The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond
Abstract
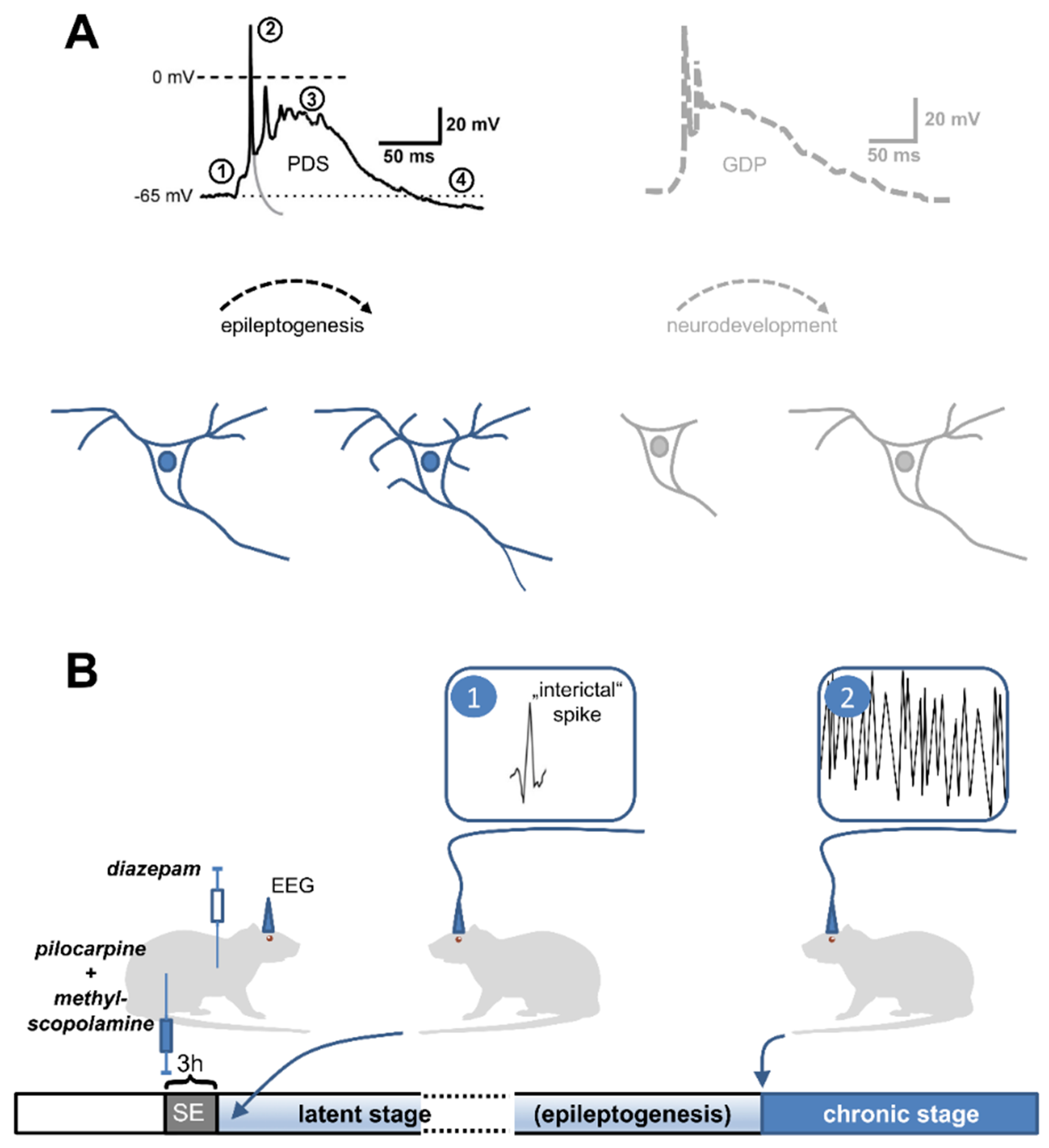
1. How can a Paroxysmal Depolarization Shift (PDS) Be Defined?
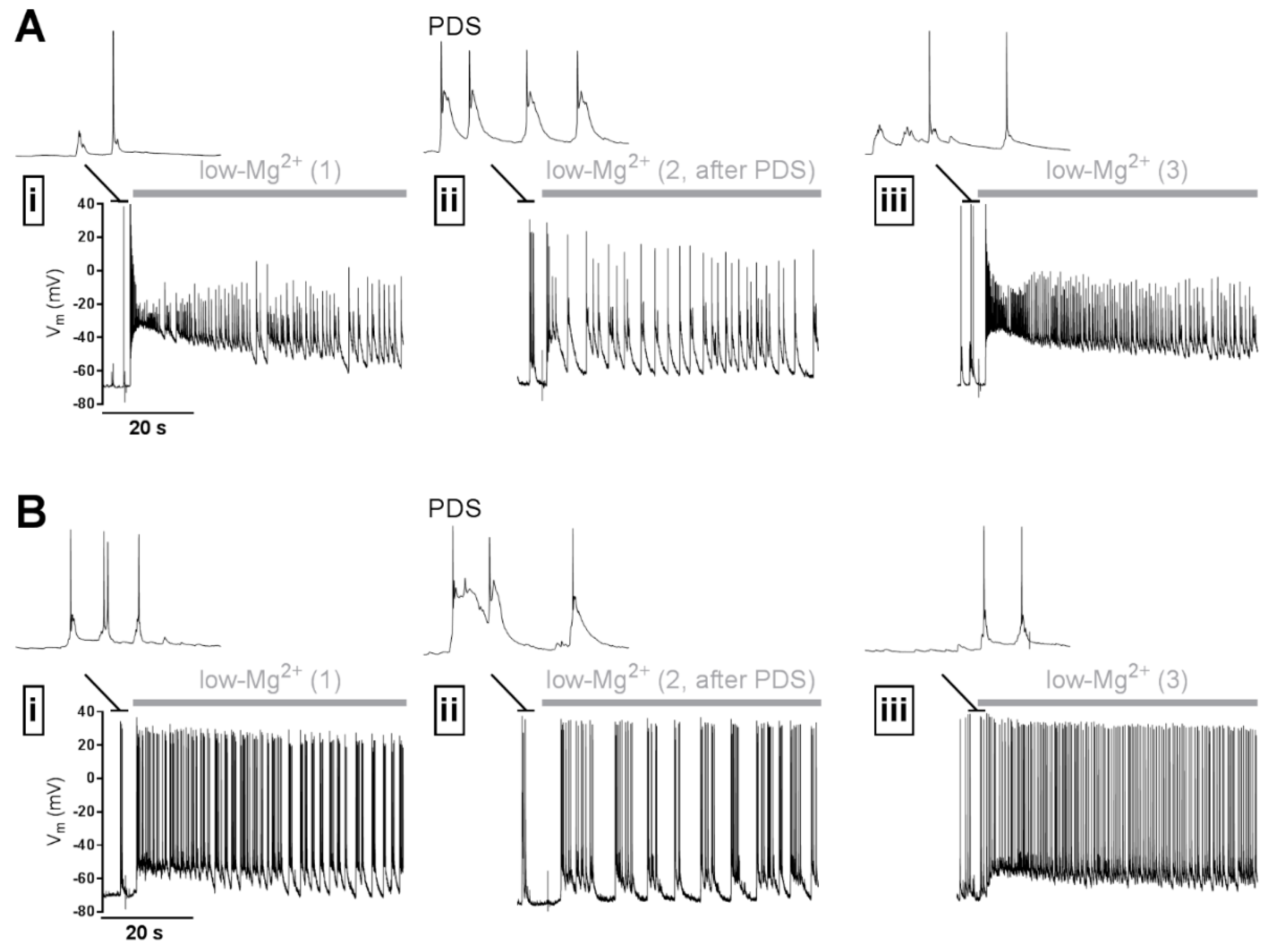
2. What Are the Mechanisms of PDS Formation?
2.1. Which Ion Conductances Underlie PDS?
2.2. How Are the Mechanisms of PDS Formation Induced?
3. Synchronization of PDS
4. Role in Epilepsy/Epileptogenesis
4.1. An Epileptogenic Role of PDS
4.2. An Anti-Ictogenic Role of PDS
5. Potential Role in Other Neurological Diseases
6. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Matsumoto, H.; Ajmone Marsan, C. Cortical cellular phenomena in experimental epilepsy: Interictal manifestations. Exp. Neurol. 1964, 9, 286–304. [Google Scholar] [CrossRef]
- Prince, D.A. The depolarization shift in “epileptic” neurons. Exp. Neurol. 1968, 21, 467–485. [Google Scholar] [CrossRef]
- Johnston, D.; Brown, T.H. Giant synaptic potential hypothesis for epileptiform activity. Science 1981, 211, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Antoniadis, A.; Müller, W.E.; Wollert, U. Inhibition of GABA and benzodiazepine receptor binding by penicillins. Neurosci. Lett. 1980, 18, 309–312. [Google Scholar] [CrossRef]
- Hablitz, J.J. Picrotoxin-induced epileptiform activity in hippocampus: Role of endogenous versus synaptic factors. J. Neurophysiol. 1984, 51, 1011–1027. [Google Scholar] [CrossRef] [PubMed]
- Bingmann, D.; Speckmann, E.J.; Baker, R.E.; Ruijter, J.; de Jong, B.M. Differential antiepileptic effects of the organic calcium antagonists verapamil and flunarizine in neurons of organotypic neocortical explants from newborn rats. Exp. Brain Res. 1988, 72, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Straub, H.; Speckmann, E.J.; Bingmann, D.; Walden, J. Paroxysmal depolarization shifts induced by bicuculline in CA3 neurons of hippocampal slices: Suppression by the organic calcium antagonist verapamil. Neurosci. Lett. 1990, 111, 99–101. [Google Scholar] [CrossRef]
- Schiller, Y. Inter-ictal- and ictal-like epileptic discharges in the dendritic tree of neocortical pyramidal neurons. J. Neurophysiol. 2002, 88, 2954–2962. [Google Scholar] [CrossRef]
- Walden, J.; Pockberger, H.; Speckmann, E.J.; Petsche, H. Paroxysmal neuronal depolarizations in the rat motorcortex in vivo: Intracellular injection of the calcium agonist BAY K 8644. Exp. Brain Res. 1986, 64, 607–609. [Google Scholar] [CrossRef]
- Witte, O.W.; Speckmann, E.J.; Walden, J. Motor cortical epileptic foci in vivo: Actions of a calcium channel blocker on paroxysmal neuronal depolarizations. Electroencephalogr. Clin. Neurophysiol. 1987, 66, 43–55. [Google Scholar] [CrossRef]
- Silva-Barrat, C.; Szente, M.; Menini, C.; Velluti, J.C.; Champagnat, J. Muscarinic Depression of Synaptic Transmission in the Epileptogenic GABA Withdrawal Syndrome Focus. J. Neurophysiol. 2001, 85, 2159–2165. [Google Scholar] [CrossRef]
- Sun, D.A.; Sombati, S.; DeLorenzo, R.J. Glutamate Injury–Induced Epileptogenesis in Hippocampal Neurons. Stroke 2001, 32, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Martella, G.; De Persis, C.; Bonsi, P.; Natoli, S.; Cuomo, D.; Bernardi, G.; Calabresi, P.; Pisani, A. Inhibition of persistent sodium current fraction and voltage-gated L-type calcium current by propofol in cortical neurons: Implications for its antiepileptic activity. Epilepsia 2005, 46, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.P.; Major, S.; Pannek, H.-W.; Woitzik, J.; Scheel, M.; Wiesenthal, D.; Martus, P.; Winkler, M.K.L.; Hartings, J.A.; Fabricius, M.; et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 2012, 135, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Calos, M.; Pilitsis, J.; Shin, D.S.-H. Deconstructing the neural and ionic involvement of seizure-like events in the striatal network. Neurobiol. Dis. 2013, 52, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, E.R.; Su, H.; Yaari, Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J. Physiol. 2001, 532, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Yaari, Y.; Yue, C.; Su, H. Recruitment of apical dendritic T-type Ca2+ channels by backpropagating spikes underlies de novo intrinsic bursting in hippocampal epileptogenesis. J. Physiol. 2007, 580, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.J.; Pitsch, J.; Sochivko, D.; Opitz, T.; Staniek, M.; Chen, C.-C.; Campbell, K.P.; Schoch, S.; Yaari, Y.; Beck, H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J. Neurosci. 2008, 28, 13341–13353. [Google Scholar] [CrossRef]
- Chen, S.; Su, H.; Yue, C.; Remy, S.; Royeck, M.; Sochivko, D.; Opitz, T.; Beck, H.; Yaari, Y. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J. Neurophysiol. 2011, 105, 117–129. [Google Scholar] [CrossRef]
- Altrup, U.; Wiemann, M. Paroxysmal depolarization shifts (PDS) induce non-synaptic responses in neighboured neurons (buccal ganglia, Helix pomatia). Brain Res. 2003, 972, 186–196. [Google Scholar] [CrossRef]
- Stanojević, M.; Lopicic, S.; Jovanovic, Z.; Pathak, D.; Pavlovic, D.V.; Spasic, S.; Nedeljkov, V.; Prostran, M. Magnesium Effects on Nonsynaptic Epileptiform Activity in Leech Retzius Neurons. Folia Biol. (Praha) 2015, 63, 301–306. [Google Scholar] [CrossRef]
- Segal, M.M.; Furshpan, E.J. Epileptiform activity in microcultures containing small numbers of hippocampal neurons. J. Neurophysiol. 1990, 64, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Silva-Barrat, C.; Velluti, J.; Szente, M.; Batini, C.; Champagnat, J. Exaggeration of epileptic-like patterns by nicotine receptor activation during the GABA withdrawal syndrome. Brain Res. 2005, 1042, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Moraidis, I.; Bingmann, D.; Lehmenkühler, A.; Speckmann, E.J. Caffeine-induced epileptic discharges in CA3 neurons of hippocampal slices of the guinea pig. Neurosci. Lett. 1991, 129, 51–54. [Google Scholar] [CrossRef]
- Straub, H.; Köhling, R.; Speckmann, E.J. Picrotoxin-induced epileptic activity in hippocampal and neocortical slices (guinea pig): Suppression by organic calcium channel blockers. Brain Res. 1994, 658, 119–126. [Google Scholar] [CrossRef]
- Traub, R.D.; Miles, R.; Jefferys, J.G. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J. Physiol. 1993, 461, 525–547. [Google Scholar] [CrossRef] [PubMed]
- Stiglbauer, V.; Hotka, M.; Ruiß, M.; Hilber, K.; Boehm, S.; Kubista, H. Ca v 1.3 channels play a crucial role in the formation of paroxysmal depolarization shifts in cultured hippocampal neurons. Epilepsia 2017, 58, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Rubi, L.; Schandl, U.; Lagler, M.; Geier, P.; Spies, D.; Gupta, K.; Boehm, S.; Kubista, H. Raised Activity of L-Type Calcium Channels Renders Neurons Prone to Form Paroxysmal Depolarization Shifts. NeuroMolecular Med. 2013, 15, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.M. Epileptiform activity in microcultures containing one excitatory hippocampal neuron. J. Neurophysiol. 1991, 65, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Speckmann, E.J.; Walden, J.; Bingmann, D. Contribution of calcium ions to epileptogenesis. J. Basic Clin. Physiol. Pharmacol. 1990, 1, 95–105. [Google Scholar] [CrossRef]
- Speckmann, E.J.; Walden, J. Anti-epileptic effects of organic calcium channel blockers. In Epilepsy: Models, Mechanisms and Concepts; Schwartzkroin, P.A., Ed.; University Press: Cambridge, UK, 1993; pp. 462–486. [Google Scholar]
- Baldino, F.; Wolfson, B.; Heinemann, U.; Gutnick, M.J. An N-methyl-D-aspartate (NMDA) receptor antagonist reduces bicuculline-induced depolarization shifts in neocortical explant cultures. Neurosci. Lett. 1986, 70, 101–105. [Google Scholar] [CrossRef]
- Jones, R.S. Epileptiform events induced by GABA-antagonists in entorhinal cortical cells in vitro are partly mediated by N-methyl-D-aspartate receptors. Brain Res. 1988, 457, 113–121. [Google Scholar] [CrossRef]
- McBain, C.J.; Boden, P.; Hill, R.G. Rat hippocampal slices “in vitro” display spontaneous epileptiform activity following long-term organotypic culture. J. Neurosci. Methods 1989, 27, 35–49. [Google Scholar] [CrossRef]
- Gean, P.W.; Chang, F.C. Ketamine suppresses synchronized discharges in the disinhibited amygdala slice. Brain Res. Bull. 1991, 26, 923–927. [Google Scholar] [PubMed]
- Hwa, G.G.; Avoli, M.; Oliver, A.; Villemure, J.G. Bicuculline-induced epileptogenesis in the human neocortex maintained in vitro. Exp. Brain Res. 1991, 83, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.L.; Hablitz, J.J. Effect of APV and ketamine on epileptiform activity in the CA1 and CA3 regions of the hippocampus. Epilepsy Res. 1990, 6, 87–94. [Google Scholar] [CrossRef]
- Lee, W.L.; Hablitz, J.J. Excitatory synaptic involvement in epileptiform bursting in the immature rat neocortex. J. Neurophysiol. 1991, 66, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Akopian, G.; Walsh, J.P. Corticostriatal paired-pulse potentiation produced by voltage-dependent activation of NMDA receptors and L-type Ca(2+) channels. J. Neurophysiol. 2002, 87, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Baginskas, A.; Kuras, A. L-type Ca2+ current in frog tectal recurrent neurons determines the NMDA receptor activation on efferent neuron. Exp. Brain Res. 2009, 193, 509–517. [Google Scholar] [CrossRef]
- Wang, D.; Grillner, S.; Wallén, P. Calcium dynamics during NMDA-induced membrane potential oscillations in lamprey spinal neurons-contribution of L-type calcium channels (CaV1.3). J. Physiol. 2013, 591, 2509–2521. [Google Scholar] [CrossRef]
- Fossat, P.; Sibon, I.; Le Masson, G.; Landry, M.; Nagy, F. L-type calcium channels and NMDA receptors: A determinant duo for short-term nociceptive plasticity. Eur. J. Neurosci. 2007, 25, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Domann, R.; Westerhoff, C.H.; Witte, O.W. Inhibitory mechanisms terminating paroxysmal depolarization shifts in hippocampal neurons of rats. Neurosci. Lett. 1994, 176, 71–74. [Google Scholar] [CrossRef]
- Holmes, G.L.; Ben-Ari, Y. The neurobiology and consequences of epilepsy in the developing brain. Pediatr. Res. 2001, 49, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Domann, R.; Dorn, T.; Witte, O.W. Afterpotentials following penicillin-induced paroxysmal depolarizations in rat hippocampal CA1 pyramidal cells in vitro. Pflugers Arch. 1991, 417, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Witte, O.W. Afterpotentials of penicillin-induced epileptiform neuronal discharges in the motor cortex of the rat in vivo. Epilepsy Res. 1994, 18, 43–55. [Google Scholar] [CrossRef]
- Witte, O.W.; Uhlig, S.; Valle, E. Separation of different types of afterpotentials following penicillin-induced paroxysmal depolarization shifts of neurons in the motor cortex of the rat. Neurosci. Lett. 1989, 101, 51–56. [Google Scholar] [CrossRef]
- Westerhoff, C.H.; Domann, R.; Witte, O.W. Inhibitory mechanisms in epileptiform activity induced by low magnesium. Pflugers Arch. 1995, 430, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Alonso, A.; Ragsdale, D.S. Increased persistent sodium currents in rat entorhinal cortex layer V neurons in a post-status epilepticus model of temporal lobe epilepsy. Epilepsia 2003, 44, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Hellier, J.L.; Patrylo, P.R.; Dou, P.; Nett, M.; Rose, G.M.; Dudek, F.E. Assessment of inhibition and epileptiform activity in the septal dentate gyrus of freely behaving rats during the first week after kainate treatment. J. Neurosci. 1999, 19, 10053–10064. [Google Scholar] [CrossRef]
- de Curtis, M.; Radici, C.; Forti, M. Cellular mechanisms underlying spontaneous interictal spikes in an acute model of focal cortical epileptogenesis. Neuroscience 1999, 88, 107–117. [Google Scholar] [CrossRef]
- Perez-Reyes, E. Molecular physiology of low-voltage-activated t-type calcium channels. Physiol. Rev. 2003, 83, 117–161. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Phelan, K.D. The role of canonical transient receptor potential channels in seizure and excitotoxicity. Cells 2014, 3, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Schiller, Y. Activation of a calcium-activated cation current during epileptiform discharges and its possible role in sustaining seizure-like events in neocortical slices. J. Neurophysiol. 2004, 92, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Hotka, M.; Kubista, H. The paroxysmal depolarization shift in epilepsy research. Int. J. Biochem. Cell Biol. 2018, 107, 77–81. [Google Scholar] [CrossRef]
- Dinocourt, C.; Petanjek, Z.; Freund, T.F.; Ben-Ari, Y.; Esclapez, M. Loss of interneurons innervating pyramidal cell dendrites and axon initial segments in the CA1 region of the hippocampus following pilocarpine-induced seizures. J. Comp. Neurol. 2003, 459, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Drexel, M.; Preidt, A.P.; Kirchmair, E.; Sperk, G. Parvalbumin interneurons and calretinin fibers arising from the thalamic nucleus reuniens degenerate in the subiculum after kainic acid-induced seizures. Neuroscience 2011, 189, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Rakhade, S.N.; Fitzgerald, E.F.; Klein, P.M.; Zhou, C.; Sun, H.; Huganir, R.L.; Hunganir, R.L.; Jensen, F.E. Glutamate receptor 1 phosphorylation at serine 831 and 845 modulates seizure susceptibility and hippocampal hyperexcitability after early life seizures. J. Neurosci. 2012, 32, 17800–17812. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.W.; Soares, F.M.S.; de Mello, N.; Nunes, J.C.; Cajado, A.G.; de Brito, D.; de Cordova, F.M.; da Cunha, R.M.S.; Walz, R.; Leal, R.B. Time-dependent modulation of AMPA receptor phosphorylation and mRNA expression of NMDA receptors and glial glutamate transporters in the rat hippocampus and cerebral cortex in a pilocarpine model of epilepsy. Exp. Brain Res. 2013, 226, 153–163. [Google Scholar] [CrossRef]
- Di Maio, R.; Mastroberardino, P.G.; Hu, X.; Montero, L.M.; Greenamyre, J.T. Thiol oxidation and altered NR2B/NMDA receptor functions in in vitro and in vivo pilocarpine models: Implications for epileptogenesis. Neurobiol. Dis. 2013, 49, 87–98. [Google Scholar] [CrossRef]
- Raza, M.; Blair, R.E.; Sombati, S.; Carter, D.S.; Deshpande, L.S.; DeLorenzo, R.J. Evidence that injury-induced changes in hippocampal neuronal calcium dynamics during epileptogenesis cause acquired epilepsy. Proc. Natl. Acad. Sci. USA 2004, 101, 17522–17527. [Google Scholar] [CrossRef]
- DeLorenzo, R.J.; Sun, D.A.; Deshpande, L.S. Erratum to “Cellular mechanisms underlying acquired epilepsy: the calcium hypothesis of the induction and maintenance of epilepsy.” [Pharmacol. Ther. 105(3) (2005) 229-266]. Pharmacol. Ther. 2006, 111, 288–325. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, R.; Mastroberardino, P.G.; Hu, X.; Montero, L.; Greenamyre, J.T. Pilocapine alters NMDA receptor expression and function in hippocampal neurons: NADPH oxidase and ERK1/2 mechanisms. Neurobiol. Dis. 2011, 42, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Waldbaum, S.; Patel, M. Mitochondrial dysfunction and oxidative stress: A contributing link to acquired epilepsy? J. Bioenerg. Biomembr. 2010, 42, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, J.; Chen, Z.; Chen, S.; Zhu, F.; Zhou, L. Long-term expressional changes of Na+ −K+ -Cl− co-transporter 1 (NKCC1) and K+ −Cl− co-transporter 2 (KCC2) in CA1 region of hippocampus following lithium-pilocarpine induced status epilepticus (PISE). Brain Res. 2008, 1221, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Barmashenko, G.; Hefft, S.; Aertsen, A.; Kirschstein, T.; Köhling, R. Positive shifts of the GABAA receptor reversal potential due to altered chloride homeostasis is widespread after status epilepticus. Epilepsia 2011, 52, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Gutnick, M.J.; Connors, B.W.; Prince, D.A. Mechanisms of neocortical epileptogenesis in vitro. J. Neurophysiol. 1982, 48, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Bingmann, D.; Speckmann, E.J. Actions of pentylenetetrazol (PTZ) on CA3 neurons in hippocampal slices of guinea pigs. Exp. Brain Res. 1986, 64, 94–104. [Google Scholar] [CrossRef]
- Raza, M.; Shaheen, F.; Choudhary, M.I.; Rahman, A.; Sombati, S.; DeLorenzo, R.J. In vitro inhibition of pentylenetetrazole and bicuculline-induced epileptiform activity in rat hippocampal pyramidal neurons by aqueous fraction isolated from Delphinium denudatum. Neurosci. Lett. 2002, 333, 103–106. [Google Scholar] [CrossRef]
- Akaishi, T.; Nakazawa, K.; Sato, K.; Saito, H.; Ohno, Y.; Ito, Y. Hydrogen peroxide modulates whole cell Ca2+ currents through L-type channels in cultured rat dentate granule cells. Neurosci. Lett. 2004, 356, 25–28. [Google Scholar] [CrossRef]
- Xu, W.; Lipscombe, D. Neuronal Ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 2001, 21, 5944–5951. [Google Scholar] [CrossRef]
- Thomas, J.R.; Lee, A. Measuring Ca2+-Dependent Modulation of Voltage-Gated Ca2+ Channels in HEK-293T Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087213. [Google Scholar] [CrossRef] [PubMed]
- Chameau, P.; Qin, Y.; Spijker, S.; Smit, A.B.; Smit, G.; Joëls, M. Glucocorticoids specifically enhance L-type calcium current amplitude and affect calcium channel subunit expression in the mouse hippocampus. J. Neurophysiol. 2007, 97, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Blair, L.A.C.; Salinas, G.D.; Needleman, L.A.; Marshall, J. Insulin-like growth factor-1 modulation of CaV1.3 calcium channels depends on Ca2+ release from IP3-sensitive stores and calcium/calmodulin kinase II phosphorylation of the alpha1 subunit EF hand. J. Neurosci. 2006, 26, 6259–6268. [Google Scholar] [CrossRef] [PubMed]
- Klassen, T.; Davis, C.; Goldman, A.; Burgess, D.; Chen, T.; Wheeler, D.; McPherson, J.; Bourquin, T.; Lewis, L.; Villasana, D.; et al. Exome sequencing of ion channel genes reveals complex profiles confounding personal risk assessment in epilepsy. Cell 2011, 145, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Pinggera, A.; Striessnig, J. Cav1.3 (CACNA1D) L-type Ca2+ channel dysfunction in CNS disorders. J. Physiol. 2016, 594, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- De Curtis, M.; Jefferys, J.G.R.; Avoli, M. Interictal Epileptiform Discharges in Partial Epilepsy Different IED Patterns in Epileptic Patients: Spikes, Spike Bursts. In Jasper’s Basic Mechanisms of the Epilepsies [Internet]; Noebels, J.L., Avoli, M., Rogawski, M.A., Olsen, R.W., Delgado-Escueta, A.V., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012; pp. 1–24. [Google Scholar]
- Jiruska, P.; de Curtis, M.; Jefferys, J.G.R.; Schevon, C.A.; Schiff, S.J.; Schindler, K. Synchronization and desynchronization in epilepsy: Controversies and hypotheses. J. Physiol. 2013, 591, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.; Lee, C.; Xie, W.; Cui, B.; Poo, M. Activity-dependent BDNF release via endocytic pathways is regulated by synaptotagmin-6 and complexin. Proc. Natl. Acad. Sci. USA 2015, 112, E4475–E4484. [Google Scholar] [CrossRef] [PubMed]
- Kafitz, K.W.; Rose, C.R.; Thoenen, H.; Konnerth, A. Neurotrophin-evoked rapid excitation through TrkB receptors. Nature 1999, 401, 918–921. [Google Scholar] [CrossRef]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef]
- Beste, C.; Kolev, V.; Yordanova, J.; Domschke, K.; Falkenstein, M.; Baune, B.T.; Konrad, C. The role of the BDNF Val66Met polymorphism for the synchronization of error-specific neural networks. J. Neurosci. 2010, 30, 10727–10733. [Google Scholar] [CrossRef]
- Zheng, K.; An, J.J.; Yang, F.; Xu, W.; Xu, Z.D.; Wu, J.; Hökfelt, T.G.M.; Fisahn, A.; Xu, B.; Lu, B. TrkB signaling in parvalbumin-positive interneurons is critical for gamma-band network synchronization in hippocampus. Proc. Natl. Acad. Sci. USA 2011, 108, 17201–17206. [Google Scholar] [CrossRef] [PubMed]
- Mongrain, V.; Warby, S.C. Determinants of cortical synchrony. Sleep 2012, 35, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lenck-Santini, P.-P.; Zhao, Q.; Holmes, G.L. Effect of interictal spikes on single-cell firing patterns in the hippocampus. Epilepsia 2007, 48, 720–731. [Google Scholar] [CrossRef] [PubMed]
- de Curtis, M.; Avanzini, G. Interictal spikes in focal epileptogenesis. Prog. Neurobiol. 2001, 63, 541–567. [Google Scholar] [CrossRef]
- Staley, K.; Hellier, J.L.; Dudek, F.E. Do interictal spikes drive epileptogenesis? Neuroscientist 2005, 11, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Staley, K.J.; White, A.; Dudek, F.E. Interictal spikes: harbingers or causes of epilepsy? Neurosci. Lett. 2011, 497, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Cherubini, E.; Corradetti, R.; Gaiarsa, J.L. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J. Physiol. 1989, 416, 303–325. [Google Scholar] [CrossRef]
- Griguoli, M.; Cherubini, E. Early Correlated Network Activity in the Hippocampus: Its Putative Role in Shaping Neuronal Circuits. Front. Cell. Neurosci. 2017, 11, 255. [Google Scholar] [CrossRef]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.G.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef]
- Tyzio, R.; Allene, C.; Nardou, R.; Picardo, M.A.; Yamamoto, S.; Sivakumaran, S.; Caiati, M.D.; Rheims, S.; Minlebaev, M.; Milh, M.; et al. Depolarizing actions of GABA in immature neurons depend neither on ketone bodies nor on pyruvate. J. Neurosci. 2011, 31, 34–45. [Google Scholar] [CrossRef]
- Mohajerani, M.H.; Sivakumaran, S.; Zacchi, P.; Aguilera, P.; Cherubini, E. Correlated network activity enhances synaptic efficacy via BDNF and the ERK pathway at immature CA3 CA1 connections in the hippocampus. Proc. Natl. Acad. Sci. USA 2007, 104, 13176–13181. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, E.; Griguoli, M.; Safiulina, V.; Lagostena, L. The depolarizing action of GABA controls early network activity in the developing hippocampus. Mol. Neurobiol. 2011, 43, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Deisseroth, K.; Mermelstein, P.G.; Xia, H.; Tsien, R.W. Signaling from synapse to nucleus: the logic behind the mechanisms. Curr. Opin. Neurobiol. 2003, 13, 354–365. [Google Scholar] [CrossRef]
- Ma, H.; Cohen, S.; Li, B.; Tsien, R.W. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci. Rep. 2012, 33, 97–101. [Google Scholar] [CrossRef]
- Hongpaisan, J.; Winters, C.A.; Andrews, S.B. Calcium-dependent mitochondrial superoxide modulates nuclear CREB phosphorylation in hippocampal neurons. Mol. Cell. Neurosci. 2003, 24, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Staley, K.J.; Dudek, F.E. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006, 6, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Rakhade, S.N.; Shah, A.K.; Agarwal, R.; Yao, B.; Asano, E.; Loeb, J.A. Activity-dependent gene expression correlates with interictal spiking in human neocortical epilepsy. Epilepsia 2007, 48 (Suppl. 5), 86–95. [Google Scholar] [CrossRef]
- Wadman, W.J.; Da Silva, F.H.; Leung, L.W. Two types of interictal transients of reversed polarity in rat hippocampus during kindling. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 314–319. [Google Scholar] [CrossRef]
- Dyhrfjeld-Johnsen, J.; Berdichevsky, Y.; Swiercz, W.; Sabolek, H.; Staley, K.J. Interictal spikes precede ictal discharges in an organotypic hippocampal slice culture model of epileptogenesis. J. Clin. Neurophysiol. 2010, 27, 418–424. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Williams, P.A.; Hellier, J.L.; Clark, S.; Dudek, F.E.; Staley, K.J. EEG spike activity precedes epilepsy after kainate-induced status epilepticus. Epilepsia 2010, 51, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Chauvière, L.; Doublet, T.; Ghestem, A.; Siyoucef, S.S.; Wendling, F.; Huys, R.; Jirsa, V.; Bartolomei, F.; Bernard, C. Changes in interictal spike features precede the onset of temporal lobe epilepsy. Ann. Neurol. 2012, 71, 805–814. [Google Scholar] [CrossRef]
- Roseman, E.; Woodhall, B. The electro-encephalogram in war wounds of the brain; with particular reference to post-traumatic epilepsy. Res. Publs. Assoc. Res. Nerv. Ment. Dis. Proc. 1946, 25, 201–219. [Google Scholar]
- Momiyama, T.; Ishihara, K.; Serikawa, T.; Moritake, K.; Sasa, M. Effect of nicardipine on abnormal excitability of CA3 pyramidal cells in hippocampal slices of spontaneously epileptic rats. Eur. J. Pharmacol. 1995, 280, 119–123. [Google Scholar] [CrossRef]
- Hanaya, R.; Sasa, M.; Kiura, Y.; Ishihara, K.; Serikawa, T.; Kurisu, K. Epileptiform burst discharges in hippocampal CA3 neurons of young but not mature Noda epileptic rats (NER). Brain Res. 2002, 950, 317–320. [Google Scholar] [CrossRef]
- McCarthy, M.M.; Auger, A.P.; Perrot-Sinal, T.S. Getting excited about GABA and sex differences in the brain. Trends Neurosci. 2002, 25, 307–312. [Google Scholar] [CrossRef]
- Danzer, S.C.; Crooks, K.R.C.; Lo, D.C.; McNamara, J.O. Increased expression of brain-derived neurotrophic factor induces formation of basal dendrites and axonal branching in dentate granule cells in hippocampal explant cultures. J. Neurosci. 2002, 22, 9754–9763. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Yamada, M.K.; Fujisawa, S.; Katoh-Semba, R.; Matsuki, N.; Ikegaya, Y. Brain-derived neurotrophic factor induces hyperexcitable reentrant circuits in the dentate gyrus. J. Neurosci. 2004, 24, 7215–7224. [Google Scholar] [CrossRef] [PubMed]
- Ikegaya, Y. Abnormal targeting of developing hippocampal mossy fibers after epileptiform activities via L-type Ca2+ channel activation in vitro. J. Neurosci. 1999, 19, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.; Goodman, J.; Macleod, A.; Phani, S.; Antonelli, C.; Croll, S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005, 192, 348–356. [Google Scholar] [CrossRef]
- Sugaya, Y.; Maru, E.; Kudo, K.; Shibasaki, T.; Kato, N. Levetiracetam suppresses development of spontaneous EEG seizures and aberrant neurogenesis following kainate-induced status epilepticus. Brain Res. 2010, 1352, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.C.; Zhan, R.; Nadler, J.V. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J. Comp. Neurol. 2011, 519, 2175–2192. [Google Scholar] [CrossRef] [PubMed]
- Scharfman, H.E.; Pierce, J.P. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia 2012, 53 (Suppl. 1), 109–115. [Google Scholar] [CrossRef]
- Botterill, J.J.; Brymer, K.J.; Caruncho, H.J.; Kalynchuk, L.E. Aberrant hippocampal neurogenesis after limbic kindling: Relationship to BDNF and hippocampal-dependent memory. Epilepsy Behav. 2015, 47, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hosford, B.E.; Liska, J.P.; Danzer, S.C. Ablation of Newly Generated Hippocampal Granule Cells Has Disease-Modifying Effects in Epilepsy. J. Neurosci. 2016, 36, 11013–11023. [Google Scholar] [CrossRef] [PubMed]
- Danzer, S.C. Neurogenesis in Epilepsy: Better to Burn Out or Fade Away? Epilepsy Curr. 2016, 16, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.A.; Figueroa-Aragon, S.; Ribak, C.E. Newly generated granule cells show rapid neuroplastic changes in the adult rat dentate gyrus during the first five days following pilocarpine-induced seizures. Eur. J. Neurosci. 2007, 26, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Hell, J.W.; Westenbroek, R.E.; Warner, C.; Ahlijanian, M.K.; Prystay, W.; Gilbert, M.M.; Snutch, T.P.; Catterall, W.A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 1993, 123, 949–962. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A.; Flockerzi, V.; Hofmann, F. Regional expression and cellular localization of the alpha1 and beta subunit of high voltage-activated calcium channels in rat brain. J. Neurosci. 1997, 17, 1339–1349. [Google Scholar] [CrossRef] [PubMed]
- Liebmann, L.; Karst, H.; Sidiropoulou, K.; van Gemert, N.; Meijer, O.C.; Poirazi, P.; Joëls, M. Differential effects of corticosterone on the slow afterhyperpolarization in the basolateral amygdala and CA1 region: Possible role of calcium channel subunits. J. Neurophysiol. 2008, 99, 958–968. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, Y.; Altier, C.; Platzer, J.; Surmeier, D.J.; Bezprozvanny, I. Ca1.2 and CaV1.3 neuronal L-type calcium channels: Differential targeting and signaling to pCREB. Eur. J. Neurosci. 2006, 23, 2297–2310. [Google Scholar] [CrossRef]
- Xu, J.H.; Long, L.; Tang, Y.C.; Hu, H.T.; Tang, F.R. Ca(v)1.2, Ca(v)1.3, and Ca(v)2.1 in the mouse hippocampus during and after pilocarpine-induced status epilepticus. Hippocampus 2007, 17, 235–251. [Google Scholar] [CrossRef]
- Mikati, M.A.; Holmes, G.L.; Werner, S.; Bakkar, N.; Carmant, L.; Liu, Z.; Stafstrom, C.E. Effects of nimodipine on the behavioral sequalae of experimental status epilepticus in prepubescent rats. Epilepsy Behav. 2004, 5, 168–174. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Liang, L.; Hellier, J.L.; Staley, K.J.; Patel, M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol. Dis. 2008, 30, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Puttachary, S.; Sharma, S.; Verma, S.; Yang, Y.; Putra, M.; Thippeswamy, A.; Luo, D.; Thippeswamy, T. 1400 W, a highly selective inducible nitric oxide synthase inhibitor is a potential disease modifier in the rat kainate model of temporal lobe epilepsy. Neurobiol. Dis. 2016, 93, 184–200. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, P.W. EEG criteria for nonconvulsive status epilepticus. Epilepsia 2007, 48 (Suppl. 8), 39–41. [Google Scholar] [CrossRef]
- Lin, L.; Drislane, F.W. Lateralized Periodic Discharges: A Literature Review. J. Clin. Neurophysiol. 2018, 35, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Mader, E.C.; Cannizzaro, L.A.; Williams, F.J.; Lalan, S.; Olejniczak, P.W. Periodic Lateralized Epileptiform Discharges can Survive Anesthesia and Result in Asymmetric Drug-induced Burst Suppression. Neurol. Int. 2017, 9, 6933. [Google Scholar] [CrossRef] [PubMed]
- Avoli, M.; Biagini, G.; de Curtis, M. Do interictal spikes sustain seizures and epileptogenesis? Epilepsy Curr. 2006, 6, 203–207. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, M.; Köhling, R.; Ricalzone, S.; Gotman, J.; Biagini, G.; Avoli, M. Antiepileptic drugs abolish ictal but not interictal epileptiform discharges in vitro. Epilepsia 2010, 51, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Terzano, M.G.; Parrino, L.; Mazzucchi, A.; Moretti, G. Confusional states with periodic lateralized epileptiform discharges (PLEDs): A peculiar epileptic syndrome in the elderly. Epilepsia 1986, 27, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.K.; Scott, R.C.; Holmes, G.L.; Roberts, D.W.; Rundle, M.M.; Testorf, M.; Lenck-Santini, P.-P.; Jobst, B.C. Hippocampal interictal epileptiform activity disrupts cognition in humans. Neurology 2013, 81, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Andraus, M.E.C.; Andraus, C.F.; Alves-Leon, S.V. Periodic EEG patterns: Importance of their recognition and clinical significance. Arq. Neuropsiquiatr. 2012, 70, 145–151. [Google Scholar] [CrossRef]
- Berdichevsky, Y.; Dzhala, V.; Mail, M.; Staley, K.J. Interictal spikes, seizures and ictal cell death are not necessary for post-traumatic epileptogenesis in vitro. Neurobiol. Dis. 2012, 45, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Segal, M.M. Endogenous bursts underlie seizurelike activity in solitary excitatory hippocampal neurons in microcultures. J. Neurophysiol. 1994, 72, 1874–1884. [Google Scholar] [CrossRef] [PubMed]
- Gotman, J. Relationships between interictal spiking and seizures: human and experimental evidence. Can. J. Neurol. Sci. 1991, 18, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.S.; Goncharova, I.I.; Duckrow, R.B.; Novotny, E.J.; Zaveri, H.P. Interictal spikes on intracranial recording: Behavior, physiology, and implications. Epilepsia 2008, 49, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Goncharova, I.I.; Alkawadri, R.; Gaspard, N.; Duckrow, R.B.; Spencer, D.D.; Hirsch, L.J.; Spencer, S.S.; Zaveri, H.P. Clinical Neurophysiology The relationship between seizures, interictal spikes and antiepileptic drugs. Clin. Neurophysiol. 2016, 127, 3180–3186. [Google Scholar] [CrossRef] [PubMed]
- Avoli, M. Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia 2001, 42 (Suppl. 3), 2–4. [Google Scholar] [CrossRef]
- Barbarosie, M.; Avoli, M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J. Neurosci. 1997, 17, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Librizzi, L.; de Curtis, M. Epileptiform ictal discharges are prevented by periodic interictal spiking in the olfactory cortex. Ann. Neurol. 2003, 53, 382–389. [Google Scholar] [CrossRef]
- de Curtis, M.; Librizzi, L.; Biella, G. Discharge threshold is enhanced for several seconds after a single interictal spike in a model of focal epileptogenesis. Eur. J. Neurosci. 2001, 14, 174–178. [Google Scholar] [CrossRef]
- Yan, W.; Mitzelfelt, J.D.; Principe, J.C.; Sanchez, J.C. The effects of interictal spikes on single neuron firing patterns in the hippocampus during the development of temporal lobe epilepsy. Conf. Proc. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Conf. 2008, 2008, 4134–4137. [Google Scholar]
- Rogawski, M.A. Point-counterpoint: Do interictal spikes trigger seizures or protect against them? Epilepsy Curr. 2006, 6, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Reibel, S.; Vivien-Roels, B.; Lê, B.T.; Larmet, Y.; Carnahan, J.; Marescaux, C.; Depaulis, A. Overexpression of neuropeptide Y induced by brain-derived neurotrophic factor in the rat hippocampus is long lasting. Eur. J. Neurosci. 2000, 12, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Kleen, J.K.; Scott, R.C.; Holmes, G.L.; Lenck-Santini, P.P. Hippocampal interictal spikes disrupt cognition in rats. Ann. Neurol. 2010, 67, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, M.; Lebreton, F.; Cho, Y.H.; Leinekugel, X. “Ectopic” theta oscillations and interictal activity during slow-wave state in the R6/1 mouse model of Huntington’s disease. Neurobiol. Dis. 2012, 48, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kam, K.; Duffy, Á.M.; Moretto, J.; LaFrancois, J.J.; Scharfman, H.E. Interictal spikes during sleep are an early defect in the Tg2576 mouse model of β-amyloid neuropathology. Sci. Rep. 2016, 6, 20119. [Google Scholar] [CrossRef]
- Foncin, J.F.; Salmon, D.; Supino-Viterbo, V.; Feldman, R.G.; Macchi, G.; Mariotti, P.; Scoppetta, C.; Caruso, G.; Bruni, A.C. Alzheimer’s presenile dementia transmitted in an extended kindred. Rev. Neurol. (Paris) 1985, 141, 194–202. [Google Scholar] [PubMed]
- Ito, M.; Echizenya, N.; Nemoto, D.; Kase, M. A case series of epilepsy-derived memory impairment resembling Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2009, 23, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; Beagle, A.J.; Rabinovici, G.D.; Shu, H.; Lee, S.E.; Naasan, G.; Hegde, M.; Cornes, S.B.; Henry, M.L.; Nelson, A.B.; et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013, 70, 1158–1166. [Google Scholar] [CrossRef]
- Vossel, K.A.; Ranasinghe, K.G.; Beagle, A.J.; Mizuiri, D.; Honma, S.M.; Dowling, A.F.; Darwish, S.M.; Van Berlo, V.; Barnes, D.E.; Mantle, M.; et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann. Neurol. 2016, 80, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.D.; Deck, G.; Goldman, A.; Eskandar, E.N.; Noebels, J.; Cole, A.J. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat. Med. 2017, 23, 678–680. [Google Scholar] [CrossRef]
- Vossel, K.A.; Tartaglia, M.C.; Nygaard, H.B.; Zeman, A.Z.; Miller, B.L. Epileptic activity in Alzheimer’s disease: Causes and clinical relevance. Lancet. Neurol. 2017, 16, 311–322. [Google Scholar] [CrossRef]
- Reyes-Marin, K.E.; Nuñez, A. Seizure susceptibility in the APP/PS1 mouse model of Alzheimer’s disease and relationship with amyloid β plaques. Brain Res. 2017, 1677, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Epilepsy and cognitive impairments in Alzheimer disease. Arch. Neurol. 2009, 66, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Noebels, J. A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia 2011, 52 (Suppl. 1), 39–46. [Google Scholar] [CrossRef]
- Binnie, C.D. Cognitive impairment during epileptiform discharges: Is it ever justifiable to treat the EEG? Lancet Neurol. 2003, 2, 725–730. [Google Scholar] [CrossRef]
- Holmes, G.L. What is more harmful, seizures or epileptic EEG abnormalities? Is there any clinical data? Epileptic Disord. 2014, 16 Spec No, S12-22. [Google Scholar]
- Pinggera, A.; Mackenroth, L.; Rump, A.; Schallner, J.; Beleggia, F.; Wollnik, B.; Striessnig, J. New gain-of-function mutation shows CACNA1D as recurrently mutated gene in autism spectrum disorders and epilepsy. Hum. Mol. Genet. 2017, 26, 2923–2932. [Google Scholar] [CrossRef] [PubMed]
- Gleichmann, M.; Chow, V.W.; Mattson, M.P. Homeostatic disinhibition in the aging brain and Alzheimer’s disease. J. Alzheimers. Dis. 2011, 24, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Larner, A.J.; Marson, A.G. Epileptic seizures in Alzheimer’s disease: Another fine MESS? J. Alzheimers. Dis. 2011, 25, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mattson, M.P. L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol. Aging 2014, 35, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef] [PubMed]
- Hartley, D.M.; Walsh, D.M.; Ye, C.P.; Diehl, T.; Vasquez, S.; Vassilev, P.M.; Teplow, D.B.; Selkoe, D.J. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 1999, 19, 8876–8884. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Walsh, D.M.; Selkoe, D.J.; Hartley, D.M. Amyloid beta-protein induced electrophysiological changes are dependent on aggregation state: N-methyl-D-aspartate (NMDA) versus non-NMDA receptor/channel activation. Neurosci. Lett. 2004, 366, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Schumacker, P.T.; Guzman, J.D.; Ilijic, E.; Yang, B.; Zampese, E. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 483, 1013–1019. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubista, H.; Boehm, S.; Hotka, M. The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond. Int. J. Mol. Sci. 2019, 20, 577. https://doi.org/10.3390/ijms20030577
Kubista H, Boehm S, Hotka M. The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond. International Journal of Molecular Sciences. 2019; 20(3):577. https://doi.org/10.3390/ijms20030577
Chicago/Turabian StyleKubista, Helmut, Stefan Boehm, and Matej Hotka. 2019. "The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond" International Journal of Molecular Sciences 20, no. 3: 577. https://doi.org/10.3390/ijms20030577
APA StyleKubista, H., Boehm, S., & Hotka, M. (2019). The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond. International Journal of Molecular Sciences, 20(3), 577. https://doi.org/10.3390/ijms20030577





