Oxidative-Inflammatory Stress in Immune Cells from Adult Mice with Premature Aging
Abstract
1. Introduction
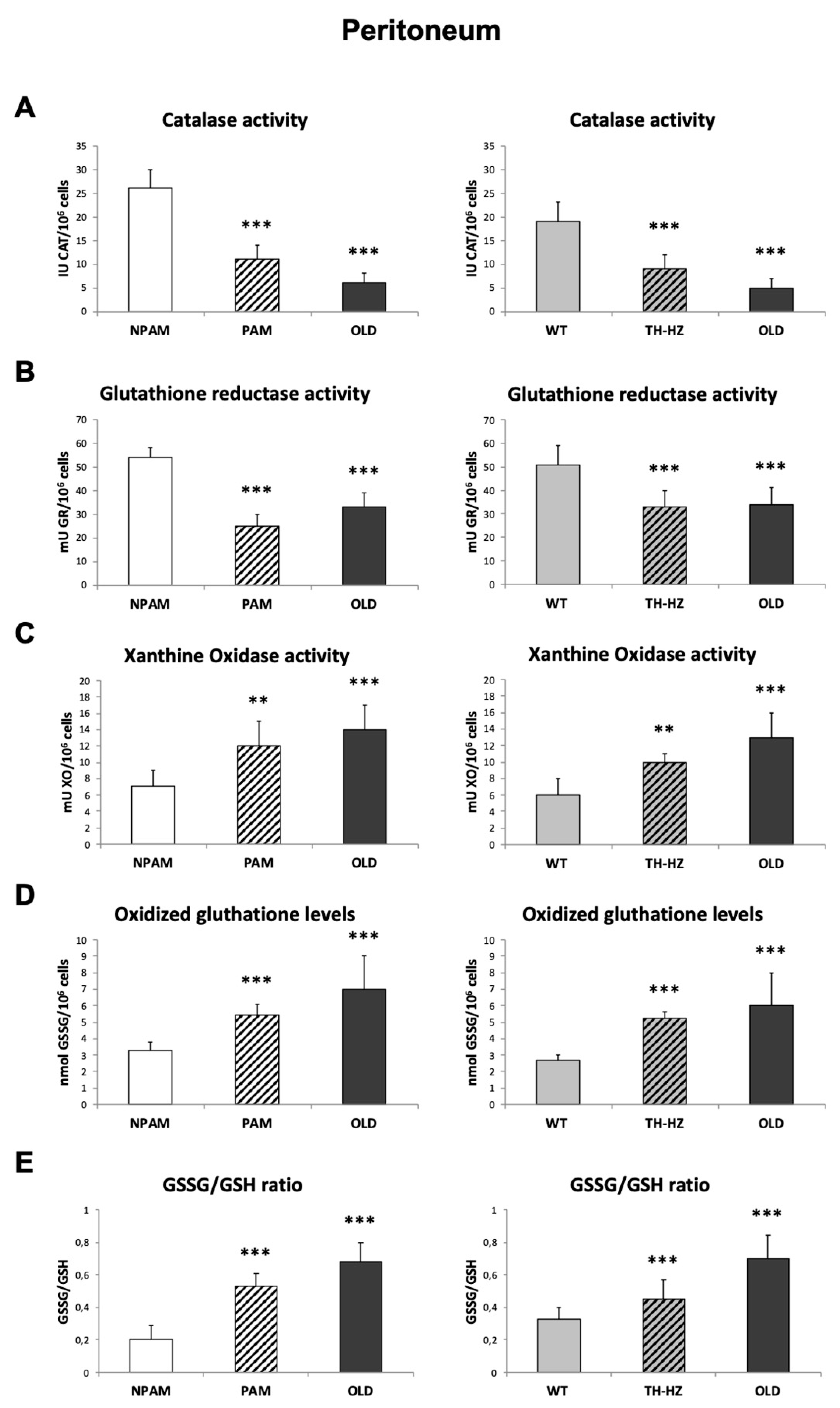
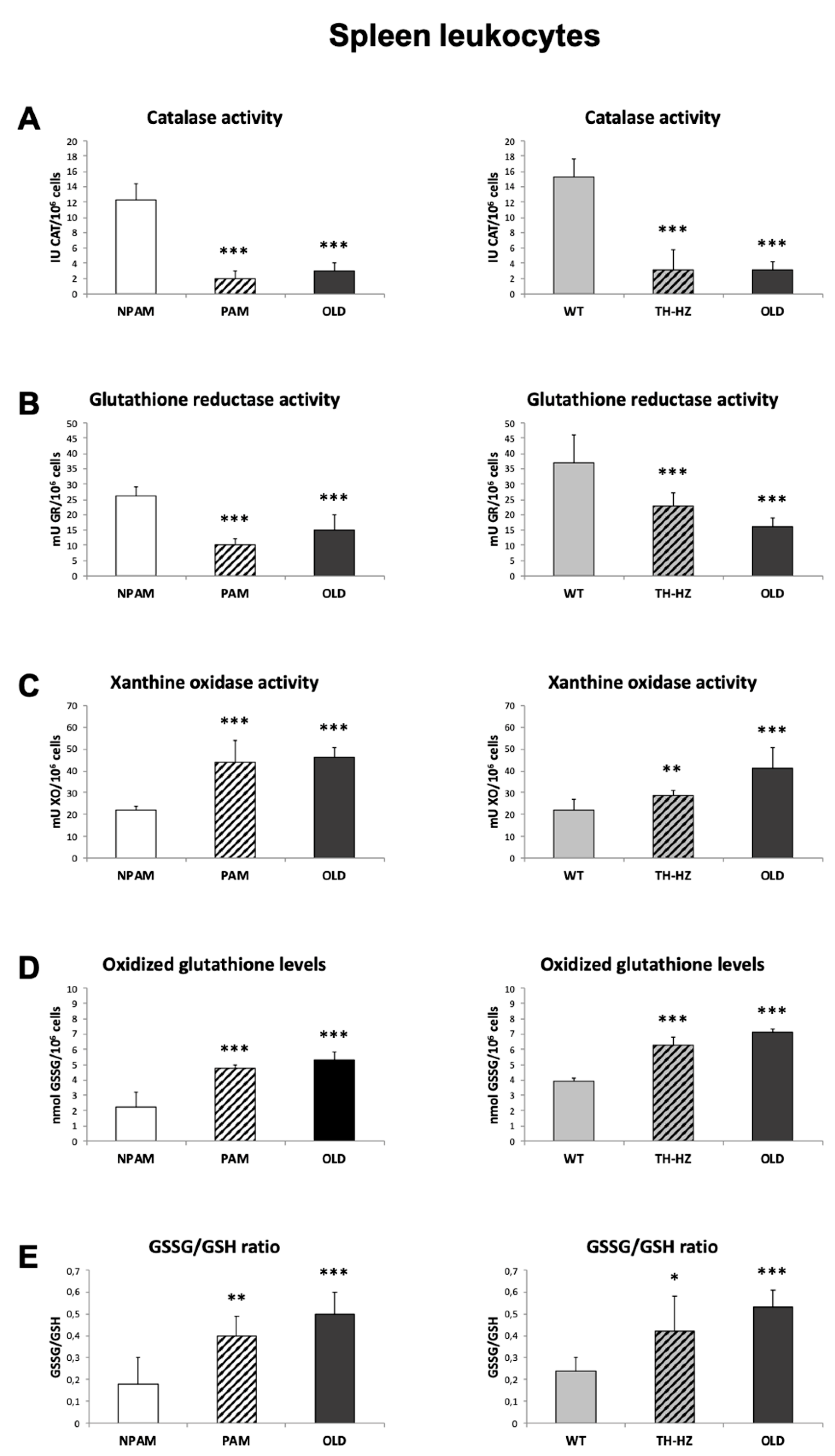
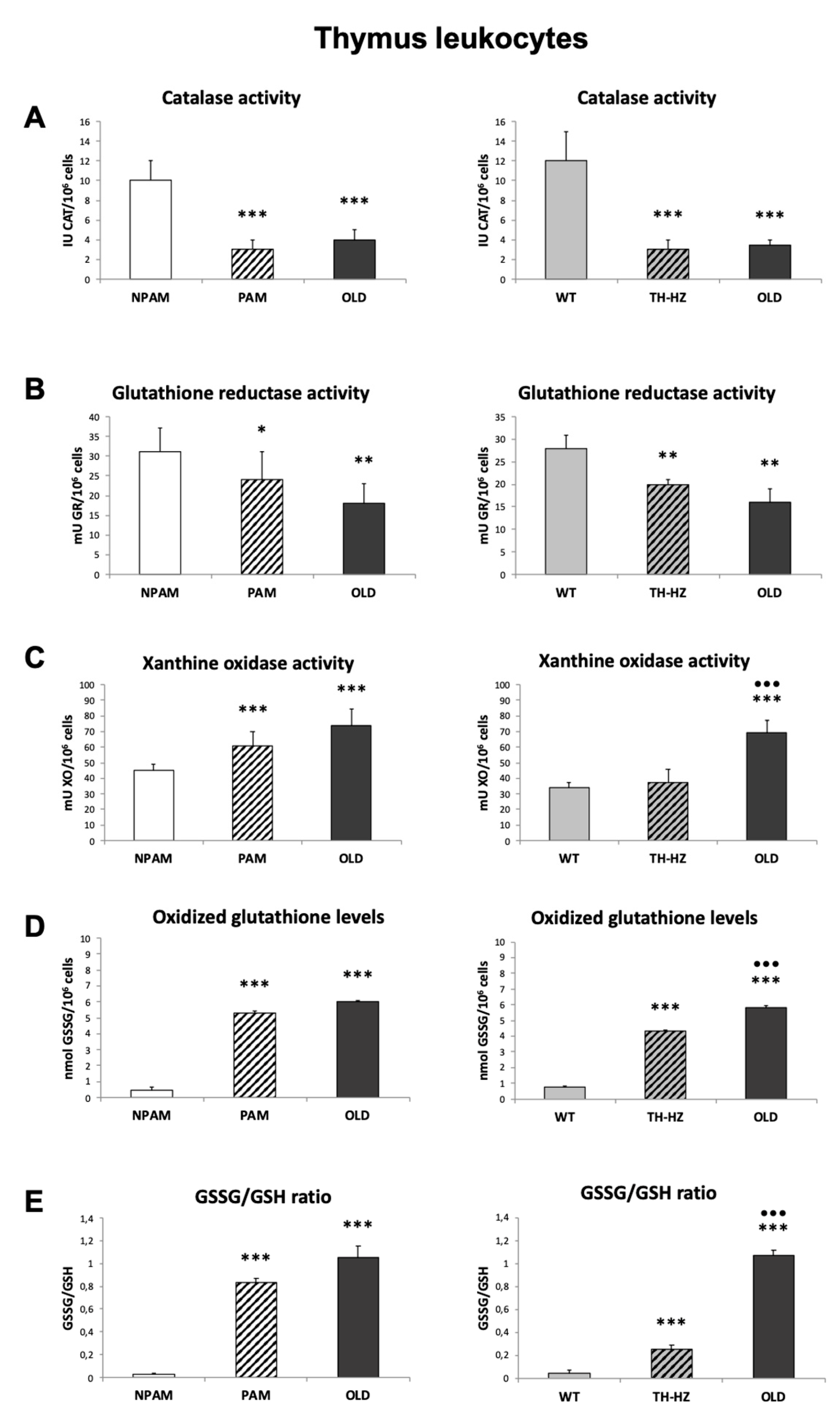
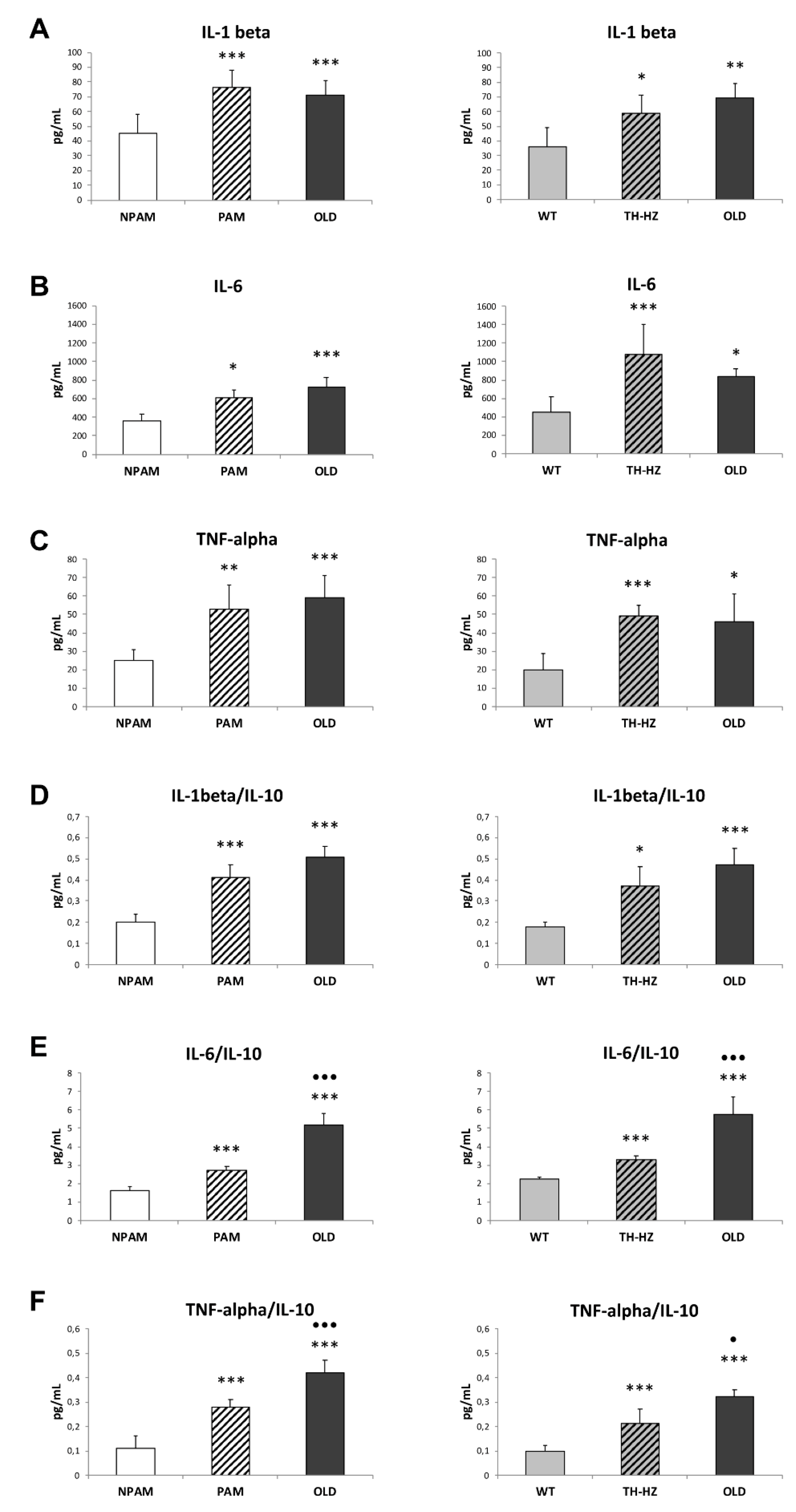
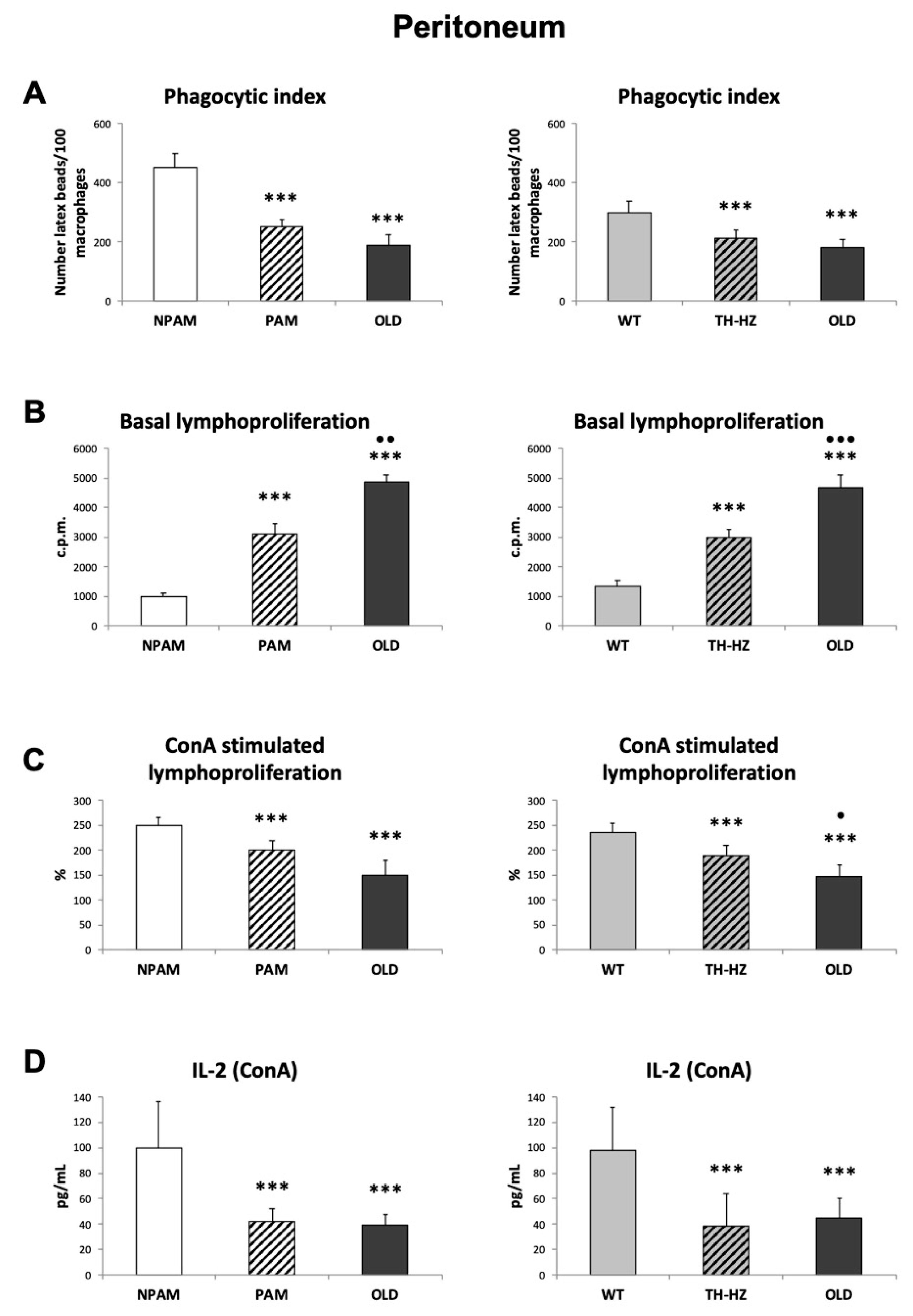
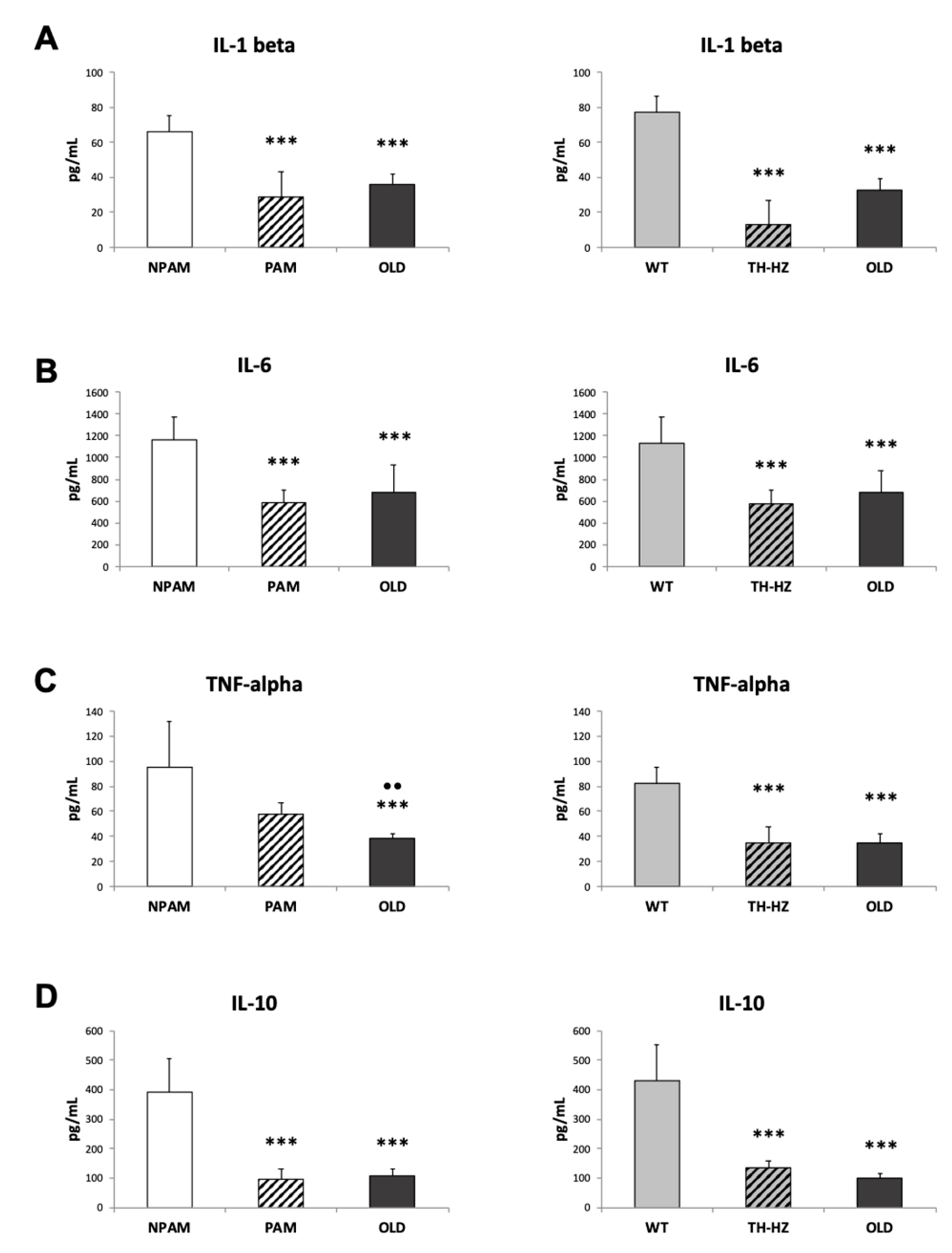
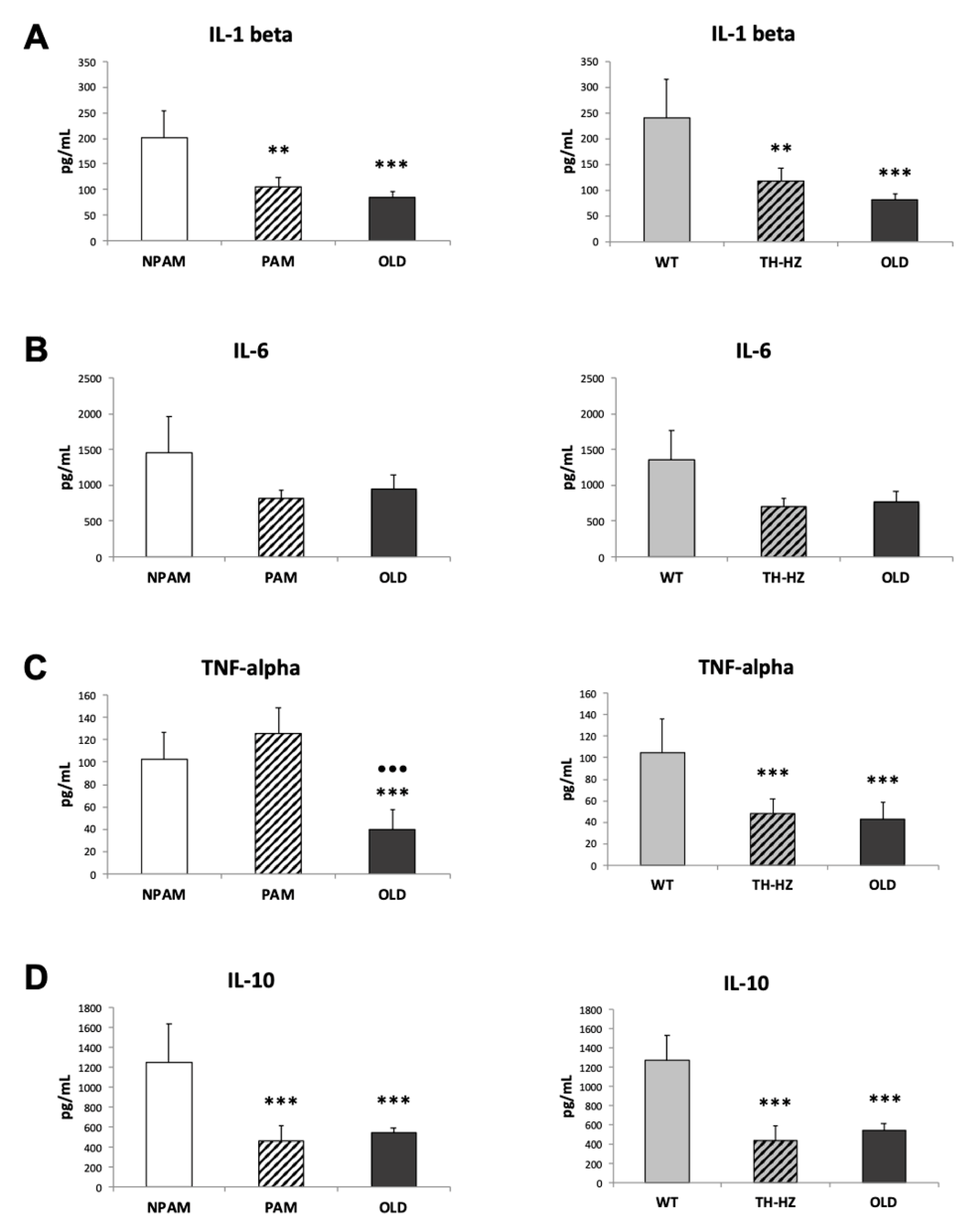
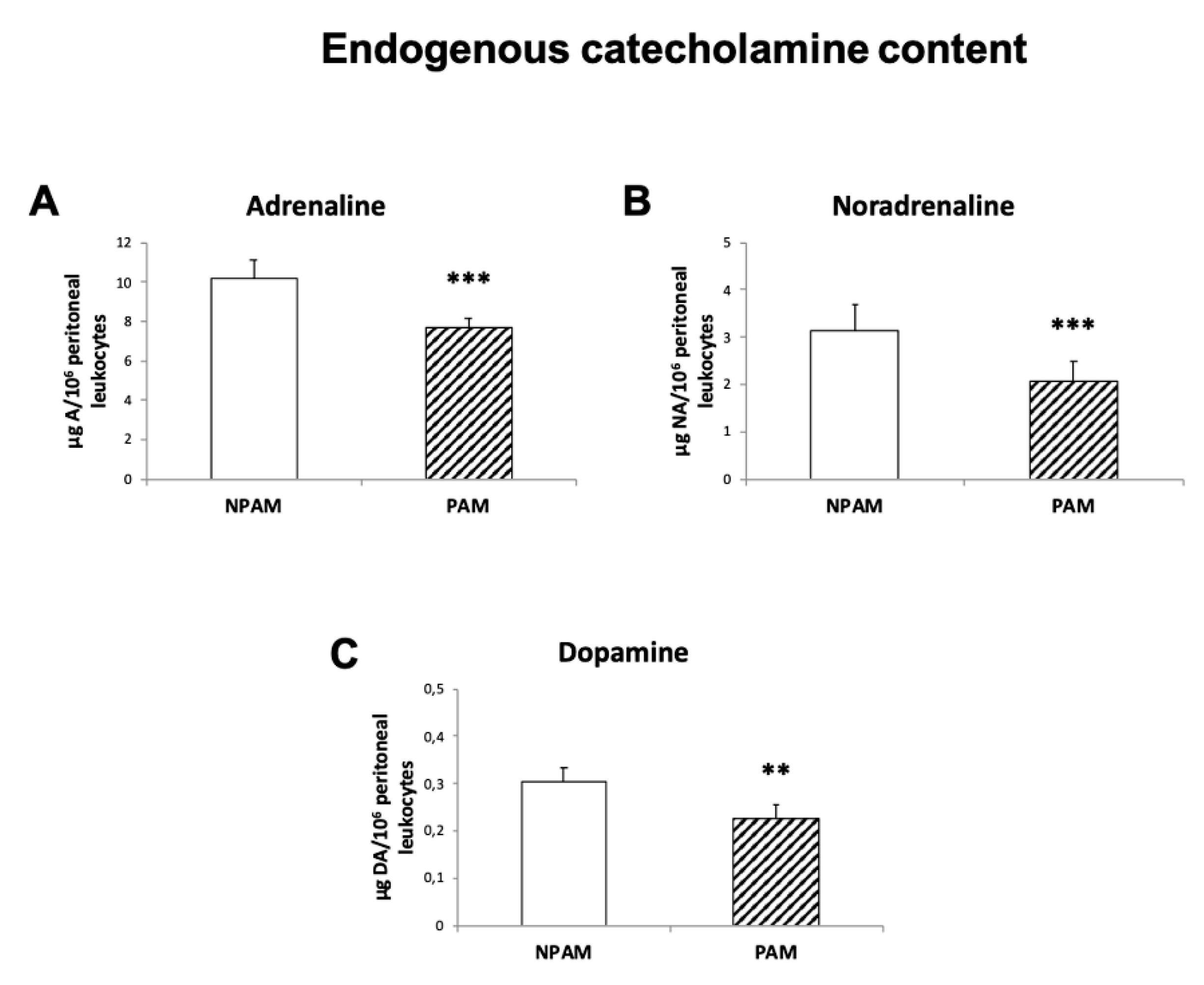
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Collection of Samples
4.2.1. Peritoneal Leukocytes
4.2.2. Spleen and Thymus Leukocytes
4.3. Oxidative Stress Parameters: Antioxidants and Oxidants, in Peritoneal, Spleen and Thymus Leukocytes
4.3.1. Catalase Activity
4.3.2. Glutathione Reductase Activity
4.3.3. Glutathione Content
4.3.4. Xanthine Oxidase Activity
4.4. Inflammatory Stress. Pro-inflammatory and Anti-Inflammatory Cytokines in Peritoneal Leukocyte Cultures
4.5. Functions of Peritoneal Immune Cells
4.5.1. Phagocytic Index
4.5.2. Lymphoproliferation
4.5.3. IL-2 Release Measurement
4.6. Endogenous Catecholamine Content
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PAM | Prematurely aging mice. |
| NPAM | Non-prematurely aging mice |
| TH-HZ | Tyrosine hydroxylase haploinsufficient mice |
| LPS | Lipopolissacharide |
| ConA | Concanavaline A |
| th | Tyrosine hydroxylase gene |
| TH | Tyrosine hydroxylase enzyme |
| A | Adrenaline |
| NA | Noradrenaline |
| DA | Dopamine |
References
- Besedovsky, H.O.; del Rey, A. Physiology of psychoneuroimmunology: A personal view. Brain Behav. Immun. 2007, 21, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The role of immunosenescence in the development of age-related diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Fulop, T.; McElhaney, J.; Pawelec, G.; Cohen, A.A.; Morais, J.A.; Dupuis, G.; Baehl, S.; Camous, X.; Witkowski, J.M.; Larbi, A. Frailty, inflammation and immunosenescence. Interdiscip. Top. Gerontol. Geriatr. 2015, 41, 26–40. [Google Scholar] [PubMed]
- Wayne, S.J.; Rhyne, R.L.; Garry, P.J.; Goodwin, J.S. Cell-mediated immunity as a predictor of morbidity and mortality in subjects over 60. J. Gerontol. 1990, 45, 45–48. [Google Scholar] [CrossRef]
- Ferguson, F.G.; Wikby, A.; Maxson, P.; Olsson, J.; Johansson, B. Immune parameters in a longitudinal study of a very old population of Swedish people: A comparison between survivors and non-survivors. J. Gerontol. A Biol. Sci. Med. Sci. 1995, 50, B378–B383. [Google Scholar] [CrossRef] [PubMed]
- De Martinis, M.; Franceschi, C.; Monti, D.; Ginaldi, L. Inflammation markers predicting frailty and mortality in the elderly. Exp. Mol. Phathol. 2006, 80, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Dewan, S.K.; Zheng, S.B.; Xia, S.J.; Bill, K. Senescent remodeling of the immune system and its contribution to the predisposition of the elderly to infections. Chin. Med. J. 2012, 125, 3325–3331. [Google Scholar]
- Fulop, T.; Larbi, A.; Kotb, R.; de Angelis, F.; Pawelec, G. Aging, immunity and cancer. Discov. Med. 2011, 11, 537–550. [Google Scholar]
- Martinez de Toda, I.; Maté, I.; Vida, C.; Cruces, J.; De la Fuente, M. Immune function parameters as markers of biological age and predictors of longevity. Aging 2016, 8, 3110–3119. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 2, 298–300. [Google Scholar] [CrossRef]
- De la Fuente, M.; Miquel, J. An update of the oxidation inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Updating the mitochondrial free radical theory of aging: An integrated view, key aspects, and confounding concepts. Antioxid. Redox Signal. 2013, 19, 1420–1445. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. The mitochondrial free radical theory of aging. Prog. Mol. Biol. Transl. Sci. 2014, 127, 1–27. [Google Scholar] [CrossRef]
- Go, Y.M.; Jones, D.P. Redox theory of aging: Implications for health and disease. Clin. Sci. 2017, 131, 1669–1688. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A. Mitochondrial reactive oxygen species: Do they extend or shorten animal lifespan? Biochim. Biophys. Acta 2016, 1857, 1116–1126. [Google Scholar] [CrossRef]
- Sanz, A.; Stefanatos, R.K. The mitocondrial free radical theory of aging: A critical view. Curr. Aging Sci. 2008, 1, 10–21. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafé, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and inflamm-aging as two sides of the same coin: Friends or foes? Front. Immunol. 2018, 10, 1960. [Google Scholar] [CrossRef]
- Salvioli, S.; Capri, M.; Valensin, S.; Tieri, P.; Monti, D.; Ottaviani, E.; Franceschi, C. Inflamm-aging, cytokines and aging: State of the art, new hypotheses on the role of mitochondria and new perspectives from systems biology. Curr. Pharm. Des. 2006, 12, 3161–3171. [Google Scholar] [CrossRef]
- Vida, C.; Gonzalez, E.M.; De la Fuente, M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr. Pharm. Des. 2014, 20, 4656–4678. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Viadero, C.; Jiménez-Sanz, M.; Fernández-Pérez, A.; Verduga Vélez, R.; Crespo Santiago, D. Inflammation and oxidation: Predictive and/or causative factors. Rev. Esp. Geriatr. Gerontol. 2016, 1, 27–33. [Google Scholar] [CrossRef]
- Bauer, M.E.; De la Fuente, M. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Dev. 2016, 156, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E. Stress, glucocorticoids and ageing of the immune system. Stress 2005, 8, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.; Bouso, E.; Ronfani, M.; Serafini, M.M.; Galasso, M.; Lanni, C.; Corsini, E. Role of hormones in the regulation of RACK1 Expression as a signaling checkpoint in immunosenescence. Int. J. Mol. Sci. 2017, 18, 1453. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; Muller, G.C.; Correa, B.L.; Vianna, P.; Turner, J.E.; Bosch, J.A. Psychoneuroendocrine interventions aimed at attenuating immunosenescence: A review. Biogerontology 2013, 14, 8–20. [Google Scholar] [CrossRef]
- Turner, J.E. Is immunosenescence influenced by our lifetime “dose” of exercise? Biogerontology 2016, 17, 581–602. [Google Scholar] [CrossRef]
- Bauer, M.E. Chronic stress and immunosenescence: A review. Neuroimmunomodulation 2008, 15, 241–250. [Google Scholar] [CrossRef]
- Cruces, J.; Venero, C.; Pereda-Pérez, J.; De la Fuente, M. The effect of psychological stress and social isolation on neuroendocrine communication. Curr. Pharm. Des. 2014, 20, 4608–4628. [Google Scholar] [CrossRef]
- De la Fuente, M. Oxidation and Inflammation in the immune and nervous systems, a link between aging and anxiety. In Handbook of Immunosenescence; Fulop, T., Franceschi, C., Hirokawa, K., Pawelec, G., Eds.; Springer: Berlin, Germany, 2018; 28p. [Google Scholar]
- Borkan, G.A.; Norris, A.H. Assessment of biological age using a profile of physical parameters. J. Gerontol. 1980, 35, 177–184. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Antikainen, R.L.; Markowe, H.L. Mortality according to a prior assessment of biological age. Curr. Aging Sci. 2009, 2, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Guayerbas, N.; De la Fuente, M. An impairment of phagocytic function is linked to a shorter life span in two strains of prematurely-aging mice. Dev. Comp. Immunol. 2003, 27, 339–350. [Google Scholar] [CrossRef]
- Viveros, M.P.; Arranz, L.; Hernanz, A.; Miquel, J.; De la Fuente, M. A model of premature aging in mice based on altered stress-related behavioral response and immunosenescence. Neuroimmunomodulation 2007, 14, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Cruces, J.; Ceprián, N.; Hernández-Sánchez, C.; De la Fuente, M. Premature aging in behavior and immune functions in tyrosine hydroxylase haploinsufficient female mice. A longitudinal study. Brain Behav. Immun. 2018, 69, 440–455. [Google Scholar] [CrossRef]
- Osada, K.; Minehira, K.; Inoue, S.; Nakamura, S.; Yamada, K.; Sugano, M. Effect of oxidized colesterol on age-associated changes to immune parameters in spleen lymphocytes and peritoneal exudate cells derived from rats. Biosci. Biotechnol. Biochem. 2000, 64, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Arranz, L.; Caamano, J.H.; Lord, J.M.; De la Fuente, M. Preserved immune functions and controlled leukocyte oxidative stress in naturally long-lived mice: Possible role of nuclear factor kappa β. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Arranz, L.; Lord, J.M.; De la Fuente, M. Preserved ex vivo inflammatory status and cytokine responses in naturally long-lived mice. Age 2010, 32, 451–466. [Google Scholar] [CrossRef]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 1, S4–S9. [Google Scholar] [CrossRef]
- Frasca, D.; Bloberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteom. 2011, 74, 2313–2323. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An update on inflamm-aging: Mechanisms, prevention and treatment. J. Immunol. Res. 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Guayerbas, N.; Puerto, M.; Victor, V.M.; Miquel, J.; De la Fuente, M. Leukocyte function and life span in a murine model of premature immunosenescence. Exp. Gerontol. 2002, 37, 249–256. [Google Scholar] [CrossRef]
- Guayerbas, N.; Catalán, M.; Victor, V.M.; Miquel, J.; De la Fuente, M. Relation of behaviour and macrophage function to life span in a murine model of premature immunosenescence. Brain Behav. Res. 2002, 134, 41–48. [Google Scholar] [CrossRef]
- Garrido, A.; Cruces, J.; Iriarte, I.; Hernández-Sánchez, C.; de Pablo, F.; De la Fuente, M. Premature immunosenescence in catecholamine synthesis defficient mice. Effect of social environment. Rev. Esp. Geriatr. Gerontol. 2017, 52, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.K.; Smith, C.A.; Sakamoto, K.; Kaminski, N.; Koff, J.L.; Goldstein, D.R. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J. Immunol. 2017, 199, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.; Carlisi, M.; Santoro, M.; Napolitano, M.; Raso, S.; Siragusa, S. Immunosenescence and lymphomagenesis. Immun. Ageing 2018, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Bergquist, J.; Tarkowski, A.; Ekman, R.; Erwing, A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via autocrine loop. Proc. Natl. Acad. Sci. USA 1994, 91, 12912–12916. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, M.; Marino, F.; Bombelli, R.; Ferrari, M.; Lecchini, S.; Frigo, G. Endogenous catecholamine synthesis, metabolism, storage and uptake in human neutrophils. Life Sci. 1999, 64, 975–981. [Google Scholar] [CrossRef]
- Qiu, Y.H.; Cheng, C.; Dai, L.; Peng, Y.P. Effect of endogenous catecholamines in lymphocytes on lymphocyte function. J. Neuroimmunol. 2005, 167, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Madden, K.S. Sympathetic neural-immune interactions regulate hematopoiesis, thermoregulation and inflammation in mammals. Dev. Comp. Immun. 2017, 66, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; Martinez de Toda, I.; Cruces, J.; Garrido, A.; González-Sánchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, C.; Álvarez, P.; Jiménez, L.; De la Fuente, M. Oxidative stress in leukocytes from young prematurely aging mice is reversed by supplementation with biscuits rich in antioxidants. Dev. Comp. Immunol. 2006, 30, 1168–1180. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.; Kequan, G.; Yasushi, A.; Susumu, I. Immune dysfunction associated with abnormal bone marrow-derived mesenchymal stroma cells in senescence accelerated mice. Int. J. Mol. Sci. 2016, 17, 183. [Google Scholar] [CrossRef]
- Caballero, B.; Vega-Naredo, I.; Sierra, V.; DeGonzalo-Calvo, D.; Medrano-Campillo, P.; Guerrero, J.M.; Tolivia, D.; Rodríguez-Colunga, M.J.; Coto-Montes, A. Autophagy upregulation and loss of NF-KappaB in oxidative stress-related immunodeficient SAMP8 mice. Mech. Ageing Dev. 2009, 130, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, T.; Asada, S.; Nishitani, S.; Hazeki, O. Age-related changes in manganese superoxide dismutase activity in the cerebral cortex of senescence-accelerated prone and resistant mouse. Neurosci. Lett. 2001, 298, 135–138. [Google Scholar] [CrossRef]
- Farr, S.A.; Poon, H.F.; Dogrokol-Ak, D.; Drake, J.; Banks, W.A.; Everman, E.; Butterfield, D.A.; Morley, J.E. The antioxidants a-lipoic and N-acetylcyteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J. Neurochem. 2003, 84, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Sureda, F.X.; Gutierrez-Cuesta, J.; Romeu, M.; Mulero, M.; Canudas, A.M.; Camins, A.; Mallol, J.; Pallás, M. Changes in oxidative stress parameters and neurodegeneration markers in the brain of the senescence-accelerated mice SAMP-8. Exp. Gerontol. 2006, 41, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Baeza, I.; De Castro, N.M.; Arranz, L.; Fdez-Tresguerres, J.; De la Fuente, M. Ovariectomy causes immunosenescence and oxi-inflamm-ageing in peritoneal leukocytes of age female mice similar to that in aged males. Biogerontology 2011, 12, 227–238. [Google Scholar] [CrossRef]
- Martínez de Toda, I.; Vida, C.; De la Fuente, M. An appropriate modulation of lymphoproliferative response and cytokine release as possible contributors to longevity. Int. J. Mol. Sci. 2017, 18, 1598. [Google Scholar] [CrossRef]
- Vassileva, V.; Piquette-Miller, M. Inflammation: The dynamic force of health and disease. Clin. Pharmacol. Ther. 2014, 96, 401–405. [Google Scholar] [CrossRef]
- Mooradian, A.D.; Reed, R.L.; Osterweil, D.; Scuderi, P. Detectable serum levels of tumor necrosis factor alpha may predict early mortality in elderly institutionalized patients. J. Am. Geriatr. Soc. 1991, 39, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.B.; Ferrucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H., Jr.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef]
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc. 1999, 47, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Behnia, F.; Sheller, S.; Menon, R. Mechanistic Differences Leading to Infectious and Sterile Inflammation. Am. J. Reprod. Immunol. 2016, 75, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, M.I.; Escames, G.; López, L.C.; López, A.; García, J.A.; Ortiz, F.; Acuña-Castroviejo, D. Chronic melatonin treatment reduces the age-dependent inflammatory process in senescence-accelerated mice. J. Pineal Res. 2007, 42, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, W.F.; Yuan, L.F.; Tu, P.F.; Ma, Z.Z. Decreasing pro-inflamatory cytokine and reversing the immunosenescence with extracts of Pu-erh tea in senescence accelerated mouse (SAM). Food Chem. 2012, 135, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, O.; Pawelec, G.; Peralbo, E.; Wikby, A.; Mariani, E.; Mocchegiani, E.; Tarazona, R.; Solana, R. Immunological biomarkers of ageing in man: Changes in both innate and adaptive immunity are associated with health and longevity. Biogerontology 2006, 7, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Sharon, N. Lectin receptors as lymphocyte surface markers. Adv. Immunol. 1983, 83, 213–298. [Google Scholar]
- Hallgren, H.M.; Bergh, N.; Rodysill, J.K.; O’Leary, J.J. Lymphocyte proliferative response to PHA and anti-CD3/Ti monoclonal antibodies T cell surface marker expression, and serum IL-2 receptor levels as biomarkers of age and health. Mech. Aging Dev. 1998, 43, 175–185. [Google Scholar] [CrossRef]
- Wikby, A.; Ferguson, F.; Forsey, R.; Thompson, J.; Strindhall, J.; Lofgren, S.; Nilsson, B.O.; Ernerudh, J.; Pawelec, G.; Johansson, B. An immune risk phenotype, cognitive impairment, and survival in very late life: Impact of allostatic load in wedish octogenarian and nonagenarian humans. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 556–565. [Google Scholar] [CrossRef]
- Pera, A.; Campos, C.; López, N.; Hassouneh, F.; Alonso, C.; Tarazona, R.; Solana, R. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas 2015, 82, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kobayasi, K.; Morita, S.; Sawada, H.; Mizuguchi, T.; Yamada, K.; Nagatsu, I.; Hata, T.; Watenabe, Y.; Fujita, K.; Nagatsu, T. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J. Biol. Chem. 1995, 270, 27235–27243. [Google Scholar] [CrossRef]
- Webster Marketon, J.I.; Glaser, R. Stress hormones and immune function. Cell. Immunol. 2008, 252, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; De la Fuente, M. Stress-related behavioural responses, immunity and ageing in animal models. Immunosenescence. In Immunosenescence: Psychosocial and Behavioral Determinant; Bosch, J.A., Phillips, C., Lord, J.M., Eds.; Springer: New York, NY, USA, 2013; pp. 125–144. [Google Scholar]
- Aguilera, G. HPA axis responsiveness to stress: Implications for healthy aging. Exp. Gerontol. 2011, 46, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Kvetnansky, R.; Sabban, E.L.; Palkovits, M. Catecholaminergic systems in stress: Structural and molecular genetic approaches. Physiol. Rev. 2009, 89, 535–606. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, P.; Alvarado, C.; Puerto, M.; Schlumberger, A.; Jiménez, L.; De la Fuente, M. Improvement of leukocyte functions in prematurely aging mice after five weeks of diet supplementation with polyphenol-rich cereals. Nutrition 2006, 22, 913–921. [Google Scholar] [CrossRef]
- Alvarado, C.; Álvarez, P.; Puerto, M.; Gausserés, N.; Jiménez, L.; De la Fuente, M. Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition 2006, 22, 767–777. [Google Scholar] [CrossRef]
- Guayerbas, N.; Puerto, M.; Álvarez, P.; De la Fuente, M. Improvement of the macrophage functions in prematurely aging mice by a diet supplemented with thiolic antioxidants. Cell. Mol. Biol. 2004, 50, OL677-81. [Google Scholar]
- Zhou, Q.Y.; Quaife, C.J.; Palmiter, R.D. Targeted disruption of the tyrosine hydroxylase gen reveals that catecholamines are required for mouse fetal development. Nature 1995, 374, 640–643. [Google Scholar] [CrossRef]
- Vázquez, P.; Robles, A.M.; de Pablo, F.; Hernández-Sánchez, C. Non-neural tyrosine hydroxylase, via modulation of endocrine pancreatic precursors, is required for normal development of beta-cells in the mouse pancreas. Diabetologia 2014, 57, 2339–2347. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [PubMed]
- Massey, V.; Williams, C. On the reaction mechanism of yeast glutathione reductase. J. Biol. Chem. 1965, 240, 4470–4481. [Google Scholar] [PubMed]
- Hissin, P.J.; Hilf, R. A fluorimetric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, A.; Cruces, J.; Ceprián, N.; Vara, E.; de la Fuente, M. Oxidative-Inflammatory Stress in Immune Cells from Adult Mice with Premature Aging. Int. J. Mol. Sci. 2019, 20, 769. https://doi.org/10.3390/ijms20030769
Garrido A, Cruces J, Ceprián N, Vara E, de la Fuente M. Oxidative-Inflammatory Stress in Immune Cells from Adult Mice with Premature Aging. International Journal of Molecular Sciences. 2019; 20(3):769. https://doi.org/10.3390/ijms20030769
Chicago/Turabian StyleGarrido, Antonio, Julia Cruces, Noemí Ceprián, Elena Vara, and Mónica de la Fuente. 2019. "Oxidative-Inflammatory Stress in Immune Cells from Adult Mice with Premature Aging" International Journal of Molecular Sciences 20, no. 3: 769. https://doi.org/10.3390/ijms20030769
APA StyleGarrido, A., Cruces, J., Ceprián, N., Vara, E., & de la Fuente, M. (2019). Oxidative-Inflammatory Stress in Immune Cells from Adult Mice with Premature Aging. International Journal of Molecular Sciences, 20(3), 769. https://doi.org/10.3390/ijms20030769






