Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti-Tumor Effects Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Rh2, R-PHQ, and S-PHQ on Cyclophosphamide-Induced Immunosuppression
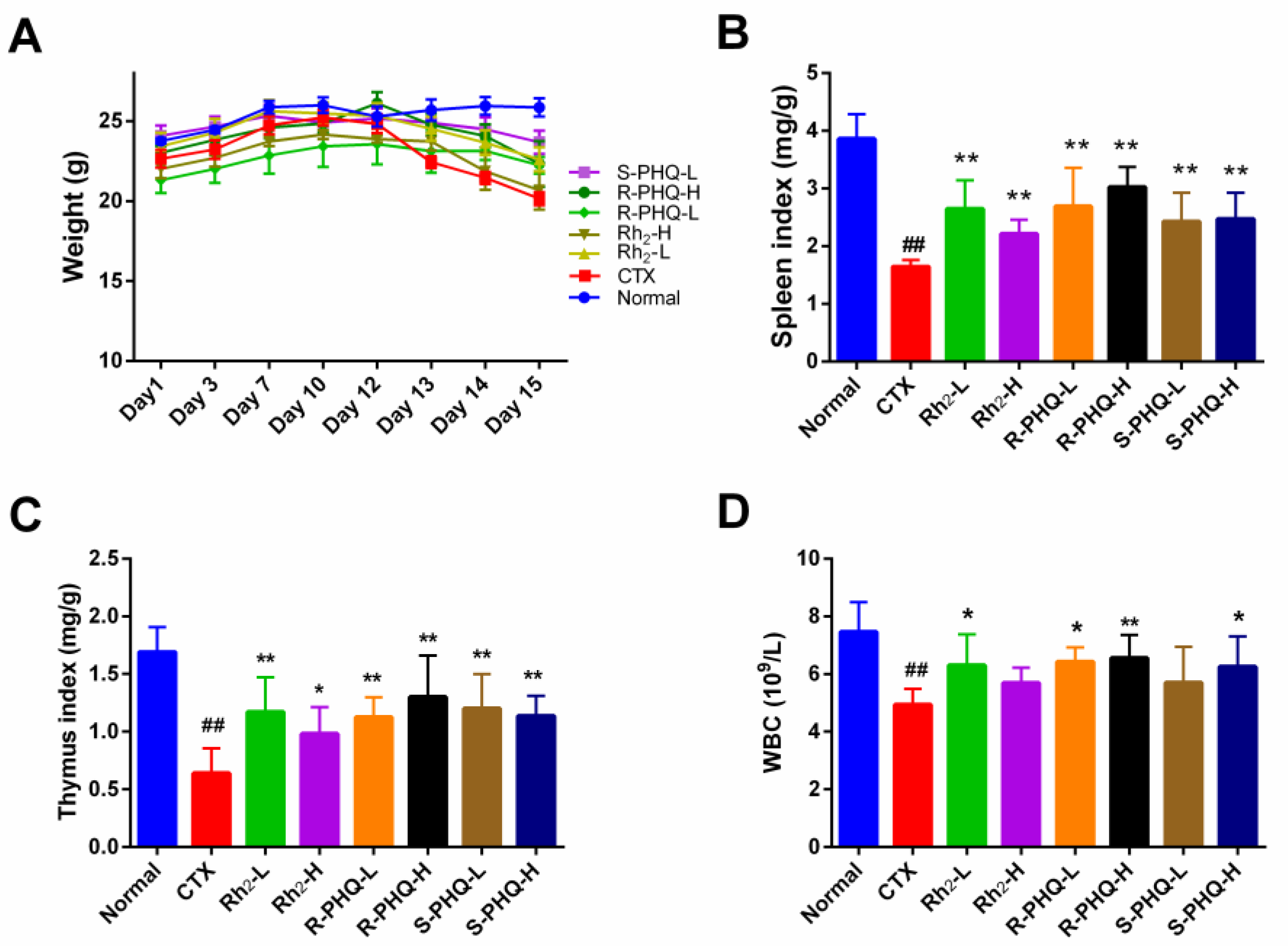
2.1.1. Effect of Rh2, R-PHQ, and S-PHQ on Body Weight and Immune Organ Indexes
2.1.2. Effect of Rh2, R-PHQ, and S-PHQ on WBC
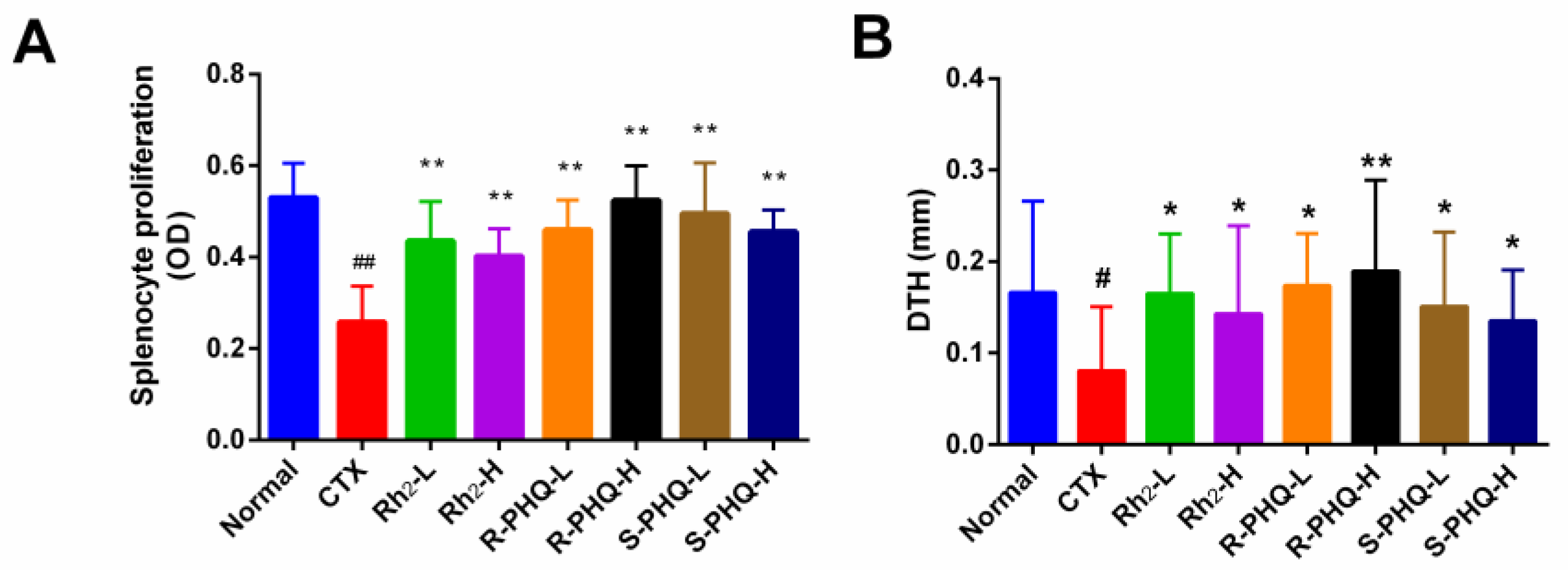
2.1.3. Effect of Rh2, R-PHQ, and S-PHQ on Cellular Immunity
Effect of Rh2, R-PHQ, and S-PHQ on Delayed-Type Hypersensitivity (DTH) to Sheep Red Blood Cells (SRBC)
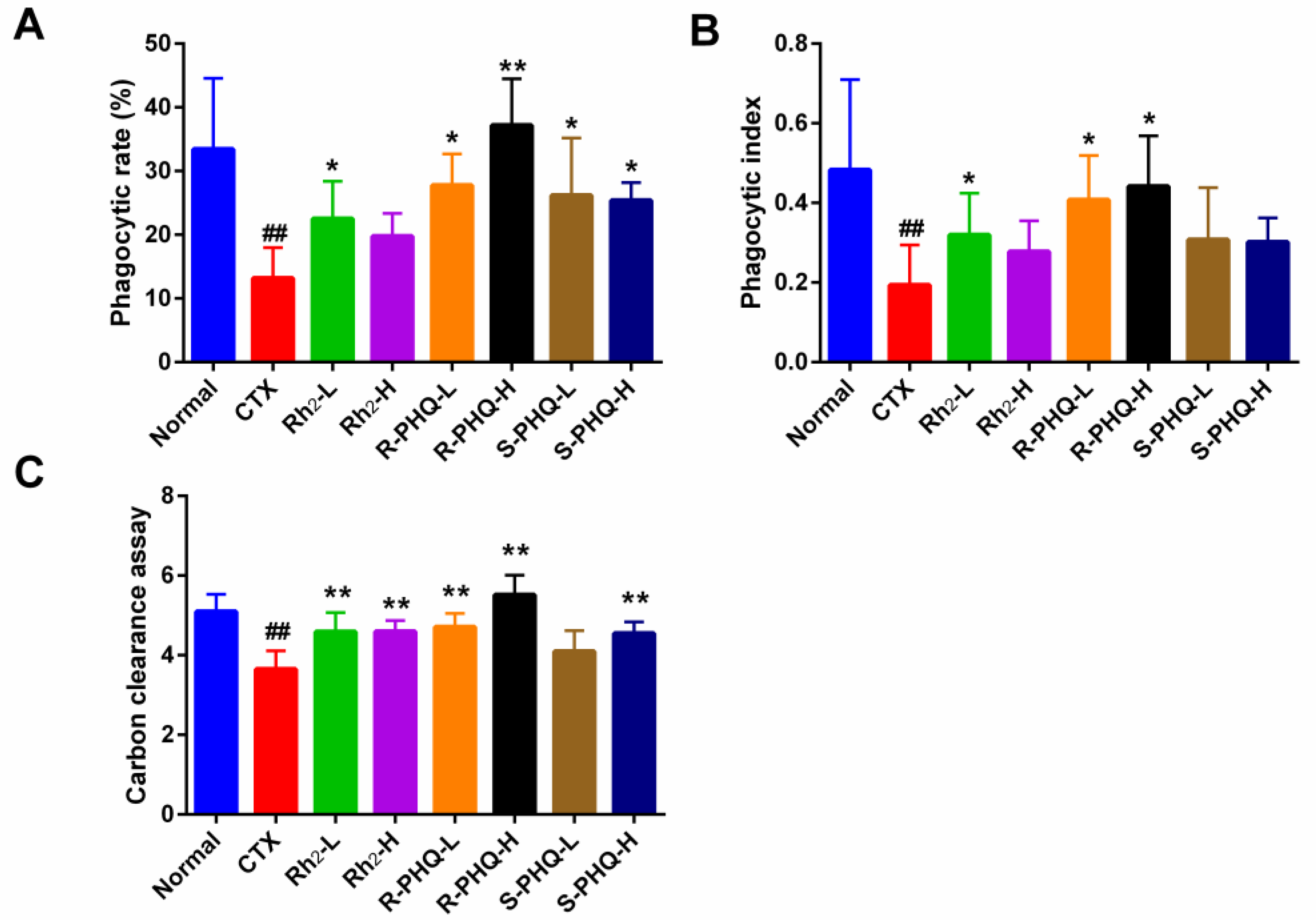
2.1.4. Effect of Rh2, R-PHQ, and S-PHQ on Macrophage Phagocytosis
Effect of Rh2, R-PHQ, and S-PHQ on the Phagocytic Capacity of Macrophage Cells
Effect of Rh2, R-PHQ, and S-PHQ on the Carbon Clearance Assay
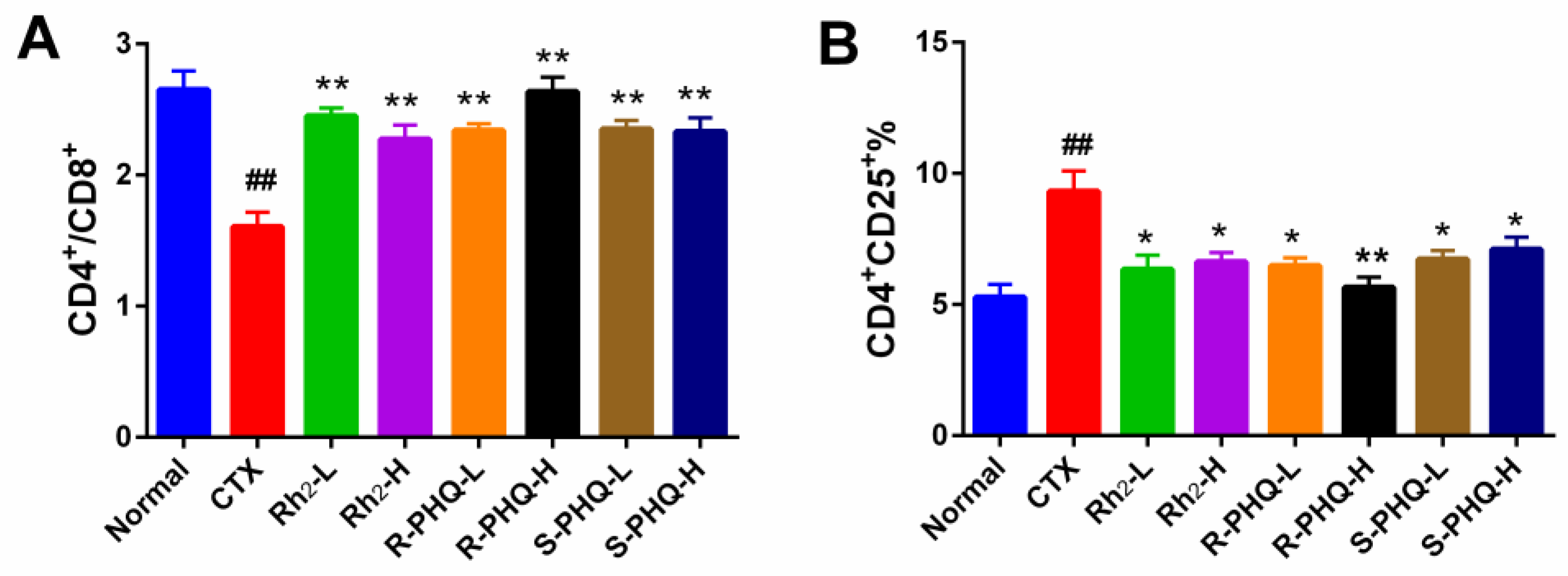
2.1.5. Effect of Rh2, R-PHQ, and S-PHQ on Splenic T-Lymphocyte Subpopulations
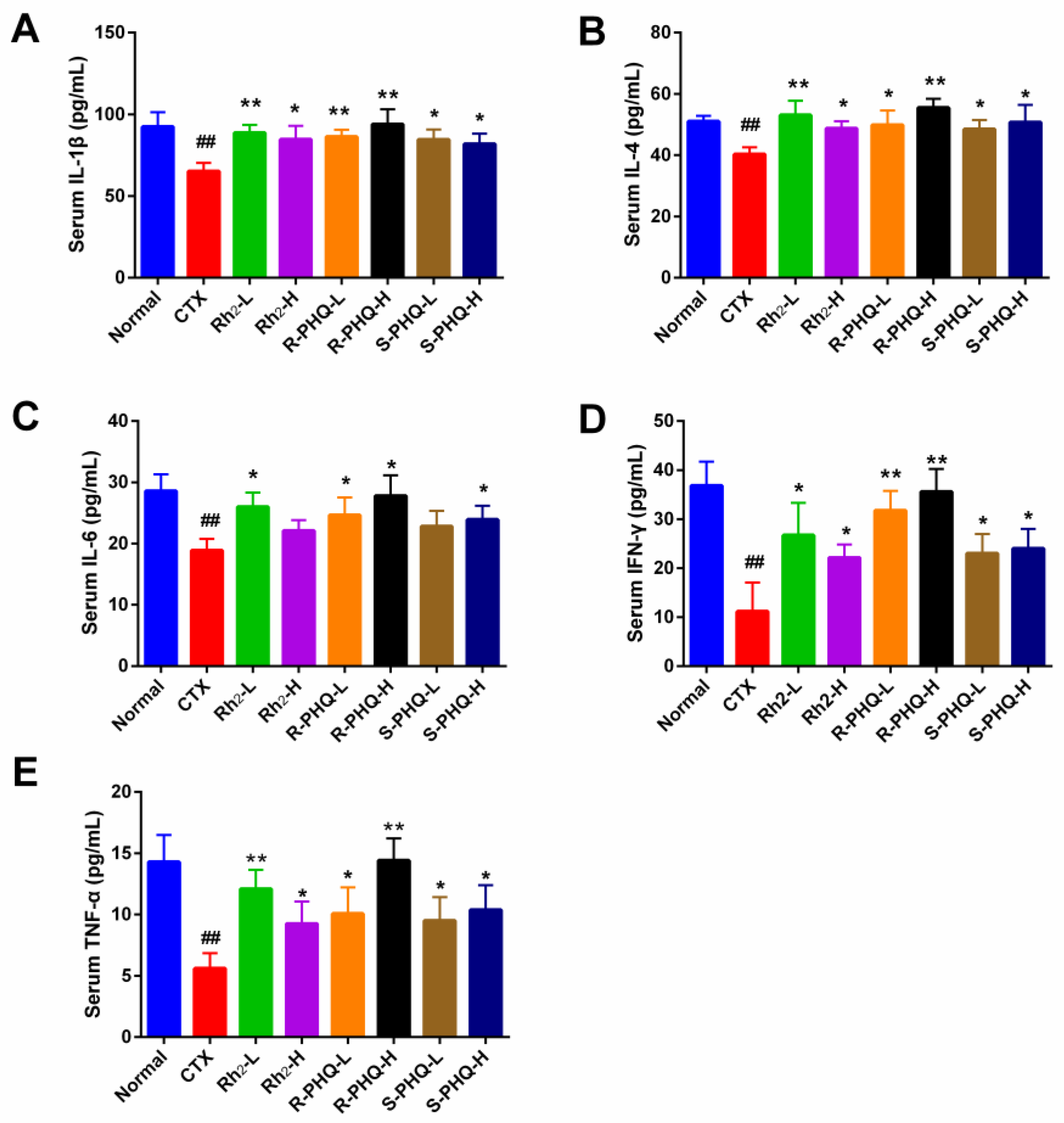
2.1.6. Effect of Rh2, R-PHQ, and S-PHQ on Cytokine Concentrations in Serum
2.2. Anti-Tumor Activity
2.2.1. Inhibition Effect of R-PHQ and S-PHQ on H22 Hepatocellular Carcinoma
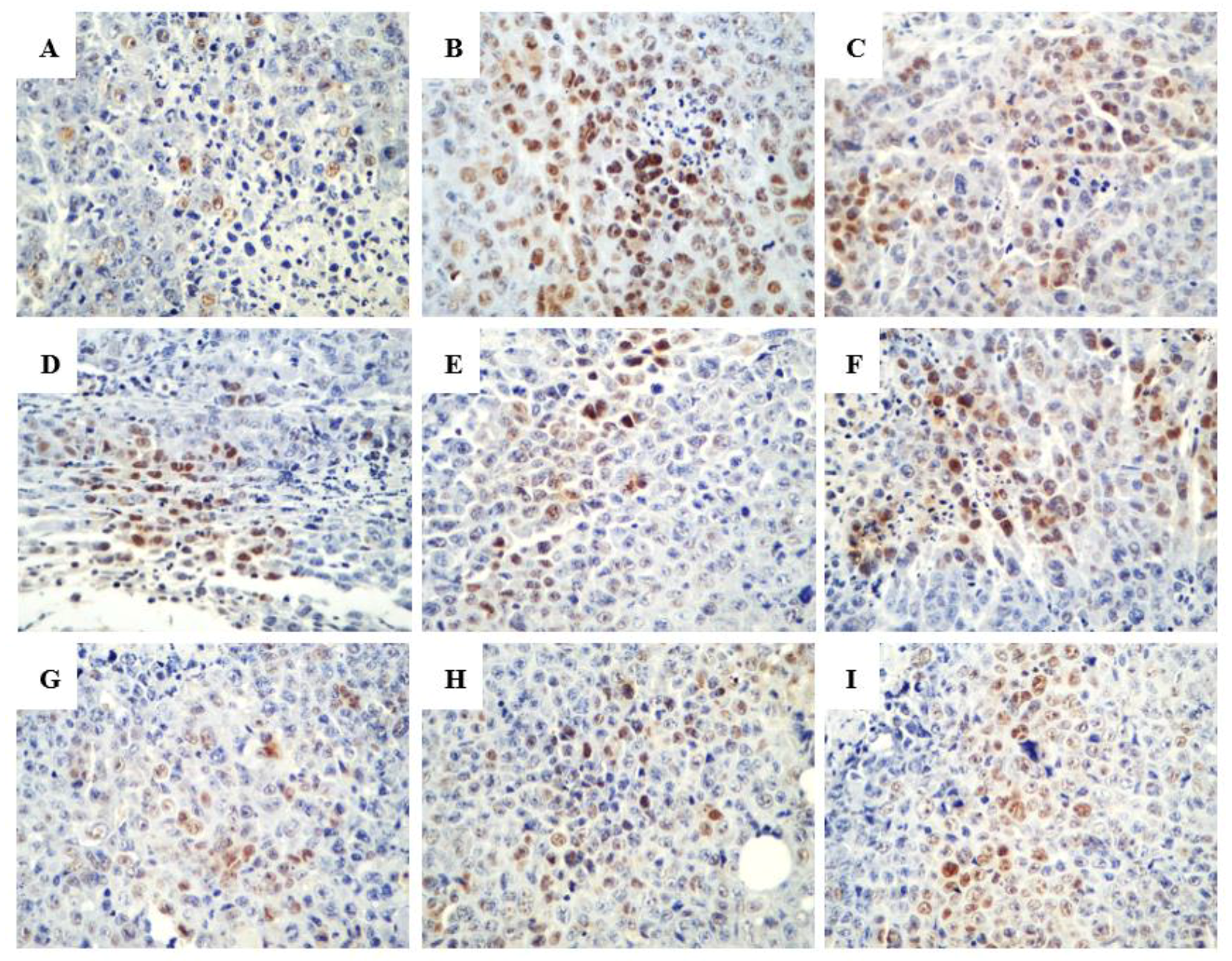
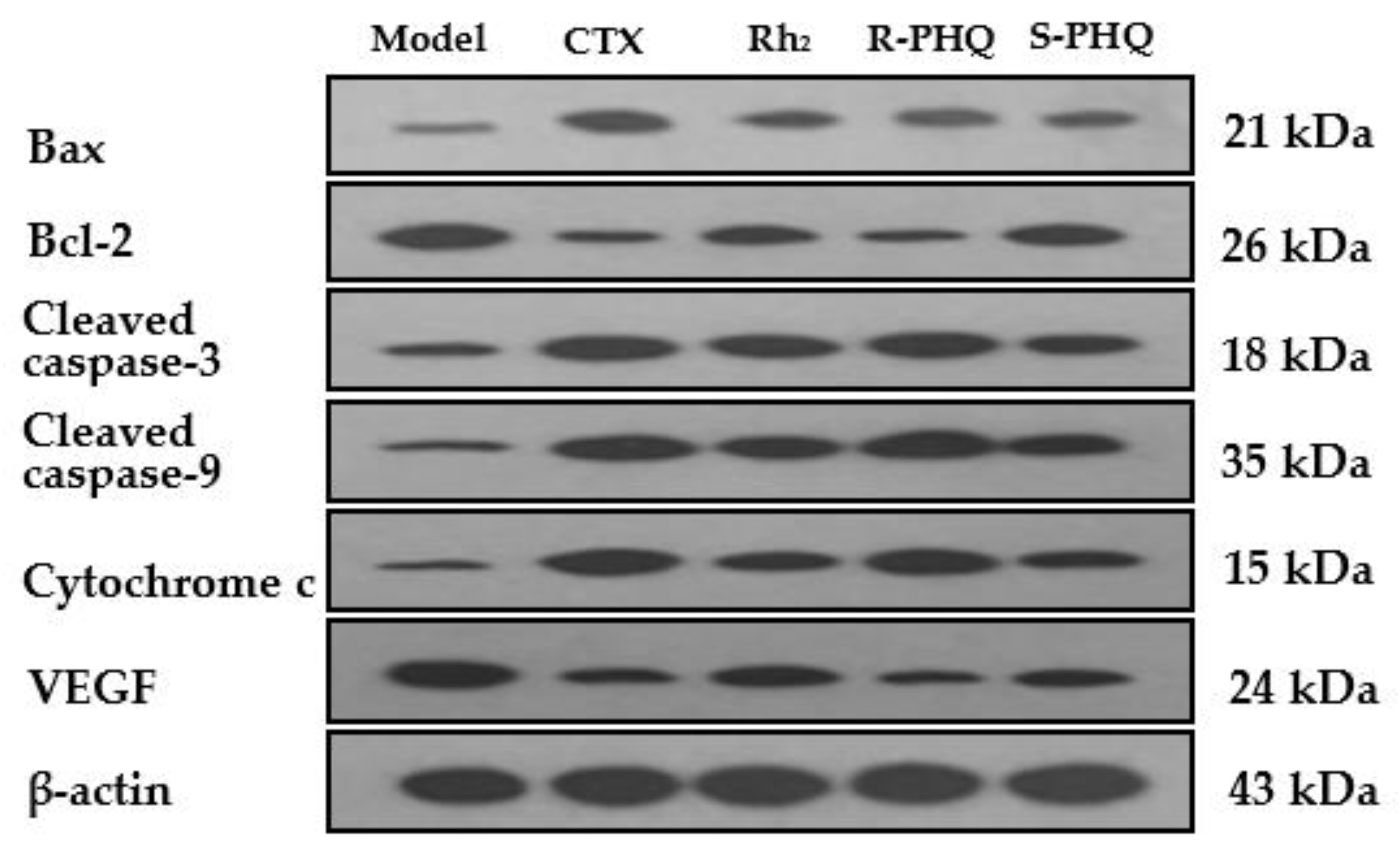
2.2.2. Promoting the Apoptotic Effect of R-PHQ and S-PHQ on H22 Hepatocellular Carcinoma
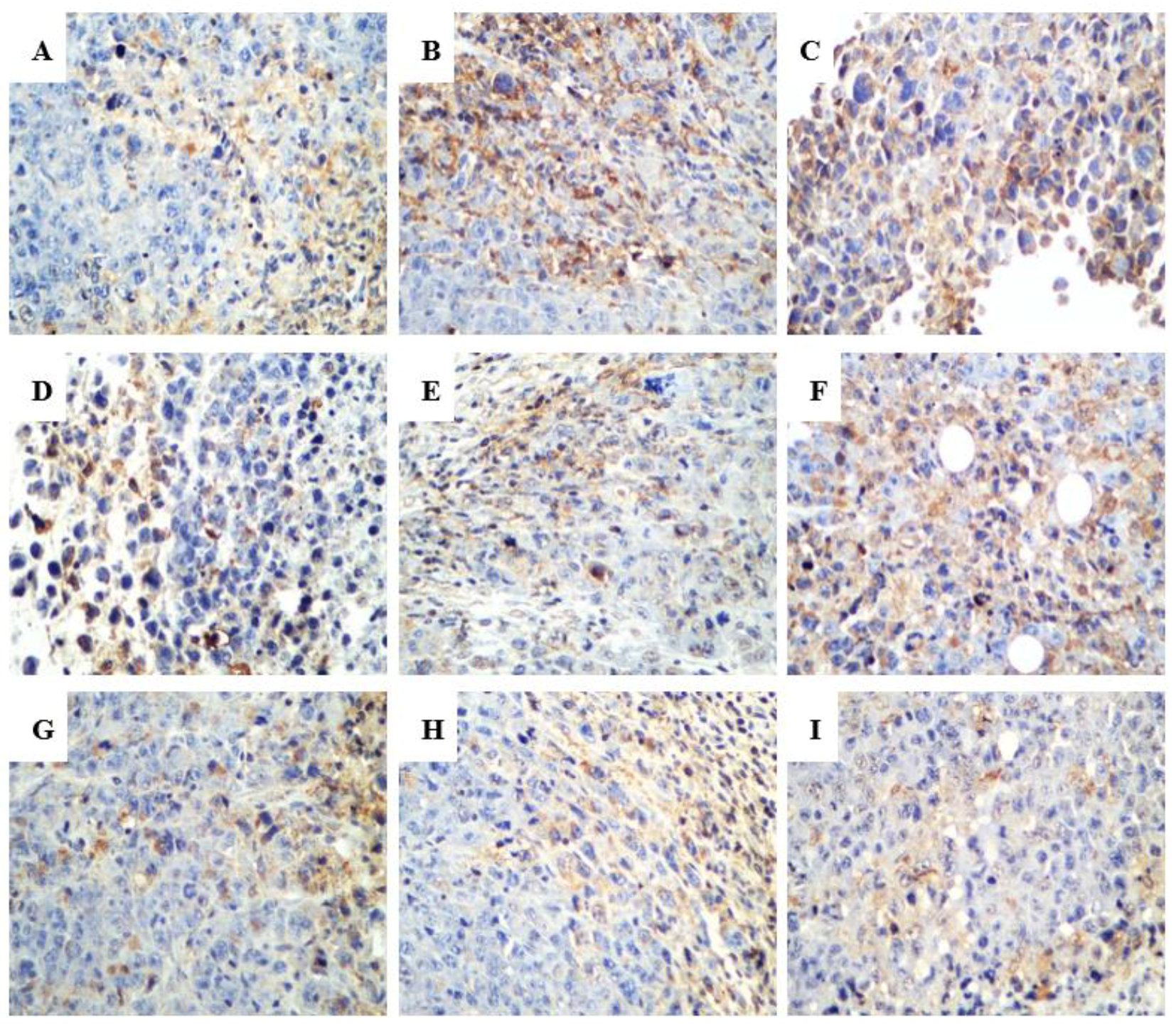
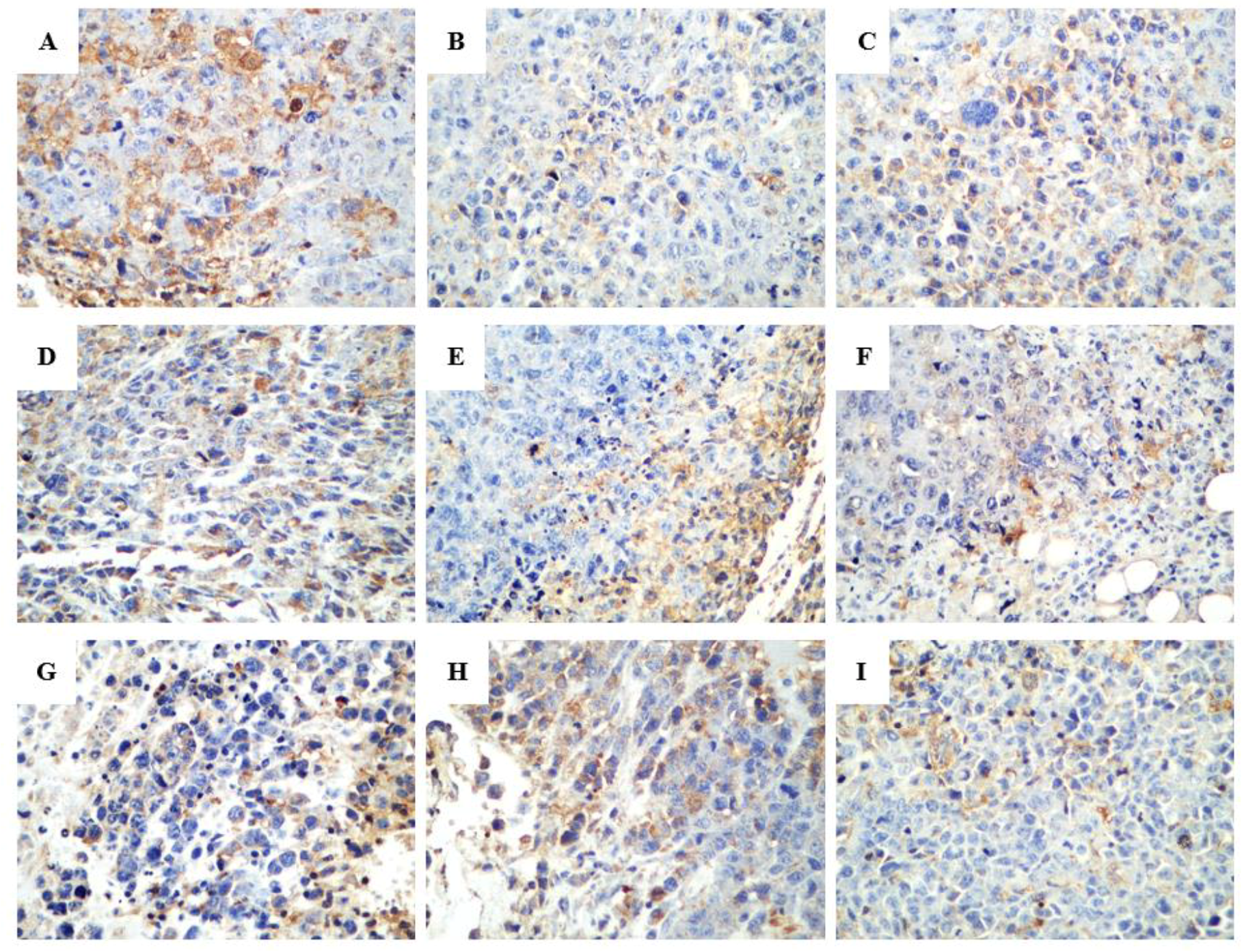
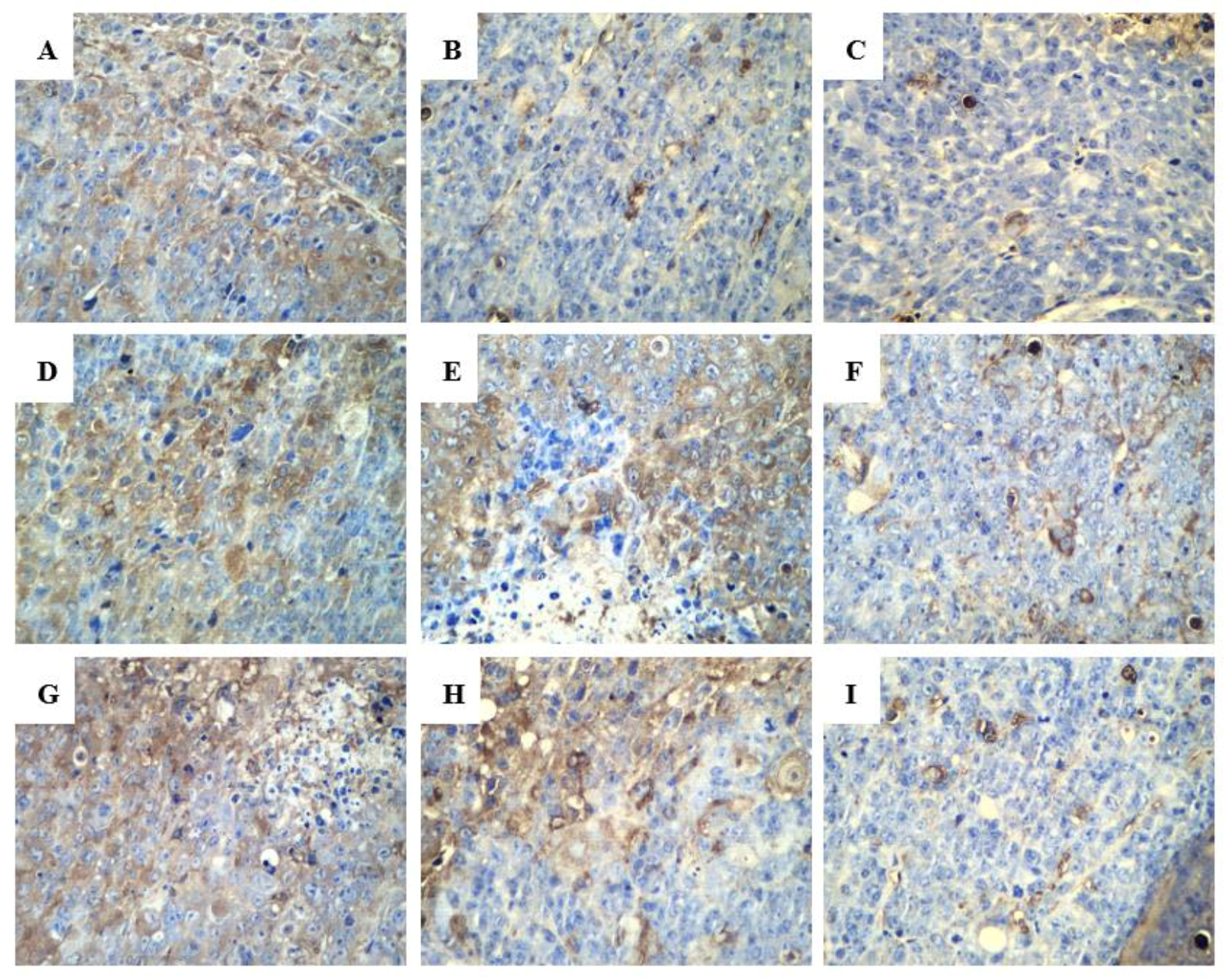
2.2.3. Effect of R-PHQ and S-PHQ on Tumor Angiogenesis
3. Materials and Methods
3.1. Reagents and Animals
3.2. Establishment of Cyclophosphamide-Induced Immunosuppression Experimental Animal Model and Drugs Evaluation
3.2.1. SRBC-Induced DTH
3.2.2. Carbon Granular Clearance assay
3.2.3. Phagocytic Function of Peritoneal Macrophage
3.2.4. Splenocyte Proliferation Assay
3.2.5. Mouse Leukocyte Assay and Splenic T-Lymphocyte Subpopulations Assay
3.2.6. Determination of Cytokines in Serum
3.3. Establishment of the Xenograft Tumor Model and Drug Treatment
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| R-PHQ | (24R)-pseudo-ginsenoside HQ |
| S-PHQ | (24S)-pseudo-ginsenoside HQ |
| Rh2 | (20S)-ginsenoside Rh2 |
| CTX | cyclophosphamide |
| PGQ | pseudo-ginsenoside GQ |
| PDQ | pseudosapogenin DQ |
| PHQ | pseudo-ginsenoside HQ |
| WBC | White blood cells |
| ConA | Concanavalin A |
| DTH | Delayed-type hypersensitivity |
| SRBC | sheep red blood cells |
| VEGF | Vascular endothelial growth factor |
References
- Wang, Y.; Qi, Q.; Li, A.; Yang, M.; Huang, W.; Xu, H.; Zhao, Z.; Li, S. Immuno-enhancement effects of Yifei Tongluo Granules on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2016, 194, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, G.; Chen, H.; Zhang, W.; Ding, Y.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.J.C.P. Se-enriched G. frondosa polysaccharide protects against immunosuppression in cyclophosphamide-induced mice via MAPKs signal transduction pathway. Carbohydr. Polym. 2018, 196, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Li, Z.; Li, W.; Liu, Y.; Wang, C.; Lin, H.; Liu, J.; Li, P. Pseudoginsengenin DQ Exhibits Therapeutic Effects in Cisplatin-Induced Acute Kidney Injury via Sirt1/NF-kappaB and Caspase Signaling Pathway without Compromising Its Antitumor Activity in Mice. Molecules 2018, 23, 3038. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.; Wang, Z.; Zhou, B.; Fu, S.; Hong, T.; Li, P.; Liu, J. A new ocotillol-type ginsenoside from stems and leaves of Panax quinquefolium L. and its anti-oxidative effect on hydrogen peroxide exposed A549 cells. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, J.; Yan, M.; Xing, J.; Liu, W.; Li, W.J.J.A.F.C. Caspase-mediated anti-apoptotic effect of Ginsenoside Rg5, a main rare ginsenoside, on Acetaminophen-induced Hepatotoxicity in Mice. J. Agric. Food Chem. 2017, 65, 9226–9236. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.Q.; Wang, C.Z.; Wang, Z.Z.; Li, Z.; Sai, J.Y.; Meng, Y.; Wang, F.; Li, P.Y.; Liu, J.P. Anti-myocardial ischaemic effect of pseudoginsenoside F11 by inhibiting expression of beta1-adrenoceptor in rats with coronary artery ligation. J. Funct. Foods 2017, 36, 224–232. [Google Scholar] [CrossRef]
- Pan, W.; Xue, B.; Yang, C.; Miao, L.; Zhou, L.; Chen, Q.; Cai, Q.; Liu, Y.; Liu, D.; He, H.J.F. Biopharmaceutical characters and bioavailability improving strategies of ginsenosides. Fitoterapia 2018, 129, 272–282. [Google Scholar] [CrossRef]
- Xiong, L.; Qi, Z.; Zheng, B.; Li, Z.; Wang, F.; Liu, J.; Li, P.J.M. Inhibitory Effect of Triterpenoids from Panax ginseng on Coagulation Factor X. Molecules 2017, 22, 649. [Google Scholar] [CrossRef]
- Wei, G.Q.; Zheng, Y.N.; Li, W.; Liu, W.C.; Lin, T.; Zhang, W.Y.; Chen, H.F.; Zeng, J.Z.; Zhang, X.K.; Chen, Q.C. Structural modification of ginsenoside Rh 2 by fatty acid esterification and its detoxification property in antitumor. Bioorg. Med. Chem. Lett. 2012, 22, 1082–1085. [Google Scholar] [CrossRef]
- Qian, G.; Wang, Z.; Zhao, J.; Li, D.; Gao, W.; Wang, B.; Sui, D.; Qu, X.; Chen, Y.J.S. Synthesis and anti-cancer cell activity of pseudo-ginsenoside Rh2. Steroids 2014, 92, 1–6. [Google Scholar] [CrossRef]
- Qian, T.; Cai, Z.; Wong, R.N.; Jiang, Z.H. Liquid chromatography/mass spectrometric analysis of rat samples for in vivo metabolism and pharmacokinetic studies of ginsenoside Rh2. Rapid Commun. Mass Spectrom. 2010, 19, 3549–3554. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Cai, Z.; Wong, R.N.; Mak, N.K.; Jiang, Z.H. In vivo rat metabolism and pharmacokinetic studies of ginsenoside Rg3. J. Chromatogr. B 2005, 816, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Tong, T.T.; Yau, L.F.; Chen, C.Y.; Bai, L.P.; Ma, J.; Hu, M.; Liu, L.; Jiang, Z.H. Characterization of oxygenated metabolites of ginsenoside Rg 1 in plasma and urine of rat. J. Chromatogr. B 2016, 1026, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.R.; Yau, L.F.; Zhang, R.; Xia, Y.; Ma, J.; Ho, H.M.; Hu, P.; Hu, M.; Liu, L.; Jiang, Z.H. Transformation of ginsenosides from notoginseng by artificial gastric juice can increase cytotoxicity toward cancer cells. J. Agric. Food Chem. 2014, 62, 2558–2573. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, X.; Sun, T.; Gao, Y.; Chen, Y.; Jin, Y.; Li, Y. Semisynthesis and bioactive evaluation of oxidized products from 20(S)-ginsenoside Rg3, Rh2, protopanaxadiol (PPD) and their 20(R)-epimers as cytotoxic agents. Steroids 2016, 106, 26–34. [Google Scholar] [CrossRef]
- Wilkinson, G.R. Drug Metabolism and Variability among Patients in Drug Response. N. Engl. J. Med. 2005, 352, 2211–2221. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the microbiota and the immune system. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Chen, F.; Sun, Y.; Zheng, S.-L.; Qin, Y.; Julian McClements, D.; Hu, J.-N.; Deng, Z.-Y. Antitumor and immunomodulatory effects of ginsenoside Rh2 and its octyl ester derivative in H22 tumor-bearing mice. J. Funct. Foods 2017, 32, 382–390. [Google Scholar] [CrossRef]
- Hou, J.; Xue, J.; Zhao, X.; Wang, Z.; Li, W.; Li, X.; Zheng, Y. Octyl ester of ginsenoside compound K as novel anti-hepatoma compound: Synthesis and evaluation on murine H22 cells in vitro and in vivo. Chem. Biol. Drug Des. 2018, 91, 951–956. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, Y.N.; Qian, S.; Gao, R.L.; Yin, L.M.; Wang, L.P.; Chong, B.H.; Zhang, S.Z. Ginseng-Derived Panaxadiol Saponins Promote Hematopoiesis Recovery in Cyclophosphamide-Induced Myelosuppressive Mice: Potential Novel Treatment of Chemotherapy-Induced Cytopenias. Chin. J. Integr. Med. 2018, 24, 200–206. [Google Scholar] [CrossRef]
- Anisimova, N.; Ustyuzhanina, N.; Bilan, M.; Donenko, F.; Usov, A.; Kiselevskiy, M.; Nifantiev, N. Fucoidan and Fucosylated Chondroitin Sulfate Stimulate Hematopoiesis in Cyclophosphamide-Induced Mice. Mar. Drugs 2017, 15, 301. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wang, Y.; Cai, E.; Zhang, L.; Zhao, Y.; Sun, N.; Zheng, X.; Wang, S. Study of the Effects and Mechanisms of Ginsenoside Compound K on Myelosuppression. J. Agric. Food Chem. 2019, 67, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.W.; Han, C.J.; Rhee, Y.K.; Lee, Y.C.; Shin, K.S.; Shin, J.S.; Lee, K.T.; Hong, H.D. Cheonggukjang polysaccharides enhance immune activities and prevent cyclophosphamide-induced immunosuppression. Int. J. Biol. Macromol. 2015, 72, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Aderem, A.; Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999, 17, 593–623. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tong, X.; Li, P.; Cao, H.; Su, W. Immuno-enhancement effects of Shenqi Fuzheng Injection on cyclophosphamide-induced immunosuppression in Balb/c mice. J. Ethnopharmacol. 2012, 139, 788–795. [Google Scholar] [CrossRef]
- Wongchana, W.; Kongkavitoon, P.; Tangtanatakul, P.; Sittplangkoon, C.; Butta, P.; Chawalitpong, S.; Pattarakankul, T.; Osborne, B.A.; Palaga, T. Notch signaling regulates the responses of lipopolysaccharide-stimulated macrophages in the presence of immune complexes. PLoS ONE 2018, 13, e0198609. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, G.; Zhang, L.; Cheng, S.; Luo, C.; Liao, Y.; Guo, S. Therapeutic efficacy of hydrogen-rich saline alone and in combination with PI3K inhibitor in non-small cell lung cancer. Mol. Med. Rep. 2018, 18, 2182–2190. [Google Scholar] [CrossRef]
- Peng, W.; Hu, C.; Shu, Z.; Han, T.; Qin, L.; Zheng, C. Antitumor activity of tatariside F isolated from roots of Fagopyrum tataricum (L.) Gaertn against H22 hepatocellular carcinoma via up-regulation of p53. Phytomedicine 2015, 22, 730–736. [Google Scholar] [CrossRef]
- Li, W.; Xu, Q.; He, Y.-F.; Liu, Y.; Yang, S.-B.; Wang, Z.; Zhang, J.; Zhao, L.-C. Anti-Tumor Effect of Steamed Codonopsis lanceolata in H22 Tumor-Bearing Mice and Its Possible Mechanism. Nutrients 2015, 7, 8294–9307. [Google Scholar] [CrossRef]
- Hu, W.-H.; Chan, G.K.-L.; Lou, J.-S.; Wu, Q.-Y.; Wang, H.-Y.; Duan, R.; Cheng, M.Y.-T.; Dong, T.T.-X.; Tsim, K.W.-K. The extract of Polygoni Cuspidati Rhizoma et Radix suppresses the vascular endothelial growth factor-induced angiogenesis. Phytomedicine 2018, 42, 135–143. [Google Scholar] [CrossRef]
- Muhammad, A.; Abubakar, I.B.; Etti, I.C.; Waziri, P.M.; Abubakar, R.M.; Mshelia, H.E. 5,6-dehydrokawain from the rhizome of Alpinia mutica Roxb. induced proangiogenic tumour-derived VEGF of HT-29 colorectal cancer AU—Malami, Ibrahim. Nat. Prod. Res. 2017, 1–4. [Google Scholar] [CrossRef]
- Souza, F.D.O.; Sorbo, J.M.; Regasini, L.O.; Bolzani, V.D.S.; Rosa, J.C.; Czernys, É.D.S.; Valente, V.; Moreira, T.F.; Navegante, G.; Fernandes, B.C.; Soares, C.P. Nitensidine B affects proteins of the glycolytic pathway and induces apoptosis in cervical carcinoma cells immortalized by HPV16. Phytomedicine 2018, 48, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Thuy, L.T.; Lee, Y.H.; Ko, K.S.; Han, J.; Choi, J.S. Polyplexes of Functional PAMAM Dendrimer/Apoptin Gene Induce Apoptosis of Human Primary Glioma Cells In Vitro. Polymers 2019, 11, 296. [Google Scholar] [CrossRef]
- Guo, X.X.; Guo, Q.; Li, Y.; Lee, S.K.; Wei, X.N.; Jin, Y.H. Ginsenoside Rh2 induces human hepatoma cell apoptosisvia bax/bak triggered cytochrome C release and caspase-9/caspase-8 activation. IJMS 2012, 13, 15523–15535. [Google Scholar] [CrossRef] [PubMed]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Dewson, G.; Kluck, R.M. Mechanisms by which Bak and Bax permeabilise mitochondria during apoptosis. J. Cell Sci. 2009, 122, 2801–2808. [Google Scholar] [CrossRef]
- Shamas-Din, A.; Kale, J.; Leber, B.; Andrews, D.W. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb. Perspect. Biol. 2013, 5, a008714. [Google Scholar] [CrossRef]
- Kalkavan, H.; Green, D.R. MOMP, cell suicide as a BCL-2 family business. Cell Death Differ. 2017, 25, 46. [Google Scholar] [CrossRef]
- Zhuang, J.; Yin, J.; Xu, C.; Mu, Y.; Lv, S. 20(S)-Ginsenoside Rh2 Induce the Apoptosis and Autophagy in U937 and K562 Cells. Nutrients 2018, 10, 328. [Google Scholar] [CrossRef]
- Wu, R.; Ru, Q.; Chen, L.; Ma, B.; Li, C. Stereospecificity of ginsenoside Rg3 in the promotion of cellular immunity in hepatoma H22-bearing mice. J. Food. Sci. 2014, 79, 1430–1435. [Google Scholar] [CrossRef]
- Qi, Z.; Li, W.; Tan, J.; Wang, C.; Lin, H.; Zhou, B.; Liu, J.; Li, P. Effect of ginsenoside Rh2 on renal apoptosis in cisplatin-induced nephrotoxicity in vivo. Phytomedicine 2019, 152862. [Google Scholar] [CrossRef]
- Lin, M.; Li, H.; Zhao, Y.; Cai, E.; Zhu, H.; Gao, Y.; Liu, S.; Yang, H.; Zhang, L.; Tang, G. 2-Naphthoic acid ergosterol ester, an ergosterol derivative, exhibits anti-tumor activity by promoting apoptosis and inhibiting angiogenesis. Steroids 2017, 122, 9–15. [Google Scholar] [CrossRef] [PubMed]
| Groups | TW (g) | TIR (%) |
|---|---|---|
| Model control | 2.46 ± 0.66 | - |
| CTX | 1.06 ± 0.20 ** | 57.09% |
| Rh2 (5 mg/kg) | 2.09 ± 0.41 | 15.12% |
| Rh2 (10 mg/kg) | 1.65 ± 0.32 ** | 32.91% |
| Rh2 (20 mg/kg) | 1.28 ± 0.30 ** | 47.83% |
| R-PHQ (5 mg/kg) | 1.54 ± 0.33 ** | 37.34% |
| R-PHQ (10 mg/kg) | 1.32 ± 0.49 ** | 46.24% |
| R-PHQ (20 mg/kg) | 1.27 ± 0.30 ** | 48.52% |
| S-PHQ (5 mg/kg) | 1.94 ± 0.50 ** | 21.25% |
| S-PHQ (10 mg/kg) | 1.65 ± 0.54 ** | 32.83% |
| S-PHQ (20 mg/kg) | 1.35 ± 0.38 ** | 44.98% |
| CTX + R-PHQ (20 mg/kg) | 0.99 ± 0.37 ** | 59.88% |
| CTX + S-PHQ (20 mg/kg) | 0.80 ± 0.47 ** | 67.57% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Chen, L.; Li, Z.; Shao, Z.; Qi, Y.; Gao, K.; Liu, S.; Sun, Y.; Li, P.; Liu, J. Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti-Tumor Effects Study. Int. J. Mol. Sci. 2019, 20, 836. https://doi.org/10.3390/ijms20040836
Qi Z, Chen L, Li Z, Shao Z, Qi Y, Gao K, Liu S, Sun Y, Li P, Liu J. Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti-Tumor Effects Study. International Journal of Molecular Sciences. 2019; 20(4):836. https://doi.org/10.3390/ijms20040836
Chicago/Turabian StyleQi, Zeng, Lixue Chen, Zhuo Li, Zijun Shao, Yuli Qi, Kun Gao, Songxin Liu, Yinshi Sun, Pingya Li, and Jinping Liu. 2019. "Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti-Tumor Effects Study" International Journal of Molecular Sciences 20, no. 4: 836. https://doi.org/10.3390/ijms20040836
APA StyleQi, Z., Chen, L., Li, Z., Shao, Z., Qi, Y., Gao, K., Liu, S., Sun, Y., Li, P., & Liu, J. (2019). Immunomodulatory Effects of (24R)-Pseudo-Ginsenoside HQ and (24S)-Pseudo-Ginsenoside HQ on Cyclophosphamide-Induced Immunosuppression and Their Anti-Tumor Effects Study. International Journal of Molecular Sciences, 20(4), 836. https://doi.org/10.3390/ijms20040836





