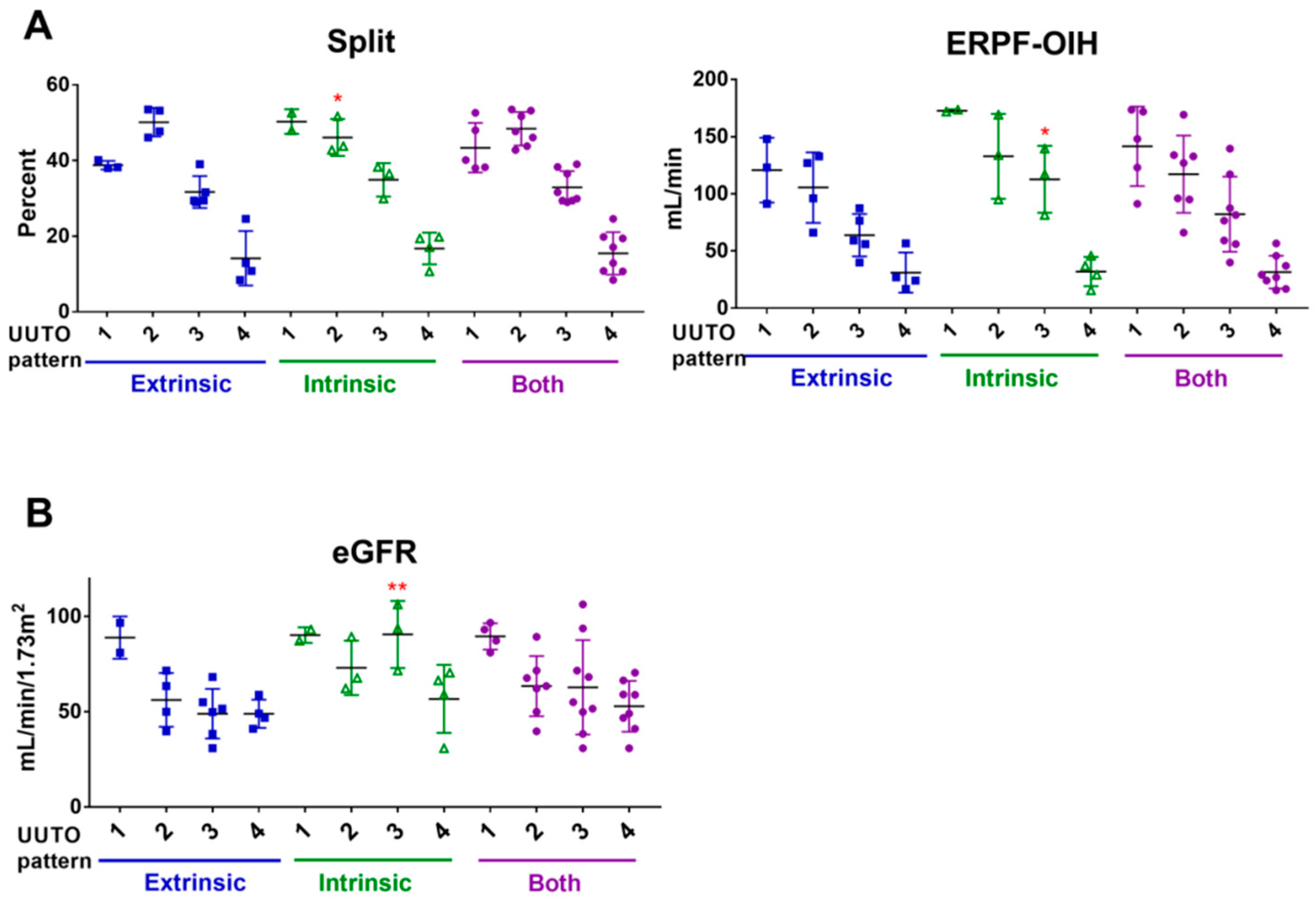
2.2. UUTO Patterns According to Tc-99m Mercaptoacetyltriglycine(MAG3) Renal Scan Results
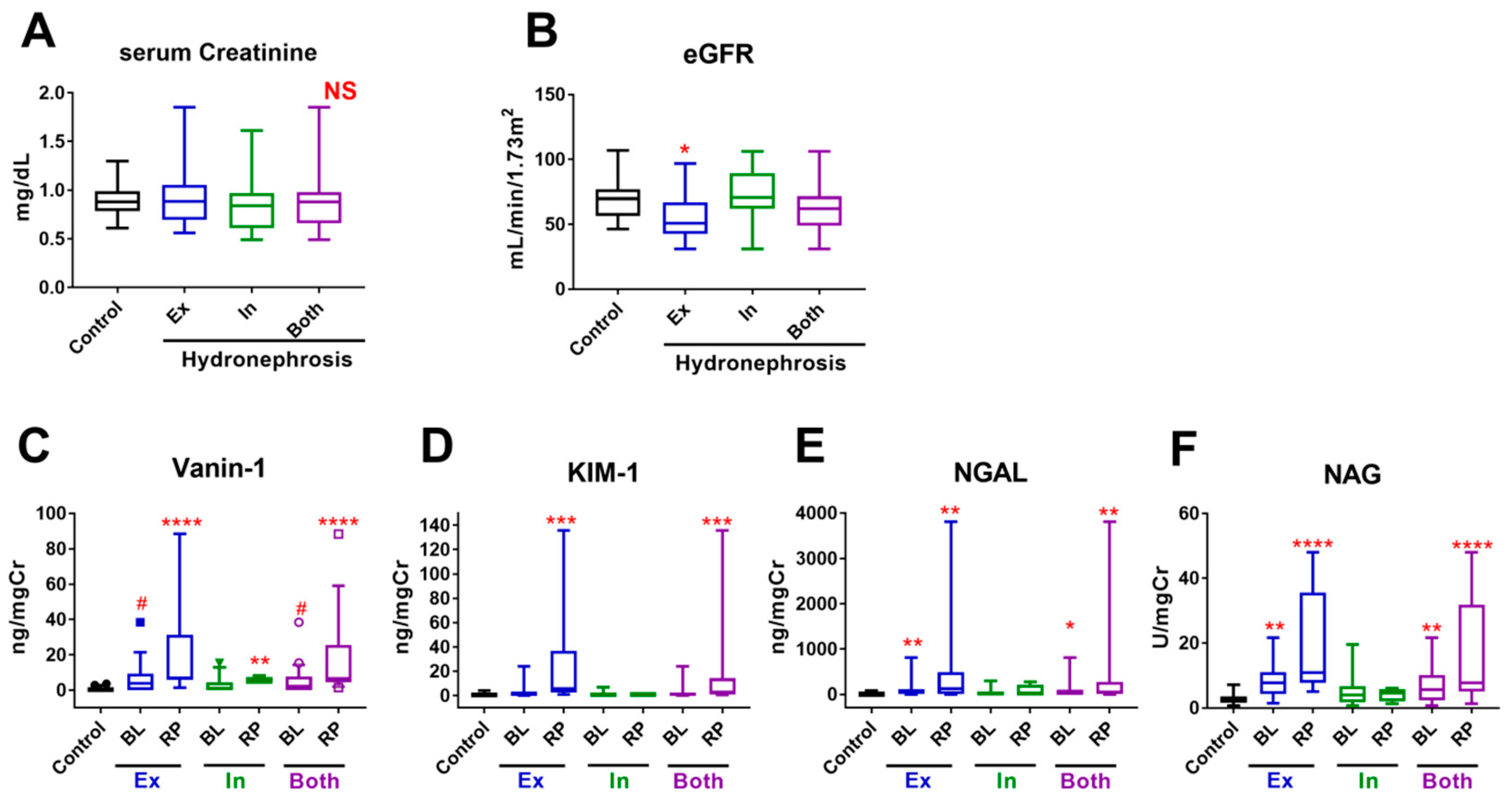
2.3. Urinary Biomarkers
2.4. Multivariate Analyses to Identify Factors that Predict HN
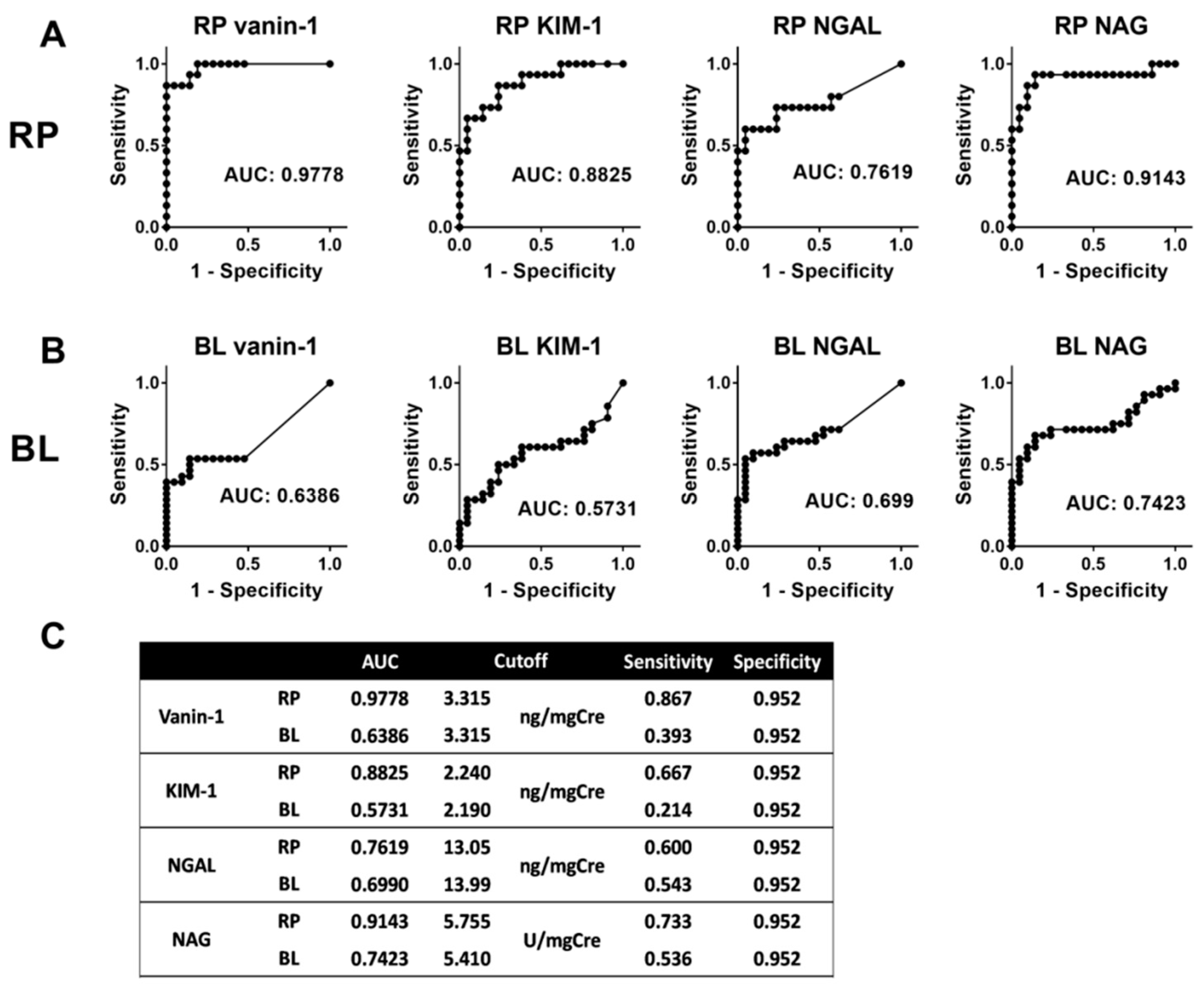
2.5. Predictive Ability of Each Biomarker
2.6. Urinary Biomarker Levels According to the UUTO Pattern
2.7. Combination of Bladder Vanin-1 and NAG
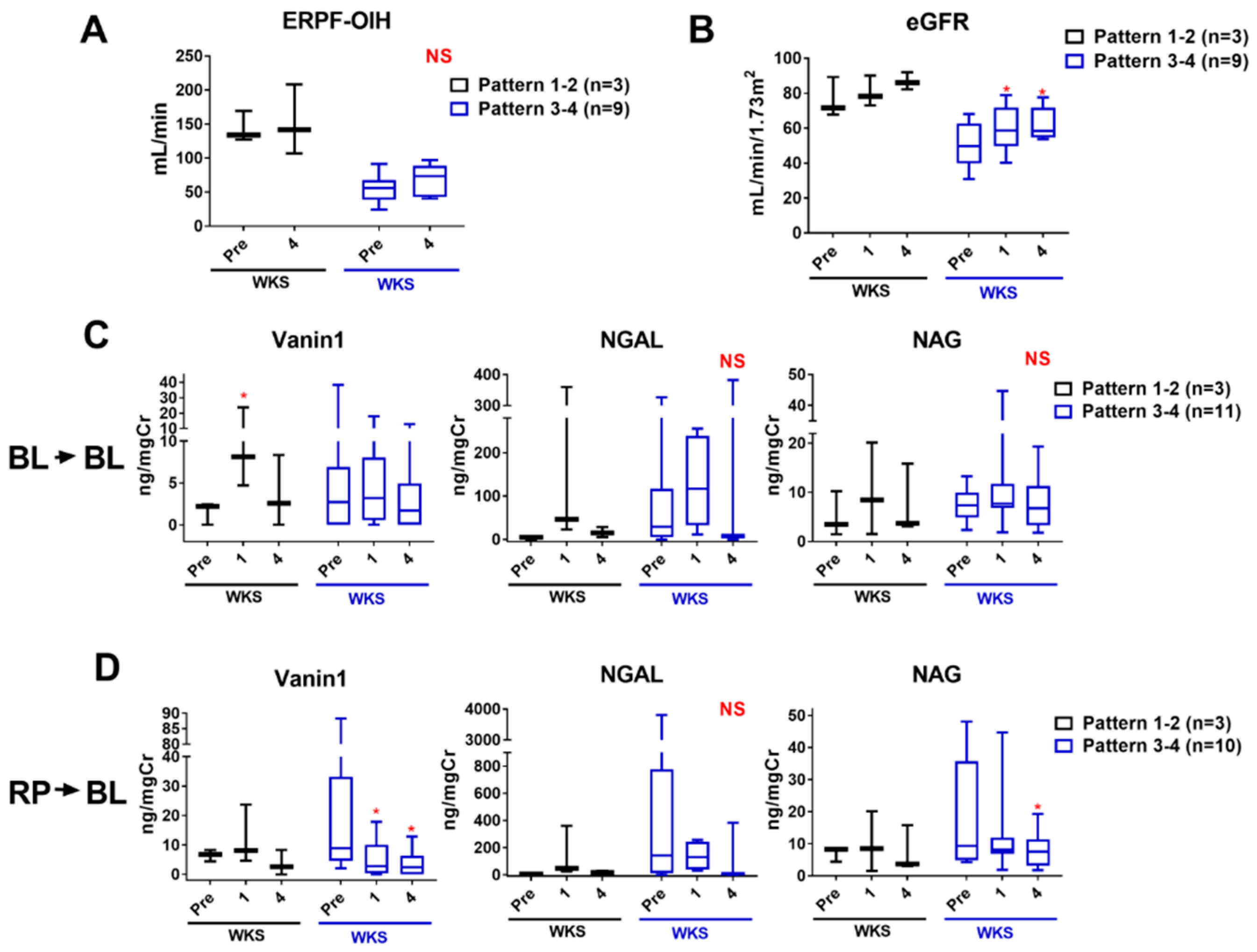
2.8. Monitoring Kidney Injury after Intervention
2.9. Case Presentation of a Patient with Asynchronous Bilateral Extrinsic UUTOs
3. Discussion
4. Patients and Methods
Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Smith, J.M.; Stablein, D.M.; Munoz, R.; Hebert, D.; McDonald, R.A. Contributions of the Transplant Registry: The 2006 Annual Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS). Pediatr. Transplant. 2007, 11, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Morrissey, J. Obstructive nephropathy and renal fibrosis. Am. J. Physiol. Ren. Physiol. 2002, 283, F861–F875. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.J.; Tarantal, A.F.; Jimenez, D.F.; Matsell, D.G. Collecting duct epithelial-mesenchymal transition in fetal urinary tract obstruction. Kidney Int. 2007, 72, 936–944. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Taranta-Janusz, K.; Debek, W.; Zoch-Zwierz, W.; Kuroczycka-Saniutycz, E. KIM-1 and NGAL: New markers of obstructive nephropathy. Pediatr. Nephrol. 2011, 26, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shao, X.; Sun, B.; Miao, C.; Li, Z.; Shi, Y. Urinary kidney injury molecule1 as an early diagnostic biomarker of obstructive acute kidney injury and development of a rapid detection method. Mol. Med. Rep. 2017, 15, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Posada, D.; Dayarathna, T.; Dion, M.; Alenezi, H.; Sener, A.; Denstedt, J.D.; Pautler, S.E.; Razvi, H. KIM-1 Is a Potential Urinary Biomarker of Obstruction: Results from a Prospective Cohort Study. J. Endourol. 2017, 31, 111–118. [Google Scholar] [CrossRef]
- Carr, M.C.; Peters, C.A.; Retik, A.B.; Mandell, J. Urinary levels of the renal tubular enzyme N-acetyl-beta-D-glucosaminidase in unilateral obstructive uropathy. J. Urol. 1994, 151, 442–445. [Google Scholar] [CrossRef]
- Devarajan, P. Neutrophil gelatinase-associated lipocalin: A promising biomarker for human acute kidney injury. Biomark Med. 2010, 4, 265–280. [Google Scholar] [CrossRef]
- Katagiri, D.; Doi, K.; Honda, K.; Negishi, K.; Fujita, T.; Hisagi, M.; Ono, M.; Matsubara, T.; Yahagi, N.; Iwagami, M.; et al. Combination of two urinary biomarkers predicts acute kidney injury after adult cardiac surgery. Ann. Thorac. Surg. 2012, 93, 577–583. [Google Scholar] [CrossRef]
- Urbschat, A.; Gauer, S.; Paulus, P.; Reissig, M.; Weipert, C.; Ramos-Lopez, E.; Hofmann, R.; Hadji, P.; Geiger, H.; Obermuller, N. Serum and urinary NGAL but not KIM-1 raises in human postrenal AKI. Eur. J. Clin. Investig. 2014, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Fugmann, T.; Borgia, B.; Revesz, C.; Godo, M.; Forsblom, C.; Hamar, P.; Holthofer, H.; Neri, D.; Roesli, C. Proteomic identification of vanin-1 as a marker of kidney damage in a rat model of type 1 diabetic nephropathy. Kidney Int. 2011, 80, 272–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosohata, K.; Ando, H.; Fujiwara, Y.; Fujimura, A. Vanin-1: A potential biomarker for nephrotoxicant-induced renal injury. Toxicology 2011, 290, 82–88. [Google Scholar] [CrossRef]
- Hosohata, K. Role of Oxidative Stress in Drug-Induced Kidney Injury. Int. J. Mol. Sci. 2016, 17, 1826. [Google Scholar] [CrossRef]
- Pitari, G.; Malergue, F.; Martin, F.; Philippe, J.M.; Massucci, M.T.; Chabret, C.; Maras, B.; Dupre, S.; Naquet, P.; Galland, F. Pantetheinase activity of membrane-bound Vanin-1: Lack of free cysteamine in tissues of Vanin-1 deficient mice. FEBS Lett. 2000, 483, 149–154. [Google Scholar] [CrossRef]
- Hosohata, K.; Jin, D.; Takai, S.; Iwanaga, K. Vanin-1 in Renal Pelvic Urine Reflects Kidney Injury in a Rat Model of Hydronephrosis. Int. J. Mol. Sci. 2018, 19, 3186. [Google Scholar] [CrossRef] [PubMed]
- Aurrand-Lions, M.; Galland, F.; Bazin, H.; Zakharyev, V.M.; Imhof, B.A.; Naquet, P. Vanin-1, a novel GPI-linked perivascular molecule involved in thymus homing. Immunity 1996, 5, 391–405. [Google Scholar] [CrossRef]
- Hosohata, K.; Ando, H.; Fujimura, A. Urinary vanin-1 as a novel biomarker for early detection of drug-induced acute kidney injury. J. Pharmacol. Exp. Ther. 2012, 341, 656–662. [Google Scholar] [CrossRef]
- Ivan, M.V.; Rogobete, A.F.; Bedreag, O.H.; Papurica, M.; Popovici, S.E.; Dinu, A.; Sandesc, M.; Beceanu, A.; Bratu, L.M.; Popoiu, C.M.; et al. New Molecular and Epigenetic Expressions as Novel Biomarkers in Critically Ill Polytrauma Patients with Acute Kidney Injury (AKI). Clin. Lab. 2018, 64, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.C.; Chen, C.C.; Chen, Y.C.; Chang, Y.S.; Chu, P.H. MicroRNAs in acute kidney injury. Hum. Genome 2016, 10, 29. [Google Scholar] [CrossRef]
- Aguado-Fraile, E.; Ramos, E.; Conde, E.; Rodriguez, M.; Martin-Gomez, L.; Lietor, A.; Candela, A.; Ponte, B.; Liano, F.; Garcia-Bermejo, M.L. A Pilot Study Identifying a Set of microRNAs as Precise Diagnostic Biomarkers of Acute Kidney Injury. PLoS ONE 2015, 10, e0127175. [Google Scholar] [CrossRef] [PubMed]
- Kengen, R.A.; Meijer, S.; Beekhuis, H.; Piers, D.A. Technetium-99m-MAG3 clearance as a parameter of effective renal plasma flow in patients with proteinuria and lowered serum albumin levels. J. Nucl. Med. 1991, 32, 1709–1712. [Google Scholar] [PubMed]
- Itoh, K.; Tsukamoto, E.; Kakizaki, H.; Nonomura, K.; Furudate, M. Comparative study of renal scintigraphy with 99Tcm-mercaptoacetyltriglycine and 123I-orthoiodohippurate. Nucl. Med. Commun. 1993, 14, 644–652. [Google Scholar] [CrossRef] [PubMed]


| Charactesitics | Details | Control | HN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 21 | Extrinsic, n = 16 | Intrinsic, n = 12 | Total, n = 28 | ||||||
| Age, mean ± SD | 67.5 ± 10.73 | 58.63 ± 13.54 | 47.48 ± 13.87 | 53.85 ± 14.55 * | |||||
| Sex, n (%) | Men | 19 | (89) | 6 | (38) | 6 | (50) | 12 | (43) |
| Women | 2 | (11) | 10 | (62) | 6 | (50) | 16 * | (57) | |
| Affected kidney, n (%) | Right (Rt) | NA | 9 | (56) | 5 | (42) | 14 | (50) | |
| Left (Lt) | NA | 7 | (44) | 7 | (58) | 14 | (50) | ||
| Kidney disease other than HN, n (%) | Diabetic nephropathy | 3 | (14) | 0 | (0) | 0 | (0) | 0 | (0) |
| Hypertensive nephropathy | 3 | (14) | 0 | (0) | 0 | (0) | 0 | (0) | |
| Drug-induced nephropathy | 0 | (0) | 1 | (6) | 1 | (8) | 2 | (7) | |
| Total | 6 | (29) | 1 | (6) | 1 | (8) | 2 | (7) | |
| Variables | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| N | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| eGFR, mL/min/1.73 m2 | 37 | (-) | (-) | 0.534 | (-) | (-) | 0.262 |
| Vanin-1, ng/mgCre | 37 | 1.445 | 1.042–2.008 | 0.028 | 1.546 | 1.046–2.283 | 0.029 |
| KIM-1, ng/mgCre | 37 | (-) | (-) | 0.319 | (-) | (-) | 0.224 |
| NGAL, ng/mgCre | 37 | 1.030 | 0.999–1.060 | 0.060 | (-) | (-) | 0.251 |
| NAG, U/mgCre | 37 | 1.447 | 1.092–1.919 | 0.010 | 1.458 | 1.067–1.992 | 0.018 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washino, S.; Hosohata, K.; Oshima, M.; Okochi, T.; Konishi, T.; Nakamura, Y.; Saito, K.; Miyagawa, T. A Novel Biomarker for Acute Kidney Injury, Vanin-1, for Obstructive Nephropathy: A Prospective Cohort Pilot Study. Int. J. Mol. Sci. 2019, 20, 899. https://doi.org/10.3390/ijms20040899
Washino S, Hosohata K, Oshima M, Okochi T, Konishi T, Nakamura Y, Saito K, Miyagawa T. A Novel Biomarker for Acute Kidney Injury, Vanin-1, for Obstructive Nephropathy: A Prospective Cohort Pilot Study. International Journal of Molecular Sciences. 2019; 20(4):899. https://doi.org/10.3390/ijms20040899
Chicago/Turabian StyleWashino, Satoshi, Keiko Hosohata, Masashi Oshima, Tomohisa Okochi, Tsuzumi Konishi, Yuhki Nakamura, Kimitoshi Saito, and Tomoaki Miyagawa. 2019. "A Novel Biomarker for Acute Kidney Injury, Vanin-1, for Obstructive Nephropathy: A Prospective Cohort Pilot Study" International Journal of Molecular Sciences 20, no. 4: 899. https://doi.org/10.3390/ijms20040899
APA StyleWashino, S., Hosohata, K., Oshima, M., Okochi, T., Konishi, T., Nakamura, Y., Saito, K., & Miyagawa, T. (2019). A Novel Biomarker for Acute Kidney Injury, Vanin-1, for Obstructive Nephropathy: A Prospective Cohort Pilot Study. International Journal of Molecular Sciences, 20(4), 899. https://doi.org/10.3390/ijms20040899






