The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence
Abstract
:1. Introduction
2. Results
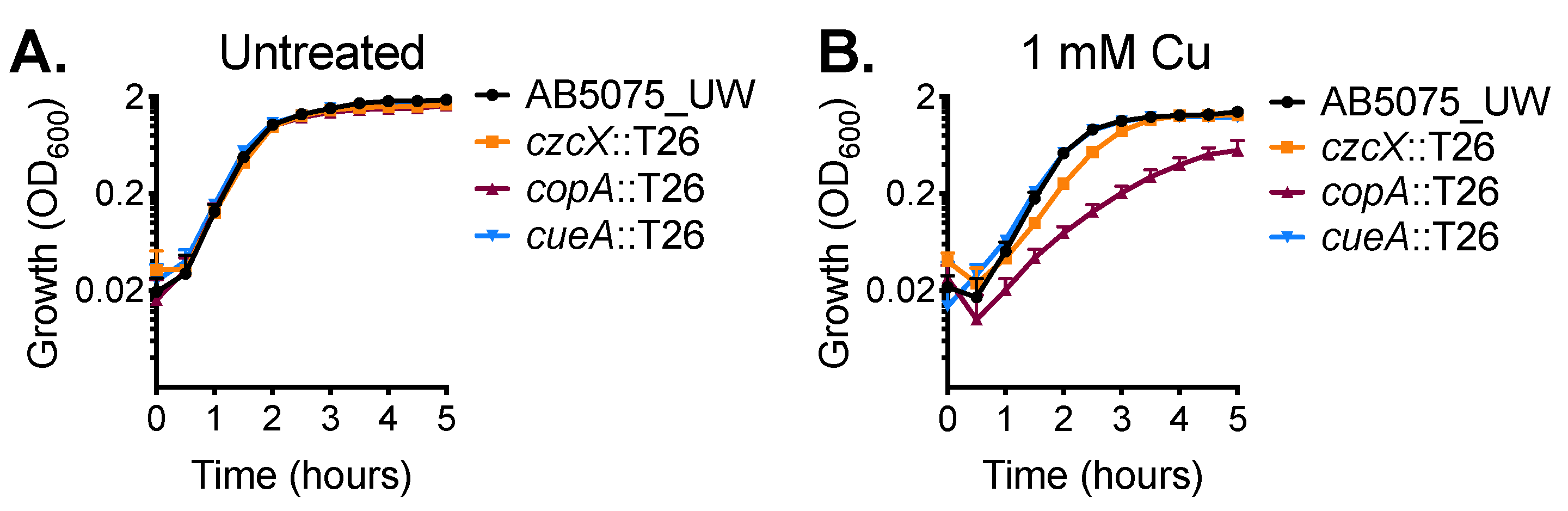
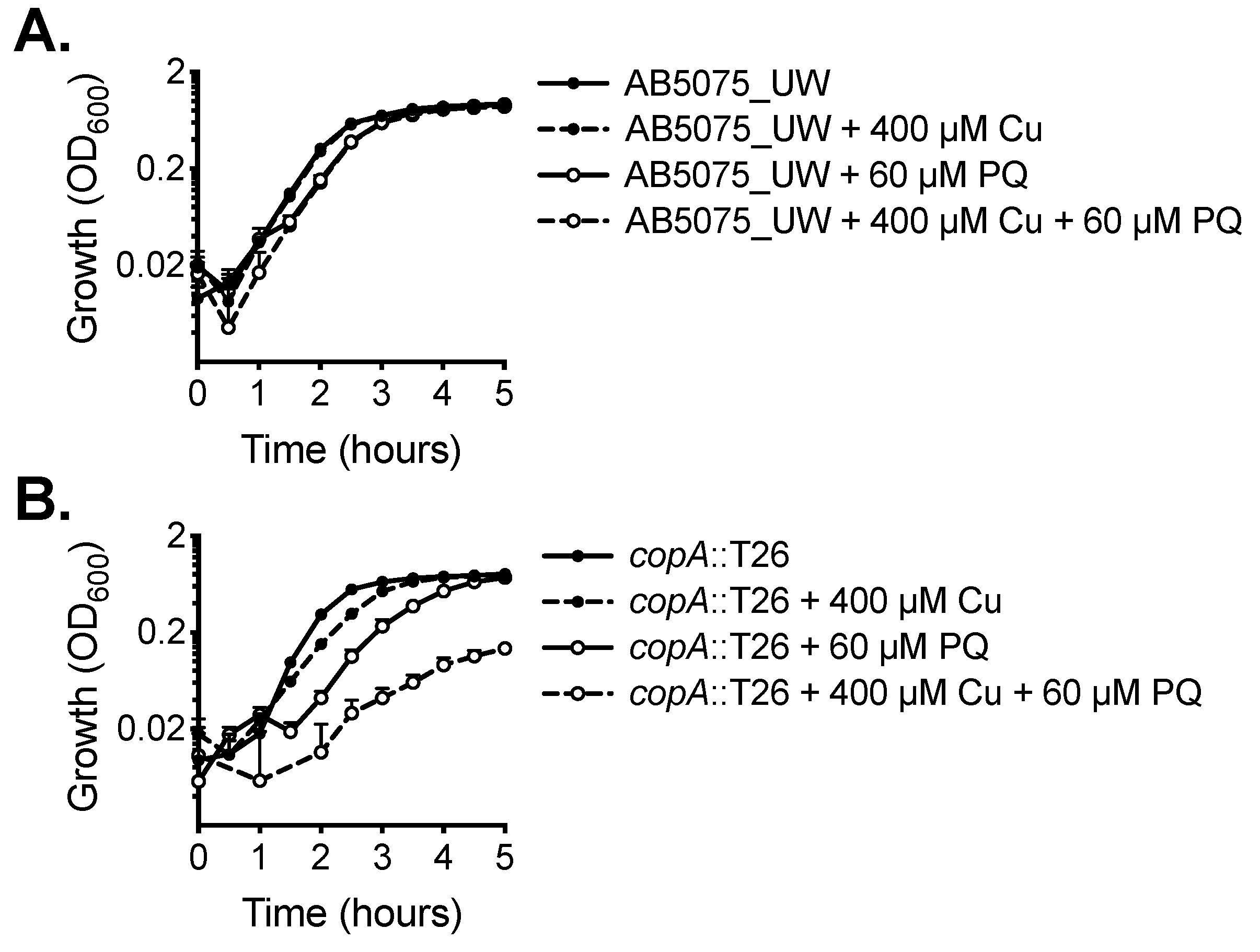
2.1. CopA Provides Copper Resistance in A. baumannii
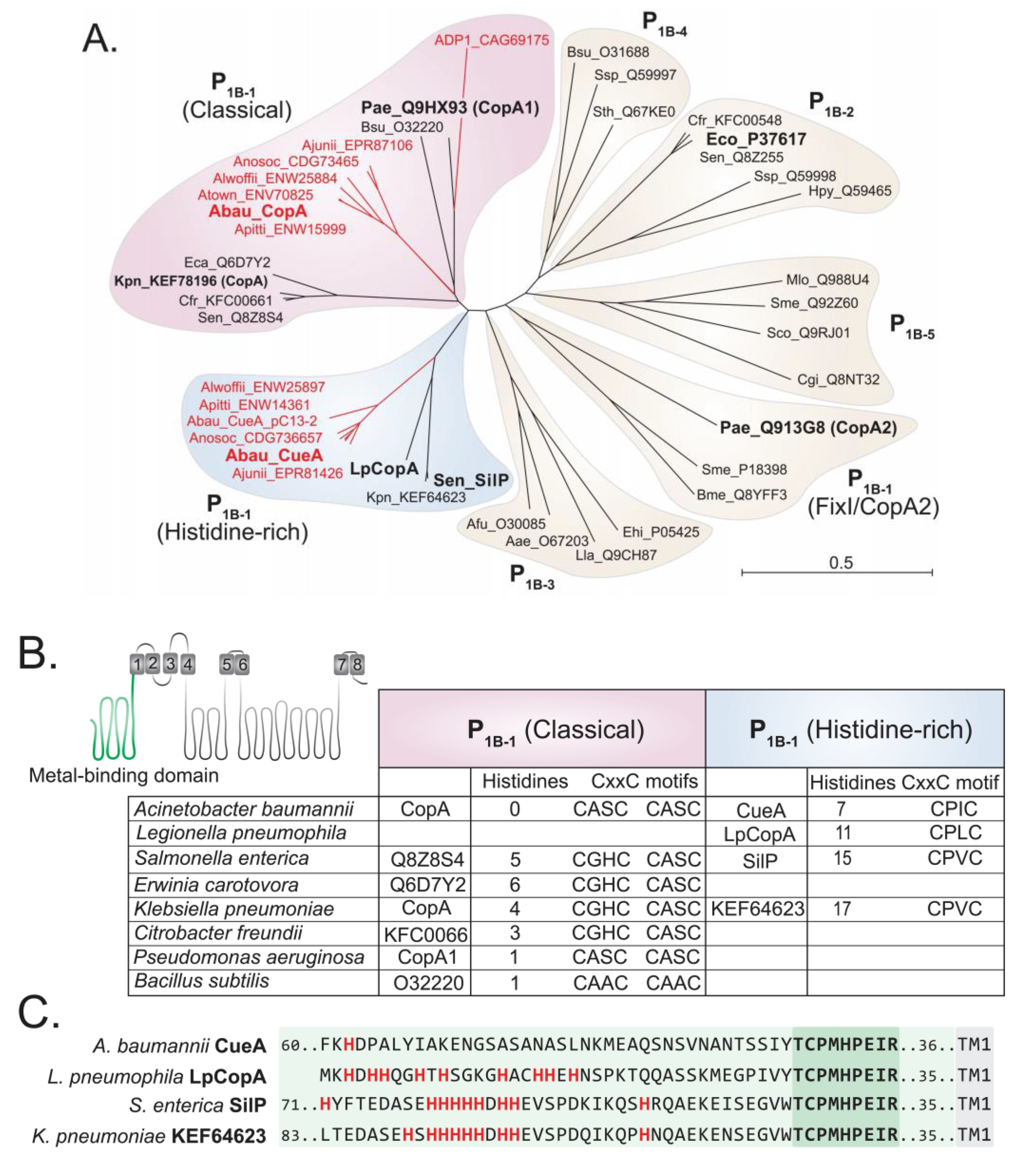
2.2. Functional Characterisation of A. baumannii CopA
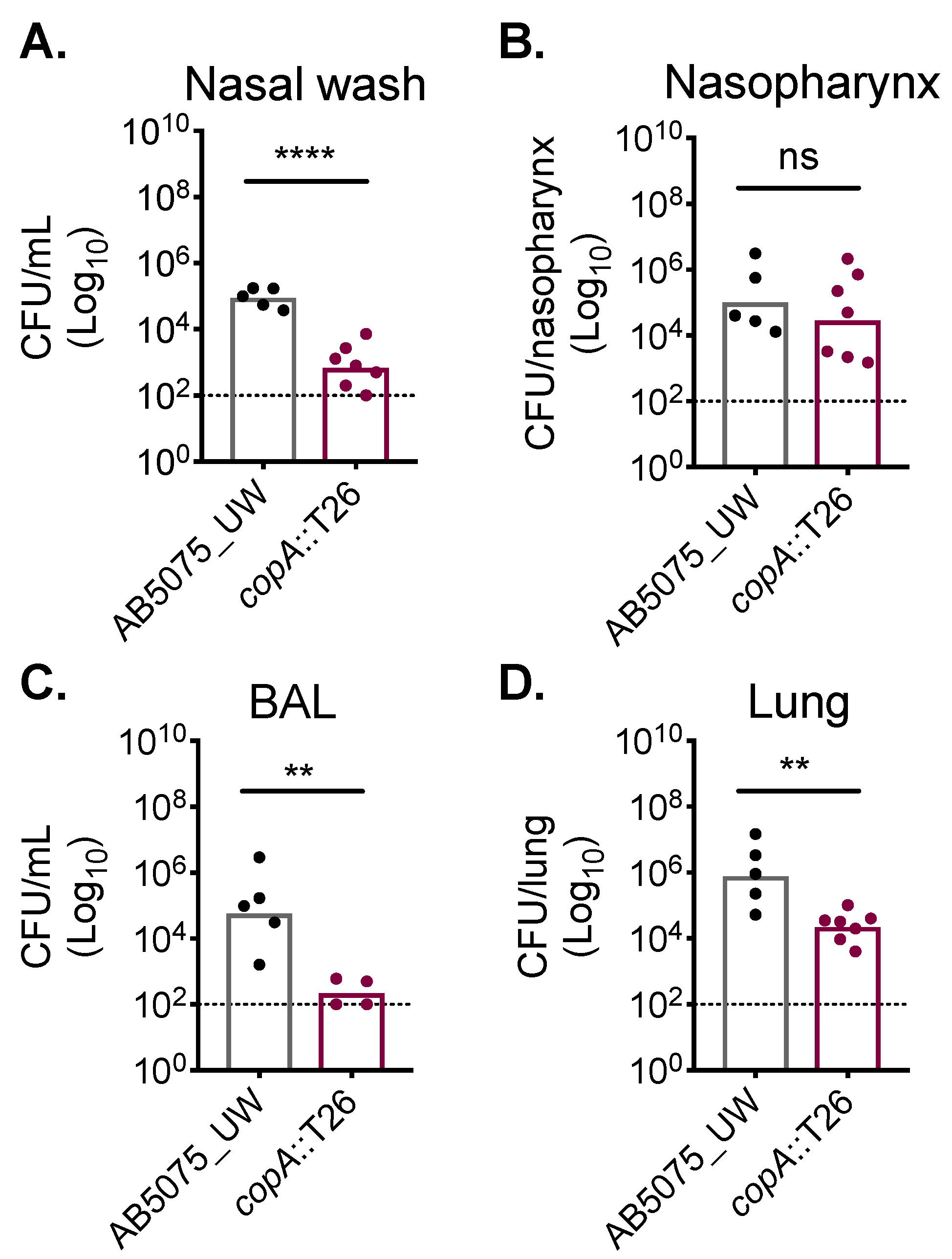
2.3. CopA Plays A Role in A. baumannii Host Colonisation
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Chemicals, Media, and Growth
4.2. Cellular Metal Ion Content Analysis
4.3. Animal Experiments
4.4. Bioinformatics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BAL | Bronchioalveolar lavage |
| CDF | Cation diffusion facilitator |
| CFU | Colony-forming unit |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| HME | Heavy metal efflux |
| RND | Resistance-nodulation-cell division |
| WT | Wild-type |
References
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Dexter, C.; Murray, G.L.; Paulsen, I.T.; Peleg, A.Y. Community-acquired Acinetobacter baumannii: Clinical characteristics, epidemiology and pathogenesis. Expert Rev. Anti-Infect. Ther. 2015, 13, 567–573. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Publishes List of Bacteria for which New Antibiotics Are Urgently Needed; WHO Media Centre: Geneva, Switzerland, 2017. [Google Scholar]
- Eijkelkamp, B.A.; Stroeher, U.H.; Hassan, K.A.; Papadimitrious, M.S.; Paulsen, I.T.; Brown, M.H. Adherence and motility characteristics of clinical Acinetobacter baumannii isolates. FEMS Microbiol. Lett. 2011, 323, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Giles, S.K.; Stroeher, U.H.; Eijkelkamp, B.A.; Brown, M.H. Identification of genes essential for pellicle formation in Acinetobacter baumannii. BMC Microbiol. 2015, 15, 116. [Google Scholar] [CrossRef]
- Williams, C.L.; Neu, H.M.; Gilbreath, J.J.; Michel, S.L.; Zurawski, D.V.; Merrell, D.S. Copper resistance of the emerging pathogen Acinetobacter baumannii. Appl. Environ. Microbiol. 2016, 82, 6174–6188. [Google Scholar] [CrossRef] [PubMed]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Rensing, C.; Moodley, A.; Cavaco, L.M.; McDevitt, S.F. Resistance to metals used in agricultural production. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Ong, C.L.; Gillen, C.M.; Barnett, T.C.; Walker, M.J.; McEwan, A.G. An antimicrobial role for zinc in innate immune defense against group A streptococcus. J. Infect. Dis. 2014, 209, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Djoko, K.Y.; Ong, C.L.; Walker, M.J.; McEwan, A.G. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J. Biol. Chem. 2015, 290, 18954–18961. [Google Scholar] [CrossRef] [PubMed]
- Achard, M.E.; Stafford, S.L.; Bokil, N.J.; Chartres, J.; Bernhardt, P.V.; Schembri, M.A.; Sweet, M.J.; McEwan, A.G. Copper redistribution in murine macrophages in response to Salmonella infection. Biochem. J. 2012, 444, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kapetanovic, R.; Bokil, N.J.; Achard, M.E.; Ong, C.Y.; Peters, K.M.; Stocks, C.J.; Phan, M.D.; Monteleone, M.; Schroder, K.; Irvine, K.M.; et al. Salmonella employs multiple mechanisms to subvert the TLR-inducible zinc-mediated antimicrobial response of human macrophages. FASEB J. 2016, 30, 1901–1912. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, S.; Nakashima, R.; Yamashita, E.; Yamaguchi, A. Crystal structure of bacterial multidrug efflux transporter AcrB. Nature 2002, 419, 587–593. [Google Scholar] [CrossRef]
- Mealman, T.D.; Blackburn, N.J.; McEvoy, M.M. Metal export by CusCFBA, the periplasmic Cu(I)/Ag(I) transport system of Escherichia coli. Curr. Top. Membr. 2012, 69, 163–196. [Google Scholar]
- Sitsel, O.; Gronberg, C.; Autzen, H.E.; Wang, K.; Meloni, G.; Nissen, P.; Gourdon, P. Structure and function of Cu(I)- and Zn(II)-ATPases. Biochemistry 2015, 54, 5673–5683. [Google Scholar] [CrossRef] [PubMed]
- Gourdon, P.; Liu, X.Y.; Skjorringe, T.; Morth, J.P.; Moller, L.B.; Pedersen, B.P.; Nissen, P. Crystal structure of a copper-transporting PIB-type ATPase. Nature 2011, 475, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kolaj-Robin, O.; Russell, D.; Hayes, K.A.; Pembroke, J.T.; Soulimane, T. Cation Diffusion Facilitator family: Structure and function. FEBS Lett. 2015, 589, 1283–1295. [Google Scholar] [CrossRef] [Green Version]
- Moore, C.M.; Gaballa, A.; Hui, M.; Ye, R.W.; Helmann, J.D. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol. Microbiol. 2005, 57, 27–40. [Google Scholar] [CrossRef]
- Wei, Y.; Fu, D. Binding and transport of metal ions at the dimer interface of the Escherichia coli metal transporter YiiP. J. Biol. Chem. 2006, 281, 23492–23502. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C. CueO is a multi-copper oxidase that confers copper tolerance in Escherichia coli. Biochem. Biophys. Res. Commun. 2001, 286, 902–908. [Google Scholar] [CrossRef]
- Cha, J.S.; Cooksey, D.A. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.S.; Cooksey, D.A. Copper hypersensitivity and uptake in Pseudomonas syringae containing cloned components of the copper resistance operon. Appl. Environ. Microbiol. 1993, 59, 1671–1674. [Google Scholar]
- Hu, Y.H.; Wang, H.L.; Zhang, M.; Sun, L. Molecular analysis of the copper-responsive CopRSCD of a pathogenic Pseudomonas fluorescens strain. J. Microbiol. 2009, 47, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Lawton, T.J.; Kenney, G.E.; Hurley, J.D.; Rosenzweig, A.C. The CopC family: Structural and bioinformatic insights into a diverse group of periplasmic copper binding proteins. Biochemistry 2016, 55, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, J.V.; Hobman, J.L.; Brown, N.L. CueR (YbbI) of Escherichia coli is a MerR family regulator controlling expression of the copper exporter CopA. Mol. Microbiol. 2001, 39, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Quintana, J.; Novoa-Aponte, L.; Arguello, J.M. Copper homeostasis networks in the bacterium Pseudomonas aeruginosa. J. Biol. Chem. 2017, 292, 15691–15704. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.A.; Pederick, V.G.; Elbourne, L.D.; Paulsen, I.T.; Paton, J.C.; McDevitt, C.A.; Eijkelkamp, B.A. Zinc stress induces copper depletion in Acinetobacter baumannii. BMC Microbiol. 2017, 17, 59. [Google Scholar] [CrossRef]
- Arguello, J.M.; Gonzalez-Guerrero, M.; Raimunda, D. Bacterial transition metal P(1B)-ATPases: Transport mechanism and roles in virulence. Biochemistry 2011, 50, 9940–9949. [Google Scholar] [CrossRef] [PubMed]
- Schwan, W.R.; Warrener, P.; Keunz, E.; Stover, C.K.; Folger, K.R. Mutations in the cueA gene encoding a copper homeostasis P-type ATPase reduce the pathogenicity of Pseudomonas aeruginosa in mice. Int. J. Med. Microbiol. 2005, 295, 237–242. [Google Scholar] [CrossRef]
- Nigro, S.J.; Hall, R.M. Loss and gain of aminoglycoside resistance in global clone 2 Acinetobacter baumannii in Australia via modification of genomic resistance islands and acquisition of plasmids. J. Antimicrob. Chemother. 2016, 71, 2432–2440. [Google Scholar] [CrossRef]
- Outten, F.W.; Huffman, D.L.; Hale, J.A.; O’Halloran, T.V. The independent cue and cus systems confer copper tolerance during aerobic and anaerobic growth in Escherichia coli. J. Biol. Chem. 2001, 276, 30670–30677. [Google Scholar] [CrossRef]
- Abdollahi, S.; Rasooli, I.; Mousavi Gargari, S.L. The role of TonB-dependent copper receptor in virulence of Acinetobacter baumannii. Infect. Genet. Evol. 2018, 60, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Cubillas, C.; Miranda-Sanchez, F.; Gonzalez-Sanchez, A.; Elizalde, J.P.; Vinuesa, P.; Brom, S.; Garcia-de Los Santos, A. A comprehensive phylogenetic analysis of copper transporting P1B ATPases from bacteria of the Rhizobiales order uncovers multiplicity, diversity and novel taxonomic subtypes. Microbiologyopen 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Miller, E.W.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. A selective turn-on fluorescent sensor for imaging copper in living cells. J. Am. Chem. Soc. 2006, 128, 10–11. [Google Scholar] [CrossRef]
- Plumptre, C.D.; Hughes, C.E.; Harvey, R.M.; Eijkelkamp, B.A.; McDevitt, C.A.; Paton, J.C. Overlapping functionality of the Pht proteins in zinc homeostasis of Streptococcus pneumoniae. Infect. Immun. 2014, 82, 4315–4324. [Google Scholar] [CrossRef]
- Eijkelkamp, B.A.; Pederick, V.G.; Plumptre, C.D.; Harvey, R.M.; Hughes, C.E.; Paton, J.C.; McDevitt, C.A. The first histidine triad motif of PhtD is critical for zinc homeostasis in Streptococcus pneumoniae. Infect. Immun. 2016, 84, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Petersen, T.N.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 4.0: Discriminating signal peptides from transmembrane regions. Nat. Methods 2011, 8, 785–786. [Google Scholar] [CrossRef]
- Cao, B.; Porollo, A.; Adamczak, R.; Jarrell, M.; Meller, J. Enhanced recognition of protein transmembrane domains with prediction-based structural profiles. Bioinformatics 2006, 22, 303–309. [Google Scholar] [CrossRef]
- Bagos, P.G.; Liakopoulos, T.D.; Spyropoulos, I.C.; Hamodrakas, S.J. PRED-TMBB: A web server for predicting the topology of beta-barrel outer membrane proteins. Nucleic Acids Res. 2004, 32, W400–W404. [Google Scholar] [CrossRef]
- Pederick, V.G.; Eijkelkamp, B.A.; Ween, M.P.; Begg, S.L.; Paton, J.C.; McDevitt, C.A. Acquisition and role of molybdate in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2014, 80, 6843–6852. [Google Scholar] [CrossRef]
- Plumptre, C.D.; Eijkelkamp, B.A.; Morey, J.R.; Behr, F.; Counago, R.M.; Ogunniyi, A.D.; Kobe, B.; O’Mara, M.L.; Paton, J.C.; McDevitt, C.A. AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae. Mol. Microbiol. 2014, 91, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Eijkelkamp, B.A.; Hassan, K.A.; Paulsen, I.T.; Brown, M.H. Investigation of the human pathogen Acinetobacter baumannii under iron limiting conditions. BMC Genomics 2011, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Pederick, V.G.; Eijkelkamp, B.A.; Begg, S.L.; Ween, M.P.; McAllister, L.J.; Paton, J.C.; McDevitt, C.A. ZnuA and zinc homeostasis in Pseudomonas aeruginosa. Sci. Rep. 2015, 5, 13139. [Google Scholar] [CrossRef]
- Kilic, S.; White, E.R.; Sagitova, D.M.; Cornish, J.P.; Erill, I. CollecTF: A database of experimentally validated transcription factor-binding sites in bacteria. Nucleic Acids Res. 2014, 42, D156–D160. [Google Scholar] [CrossRef]
- Finn, R.D.; Clements, J.; Eddy, S.R. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011, 39, W29–W37. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alquethamy, S.F.; Khorvash, M.; Pederick, V.G.; Whittall, J.J.; Paton, J.C.; Paulsen, I.T.; Hassan, K.A.; McDevitt, C.A.; Eijkelkamp, B.A. The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence. Int. J. Mol. Sci. 2019, 20, 575. https://doi.org/10.3390/ijms20030575
Alquethamy SF, Khorvash M, Pederick VG, Whittall JJ, Paton JC, Paulsen IT, Hassan KA, McDevitt CA, Eijkelkamp BA. The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence. International Journal of Molecular Sciences. 2019; 20(3):575. https://doi.org/10.3390/ijms20030575
Chicago/Turabian StyleAlquethamy, Saleh F., Marjan Khorvash, Victoria G. Pederick, Jonathan J. Whittall, James C. Paton, Ian T. Paulsen, Karl A. Hassan, Christopher A. McDevitt, and Bart A. Eijkelkamp. 2019. "The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence" International Journal of Molecular Sciences 20, no. 3: 575. https://doi.org/10.3390/ijms20030575
APA StyleAlquethamy, S. F., Khorvash, M., Pederick, V. G., Whittall, J. J., Paton, J. C., Paulsen, I. T., Hassan, K. A., McDevitt, C. A., & Eijkelkamp, B. A. (2019). The Role of the CopA Copper Efflux System in Acinetobacter baumannii Virulence. International Journal of Molecular Sciences, 20(3), 575. https://doi.org/10.3390/ijms20030575





