Muscle Insulin Resistance and the Inflamed Microvasculature: Fire from Within
Abstract
1. Introduction
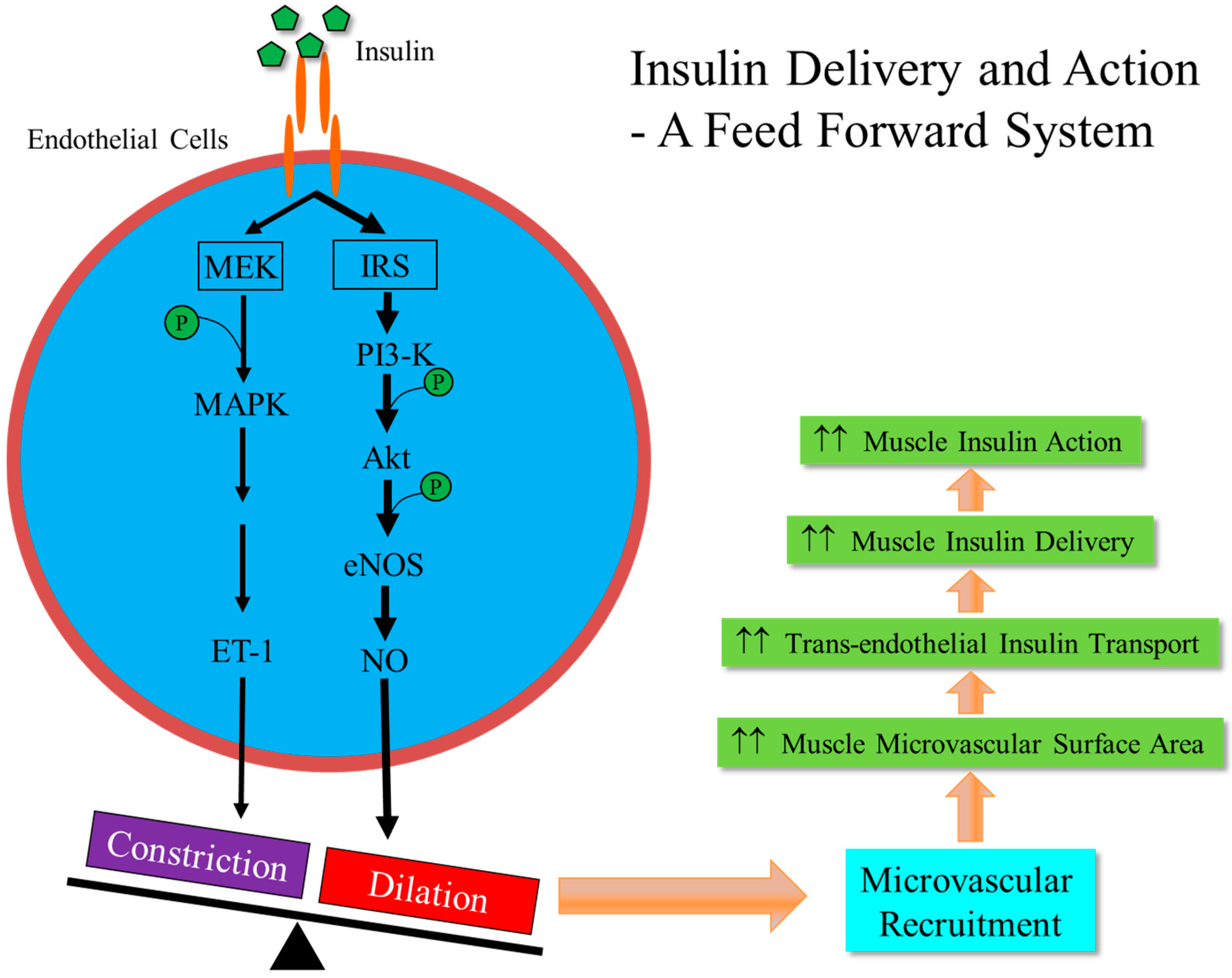
2. Vasculature is a Target for Insulin Action
3. Microvasculature Critically Regulates Insulin Action in Muscle
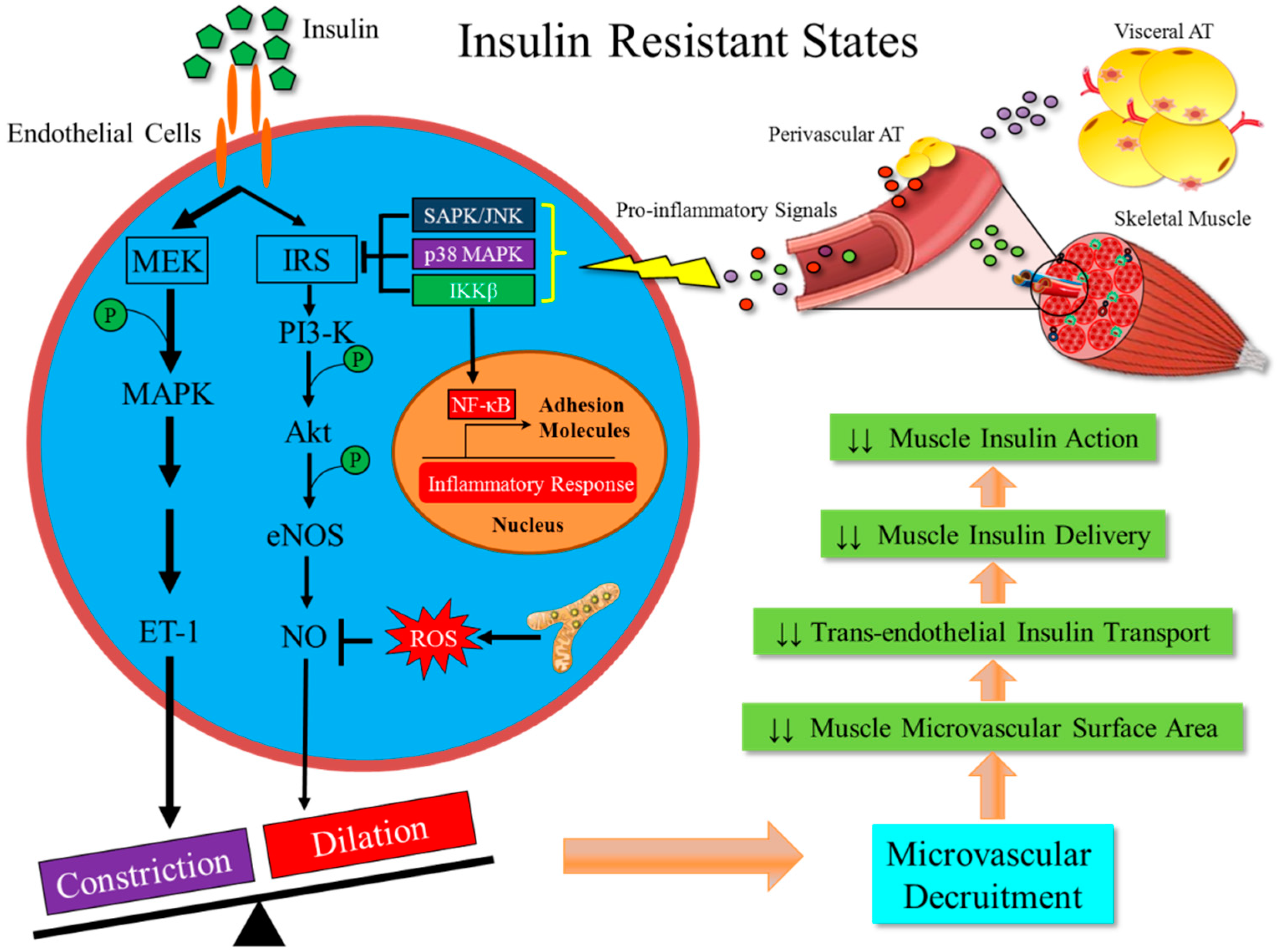
4. Metabolic Insulin Resistance Coexists with Microvascular Insulin Resistance
5. Metabolic Insulin Resistance is Associated with Inflammation in the Skeletal Muscle
6. Inflammation-Induced Insulin Resistance in Muscle Microvasculature is an Early Event in the Development of Obesity and T2DM
7. Conclusions
Funding
Conflicts of Interest
References
- Unnikrishnan, R.; Pradeepa, R.; Joshi, S.R.; Mohan, V. Type 2 diabetes: Demystifying the global epidemic. Diabetes 2017, 66, 1432–1442. [Google Scholar] [CrossRef]
- Kahn, B.B.; Flier, J.S. Obesity and insulin resistance. J. Clin. Investig. 2000, 106, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. 10. Cardiovascular disease and risk management: Standards of medical care in diabetes—2019. Diabetes Care 2019, 42, S103–S123. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.J.; Liu, Z.; Khamaisi, M.; King, G.L.; Klein, R.; Klein, B.E.K.; Hughes, T.M.; Craft, S.; Freedman, B.I.; Bowden, D.W.; et al. Diabetic microvascular disease: An Endocrine Society scientific statement. J. Clin. Endocrinol. Metab. 2017, 102, 4343–4410. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical nodes in signalling pathways: Insights into insulin action. Nat. Rev. Mol. Cell. Biol. 2006, 7, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef]
- Barrett, E.J.; Wang, H.; Upchurch, C.T.; Liu, Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am. J. Physiol. 2011, 301, E252–E263. [Google Scholar] [CrossRef]
- Barrett, E.J.; Eggleston, E.M.; Inyard, A.C.; Wang, H.; Li, G.; Liu, Z. The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia 2009, 52, 752–764. [Google Scholar] [CrossRef]
- Clark, M.G.; Wallis, M.G.; Barrett, E.J.; Vincent, M.A.; Richards, S.M.; Clerk, L.H.; Rattigan, S. Blood flow and muscle metabolism: A focus on insulin action. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E241–E258. [Google Scholar] [CrossRef]
- Kim, J.A.; Montagnani, M.; Koh, K.K.; Quon, M.J. Reciprocal relationships between insulin resistance and endothelial dysfunction: Molecular and pathophysiological mechanisms. Circulation 2006, 113, 1888–1904. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, W.A.; Quinones, M.J. Role of endothelial dysfunction in insulin resistance. Am. J. Cardiol. 2003, 92, 10J–17J. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J. Role of insulin resistance in endothelial dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Barrett, E.J.; Ko, S.H.; Cao, W.; Liu, Z. Insulin and insulin-like growth factor-I receptors differentially mediate insulin-stimulated adhesion molecule production by endothelial cells. Endocrinology 2009, 150, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Barrett, E.J.; Wang, H.; Chai, W.; Liu, Z. Insulin at physiological concentrations selectively activates insulin but not insulin-like growth factor I (IGF-I) or insulin/IGF-I hybrid receptors in endothelial cells. Endocrinology 2005, 146, 4690–4696. [Google Scholar] [CrossRef]
- Chisalita, S.I.; Arnqvist, H.J. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E896–E901. [Google Scholar] [CrossRef] [PubMed]
- Dekker Nitert, M.; Chisalita, S.I.; Olsson, K.; Bornfeldt, K.E.; Arnqvist, H.J. IGF-I/insulin hybrid receptors in human endothelial cells. Mol. Cell. Endocrinol. 2005, 229, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Nystrom, F.H.; Ravichandran, L.V.; Cong, L.N.; Kirby, M.; Mostowski, H.; Quon, M.J. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation 2000, 101, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Quon, M.J. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J. Clin. Investig. 1996, 98, 894–898. [Google Scholar] [CrossRef]
- Oliver, F.J.; de la Rubia, G.; Feener, E.P.; Lee, M.E.; Loeken, M.R.; Shiba, T.; Quertermous, T.; King, G.L. Stimulation of endothelin-1 gene expression by insulin in endothelial cells. J. Biol. Chem. 1991, 266, 23251–23256. [Google Scholar]
- Eringa, E.C.; Stehouwer, C.D.A.; van Nieuw Amerongen, G.P.; Ouwehand, L.; Westerhof, N.; Sipkema, P. Vasoconstrictor effects of insulin in skeletal muscle arterioles are mediated by ERK1/2 activation in endothelium. Am. J. Physiol. Heart Circl. Physiol. 2004, 287, H2043–H2048. [Google Scholar] [CrossRef] [PubMed]
- Eringa, E.C.; Stehouwer, C.D.A.; Merlijn, T.; Westerhof, N.; Sipkema, P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc. Res. 2002, 56, 464–471. [Google Scholar] [CrossRef]
- Tamminen, M.; Westerbacka, J.; Vehkavaara, S.; Yki-Järvinen, H. Insulin-Induced Decreases in Aortic Wave Reflection and Central Systolic Pressure Are Impaired in Type 2 Diabetes. Diabetes Care 2002, 25, 2314–2319. [Google Scholar] [CrossRef] [PubMed]
- Westerbacka, J.; Vehkavaara, S.; Bergholm, R.; Wilkinson, I.; Cockcroft, J.; Yki-Jarvinen, H. Marked resistance of the ability of insulin to decrease arterial stiffness characterizes human obesity. Diabetes 1999, 48, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Tamminen, M.; Seppala-Lindroos, A.; Yki-Jarvinen, H. Resistance to Acute Insulin Induced Decreases in Large Artery Stiffness Accompanies the Insulin Resistance Syndrome. J. Clin. Endocrinol. Metab. 2001, 86, 5262–5268. [Google Scholar]
- Steinberg, H.O.; Brechtel, G.; Johnson, A.; Fineberg, N.; Baron, A.D. Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J. Clin. Investig. 1994, 94, 1172–1179. [Google Scholar] [CrossRef]
- Steinberg, H.O.; Paradisi, G.; Hook, G.; Crowder, K.; Cronin, J.; Baron, A.D. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes 2000, 49, 1231–1238. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Lin, Y.W.; Clemont, A.; Feener, E.P.; Hein, K.D.; Igarashi, M.; Yamauchi, T.; White, M.F.; King, G.L. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J. Clin. Investig. 1999, 104, 447–457. [Google Scholar] [CrossRef]
- Eringa, E.C.; Stehouwer, C.D.A.; Roos, M.H.; Westerhof, N.; Sipkema, P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1134–E1139. [Google Scholar] [CrossRef]
- Mather, K.J.; Mirzamohammadi, B.; Lteif, A.; Steinberg, H.O.; Baron, A.D. Endothelin contributes to basal vascular tone and endothelial dysfunction in human obesity and type 2 diabetes. Diabetes 2002, 51, 3517–3523. [Google Scholar] [CrossRef]
- Ferrannini, E.; Simonson, D.C.; Katz, L.D.; Reichard, G., Jr.; Bevilacgua, S.; Barrett, E.J.; Olsson, M.; DeFronzo, R.A. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism 1988, 37, 79–85. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Gunnarsson, R.; Bjorkman, O.; Olsson, M.; Wahren, J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin-dependent (type II) diabetes mellitus. J. Clin. Investig. 1985, 76, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Hope, I.D.; Ader, M.; Bergman, R.N. Insulin transport across capillaries is rate limiting for insulin action in dogs. J. Clin. Investig. 1989, 84, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Herkner, H.; Klein, N.; Joukhadar, C.; Lackner, E.; Langenberger, H.; Frossard, M.; Bieglmayer, C.; Wagner, O.; Roden, M.; Müller, M. Transcapillary insulin transfer in human skeletal muscle. Eur. J. Clin. Investig. 2003, 33, 141–146. [Google Scholar] [CrossRef]
- Holmäng, A.; Mimura, K.; Björntorp, P.; Lsönroth, P. Interstitial muscle insulin and glucose levels in normal and insulin-resistant Zucker rats. Diabetes 1997, 46, 1799–1804. [Google Scholar] [CrossRef]
- Castillo, C.; Bogardus, C.; Bergman, R.; Thuillez, P.; Lillioja, S. Interstitial insulin concentrations determine glucose uptake rates but not insulin resistance in lean and obese men. J. Clin. Investig. 1994, 93, 10–16. [Google Scholar] [CrossRef]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Klibanov, A.L.; Clark, M.G.; Rattigan, S.; Barrett, E.J. Microvascular recruitment is an early insulin effect that regulates skeletal muscle glucose uptake in vivo. Diabetes 2004, 53, 1418–1423. [Google Scholar] [CrossRef]
- Vincent, M.A.; Dawson, D.; Clark, A.D.H.; Linder, J.R.; Rattigan, S.; Clark, M.G.; Barrett, E.J. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 2002, 51, 42–48. [Google Scholar] [CrossRef]
- Eggleston, E.M.; Jahn, L.A.; Barrett, E.J. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: Evidence that a saturable process mediates muscle insulin uptake. Diabetes 2007, 56, 2958–2963. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Li, G.; Barrett, E.J. The vascular endothelial cell mediates insulin transport into skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E323–E332. [Google Scholar] [CrossRef]
- Honig, C.R.; Odoroff, C.L.; Frierson, J.L. Active and passive capillary control in red muscle at rest and in exercise. Am. J. Physiol. 1982, 243, H196–H206. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.A.; Barrett, E.J.; Lindner, J.R.; Clark, M.G.; Rattigan, S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E123–E129. [Google Scholar] [CrossRef] [PubMed]
- Inyard, A.C.; Chong, D.G.; Klibanov, A.L.; Barrett, E.J. Muscle contraction, but not insulin, increases microvascular blood volume in the presence of free fatty acid-induced insulin resistance. Diabetes 2009, 58, 2457–2463. [Google Scholar] [CrossRef] [PubMed]
- Inyard, A.C.; Clerk, L.H.; Vincent, M.A.; Barrett, E.J. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 2007, 56, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Jahn, L.A.; Fowler, D.E.; Barrett, E.J. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J. Clin. Endocrinol. Metab. 2009, 94, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.A.; Clerk, L.H.; Lindner, J.R.; Price, W.J.; Jahn, L.A.; Leong-Poi, H.; Barrett, E.J. Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1191–E1197. [Google Scholar] [CrossRef]
- Wang, N.; Ko, S.H.; Chai, W.; Li, G.; Barrett, E.J.; Tao, L.; Cao, W.; Liu, Z. Resveratrol recruits rat muscle microvasculature via a nitric oxide-dependent mechanism that is blocked by TNFa. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E195–E201. [Google Scholar] [CrossRef]
- Fu, Z.; Zhao, L.; Chai, W.; Dong, Z.; Cao, W.; Liu, Z. Ranolazine recruits muscle microvasculature and enhances insulin action in rats. J. Physiol. 2013, 591, 5235–5249. [Google Scholar] [CrossRef]
- Chai, W.; Wang, W.; Dong, Z.; Cao, W.; Liu, Z. Angiotensin II receptors modulate muscle microvascular and metabolic responses to insulin in vivo. Diabetes 2011, 60, 2939–2946. [Google Scholar] [CrossRef]
- Chai, W.; Wang, W.; Liu, J.; Barrett, E.J.; Carey, R.M.; Cao, W.; Liu, Z. Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension 2010, 55, 523–530. [Google Scholar] [CrossRef]
- Wang, N.; Chai, W.; Zhao, L.; Tao, L.; Cao, W.; Liu, Z. Losartan increases muscle insulin delivery and rescues insulin’s metabolic action during lipid infusion via microvascular recruitment. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E538–E545. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Zhao, L.; Aylor, K.W.; Carey, R.M.; Barrett, E.J.; Liu, Z. Angiotensin-(1–7) recruits muscle microvasculature and enhances insulin’s metabolic action via Mas receptor. Hypertension 2014, 63, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chai, W.; Fu, Z.; Dong, Z.; Aylor, K.W.; Barrett, E.J.; Liu, Z. Globular adiponectin enhances muscle insulin action via microvascular recruitment and increased insulin delivery. Circul. Res. 2013, 112, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fu, Z.; Wu, J.; Aylor, K.W.; Barrett, E.J.; Cao, W.; Liu, Z. Globular adiponectin ameliorates metabolic insulin resistance via AMPK-mediated restoration of microvascular insulin responses. J. Physiol. 2015, 593, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Zhang, X.; Barrett, E.J.; Liu, Z. Glucagon-like peptide 1 recruits muscle microvasculature and improves insulin’s metabolic action in the presence of insulin resistance. Diabetes 2014, 63, 2788–2799. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Dong, Z.; Wang, N.; Tao, L.; Cao, W.; Liu, Z. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes 2012, 61, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Yuan, Z.; Wang, N.; Carey, R.M.; Aylor, K.W.; Chen, L.; Zhou, X.; Liu, Z. Direct activation of angiotensin II type 2 receptors enhances muscle microvascular perfusion, oxygenation, and insulin delivery in male rats. Endocrinology 2018, 159, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, A.X.; Liu, Z.; Barrett, E.J. Insulin signaling stimulates insulin transport by bovine aortic endothelial cells. Diabetes 2008, 57, 540–547. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Johnson, S.M. Receptor-mediated transport of insulin across endothelial cells. Science 1985, 227, 1583–1586. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.D.; Levisetti, M.; Reichart, D.; Khoursheed, M.; Moossa, A.R.; Olefsky, J.M. Kinetics of insulin action in vivo. Identification of rate-limiting steps. Diabetes 1995, 44, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.J.; Ludvik, B.; Baloga, J.; Reichart, D.; Olefsky, J.M. Mechanisms of the kinetic defect in insulin action in obesity and NIDDM. Diabetes 1997, 46, 994–1000. [Google Scholar] [CrossRef] [PubMed]
- Clerk, L.H.; Vincent, M.A.; Jahn, L.A.; Liu, Z.; Lindner, J.R.; Barrett, E.J. Obesity blunts insulin-mediated microvascular recruitment in human forearm muscle. Diabetes 2006, 55, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jahn, L.A.; Fowler, D.E.; Barrett, E.J.; Cao, W.; Liu, Z. Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J. Clin. Endocrinol. Metab. 2011, 96, 438–446. [Google Scholar] [CrossRef]
- Chai, W.; Fu, Z.; Aylor, K.W.; Barrett, E.J.; Liu, Z. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E640–E648. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Fu, Z.; Wu, J.; Aylor, K.W.; Barrett, E.J.; Cao, W.; Liu, Z. Inflammation-induced microvascular insulin resistance is an early event in diet-induced obesity. Clin. Sci. 2015, 129, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wheatley, C.M.; Richards, S.M.; Barrett, E.J.; Clark, M.G.; Rattigan, S. TNF-a acutely inhibits vascular effects of physiological but not high insulin or contraction. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E654–E660. [Google Scholar] [CrossRef]
- Wheatley, C.M.; Rattigan, S.; Richards, S.M.; Barrett, E.J.; Clark, M.G. Skeletal muscle contraction stimulates capillary recruitment and glucose uptake in insulin-resistant obese Zucker rats. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E804–E809. [Google Scholar] [CrossRef]
- Coggins, M.; Lindner, J.; Rattigan, S.; Jahn, L.; Fasy, E.; Kaul, S.; Barrett, E.J. Physiologic hyperinsulinemia enhances human skeletal muscle perfusion by capillary recruitment. Diabetes 2001, 50, 2682–2690. [Google Scholar] [CrossRef]
- Clerk, L.H.; Vincent, M.A.; Barrett, E.J.; Lankford, M.F.; Lindner, J.R. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiogensin-converting enzyme inhibition. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1804–E1809. [Google Scholar] [CrossRef]
- Youd, J.M.; Rattigan, S.; Clark, M.G. Acute impairment of insulin-mediated capillary recruitment and glucose uptake in rat skeletal muscle in vivo by TNF-a. Diabetes 2000, 49, 1904–1909. [Google Scholar] [CrossRef]
- Clerk, L.H.; Rattigan, S.; Clark, M.G. Lipid infusion impairs physiologic insulin-mediated capillary recruitment and muscle glucose uptake in vivo. Diabetes 2002, 51, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Peng, I.C.; Sun, W.; Su, M.I.; Hsu, P.H.; Fu, Y.; Zhu, Y.; DeFea, K.; Pan, S.; Tsai, M.D.; et al. AMP-Activated Protein Kinase Functionally Phosphorylates Endothelial Nitric Oxide Synthase Ser633. Circul. Res. 2009, 104, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Mitchelhill, K.I.; Michell, B.J.; Stapleton, D.; Rodriguez-Crespo, I.; Witters, L.A.; Power, D.A.; Ortiz de Montellano, P.R.; Kemp, B.E. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999, 443, 285–289. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, insulin resistance, and the metabolic syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef] [PubMed]
- Fisslthaler, B.; Fleming, I. Activation and Signaling by the AMP-Activated Protein Kinase in Endothelial Cells. Circ Res. 2009, 105, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Investig. 2000, 106, 453–458. [Google Scholar] [CrossRef]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Samuel, V.T.; Petersen, K.F.; Shulman, G.I. Lipid-induced insulin resistance: Unravelling the mechanism. Lancet 2010, 375, 2267–2277. [Google Scholar] [CrossRef]
- de Jongh, R.T.; Serne, E.H.; IJzerman, R.G.; de Vries, G.; Stehouwer, C.D.A. Free fatty acid levels modulate microvascular function: Relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes 2004, 53, 2873–2882. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of insulin action and insulin resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, T.; Church, C.; Baker, D.J.; Jones, S.W. The role of adipokines in skeletal muscle inflammation and insulin sensitivity. J. Inflamm. 2018, 15, 9. [Google Scholar] [CrossRef]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. The, J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol. Rev. 2008, 88, 1379–1406. [Google Scholar] [CrossRef]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef]
- Khan, I.M.; Perrard, X.Y.; Brunner, G.; Liu, H.; Sparks, L.M.; Smith, S.R.; Wang, X.; Shi, Z.Z.; Lewis, D.E.; Wu, H.; et al. Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance. Int. J. Obes. 2015, 39, 1607. [Google Scholar] [CrossRef] [PubMed]
- Olefsky, J.M.; Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Förstermann, U.; Xia, N.; Li, H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circul. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Yin, M.J.; Yamamoto, Y.; Gaynor, R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IkB kinase-b. Nature 1998, 396, 77–80. [Google Scholar] [CrossRef]
- Yuan, M.; Konstantopoulos, N.; Lee, J.; Hansen, L.; Li, Z.W.; Karin, M.; Shoelson, S.E. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkb. Science 2001, 293, 1673–1677. [Google Scholar] [CrossRef]
- Kim, F.; Pham, M.; Maloney, E.; Rizzo, N.O.; Morton, G.J.; Wisse, B.E.; Kirk, E.A.; Chait, A.; Schwartz, M.W. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1982–1988. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.; Liu, J.; Jahn, L.A.; Fowler, D.E.; Barrett, E.J.; Liu, Z. Salsalate attenuates free fatty acid-induced microvascular and metabolic insulin resistance in humans. Diabetes Care 2011, 34, 1634–1638. [Google Scholar] [CrossRef]
- Shiraki, A.; Oyama, J.I.; Komoda, H.; Asaka, M.; Komatsu, A.; Sakuma, M.; Kodama, K.; Sakamoto, Y.; Kotooka, N.; Hirase, T.; et al. The glucagon-like peptide 1 analog liraglutide reduces TNF-α-induced oxidative stress and inflammation in endothelial cells. Atherosclerosis 2012, 221, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Schisano, B.; Harte, A.L.; Lois, K.; Saravanan, P.; Al-Daghri, N.; AI-Attas, O.; Knudsen, L.B.; Mc Ternan, P.G.; Ceriello, A.; Tripathi, G. GLP-1 analogue, Liraglutide protects human umbilical vein endothelial cells against high glucose induced endoplasmic reticulum stress. Regul. Peptides 2012, 174, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, A.X.; Aylor, K.; Barrett, E.J. Caveolin-1 phosphorylation regulates vascular endothelial insulin uptake and is impaired by insulin resistance in rats. Diabetologia 2015, 58, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Matsui-Hirai, H.; Miyazaki-Akita, A.; Fukatsu, A.; Funami, J.; Ding, Q.F.; Kamalanathan, S.; Hattori, Y.; Ignarro, L.J.; Iguchi, A. Endothelial cellular senescence is inhibited by nitric oxide: Implications in atherosclerosis associated with menopause and diabetes. Proc. Natl. Acad. Sci. USA 2006, 103, 17018–17023. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Y.; Awad, E.M.; Oszwald, A.; Mayr, M.; Yin, X.; Waltenberger, B.; Stuppner, H.; Lipovac, M.; Uhrin, P.; Breuss, J.M. Premature senescence of endothelial cells upon chronic exposure to TNFα can be prevented by N-acetyl cysteine and plumericin. Sci. Rep. 2017, 7, 39501. [Google Scholar] [CrossRef]
- Matsui-Hirai, H.; Hayashi, T.; Yamamoto, S.; Ina, K.; Maeda, M.; Kotani, H.; Ignarro, L.J.; Hattori, Y. Dose-dependent modulatory effects of insulin on glucose-induced endothelial senescence in vitro and in vivo: A relationship between telomeres and nitric oxide. J. Pharmacol. Exp. Ther. 2011, 337, 591–599. [Google Scholar] [CrossRef]
- Eringa, E.C.; Bakker, W.; Smulders, Y.M.; Serne, E.H.; Yudkin, J.S.; Stehouwer, C.D.A. Regulation of vascular function and insulin sensitivity by adipose tissue: Focus on perivascular adipose tissue. Microcirculation 2007, 14, 389–402. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Tarun, A.; Antoniades, C. Perivascular adipose tissue as a regulator of vascular disease pathogenesis: Identifying novel therapeutic targets. Br. J. Pharmacol. 2017, 174, 3411–3424. [Google Scholar] [CrossRef]
- Xia, N.; Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017, 174, 3425–3442. [Google Scholar] [CrossRef] [PubMed]
- Virdis, A.; Duranti, E.; Rossi, C.; Dell’Agnello, U.; Santini, E.; Anselmino, M.; Chiarugi, M.; Taddei, S.; Solini, A. Tumour necrosis factor-alpha participates on the endothelin-1/nitric oxide imbalance in small arteries from obese patients: Role of perivascular adipose tissue. Eur. Heart J. 2015, 36, 784–794. [Google Scholar] [CrossRef]
- Meijer, R.I.; Bakker, W.; Alta, C.L.; Sipkema, P.; Yudkin, J.S.; Viollet, B.; Richter, E.A.; Smulders, Y.M.; van Hinsbergh, V.W.; Serné, E.H.; et al. Perivascular Adipose Tissue Control of Insulin-Induced Vasoreactivity in Muscle Is Impaired in db/db Mice. Diabetes 2013, 62, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.I.; Serné, E.H.; Korkmaz, H.I.; van der Peet, D.L.; de Boer, M.P.; Niessen, H.W.; van Hinsbergh, V.W.; Yudkin, J.S.; Smulders, Y.M.; Eringa, E.C. Insulin-induced changes in skeletal muscle microvascular perfusion are dependent upon perivascular adipose tissue in women. Diabetologia 2015, 58, 1907–1915. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, Z. Muscle Insulin Resistance and the Inflamed Microvasculature: Fire from Within. Int. J. Mol. Sci. 2019, 20, 562. https://doi.org/10.3390/ijms20030562
Liu J, Liu Z. Muscle Insulin Resistance and the Inflamed Microvasculature: Fire from Within. International Journal of Molecular Sciences. 2019; 20(3):562. https://doi.org/10.3390/ijms20030562
Chicago/Turabian StyleLiu, Jia, and Zhenqi Liu. 2019. "Muscle Insulin Resistance and the Inflamed Microvasculature: Fire from Within" International Journal of Molecular Sciences 20, no. 3: 562. https://doi.org/10.3390/ijms20030562
APA StyleLiu, J., & Liu, Z. (2019). Muscle Insulin Resistance and the Inflamed Microvasculature: Fire from Within. International Journal of Molecular Sciences, 20(3), 562. https://doi.org/10.3390/ijms20030562




