Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines
Abstract
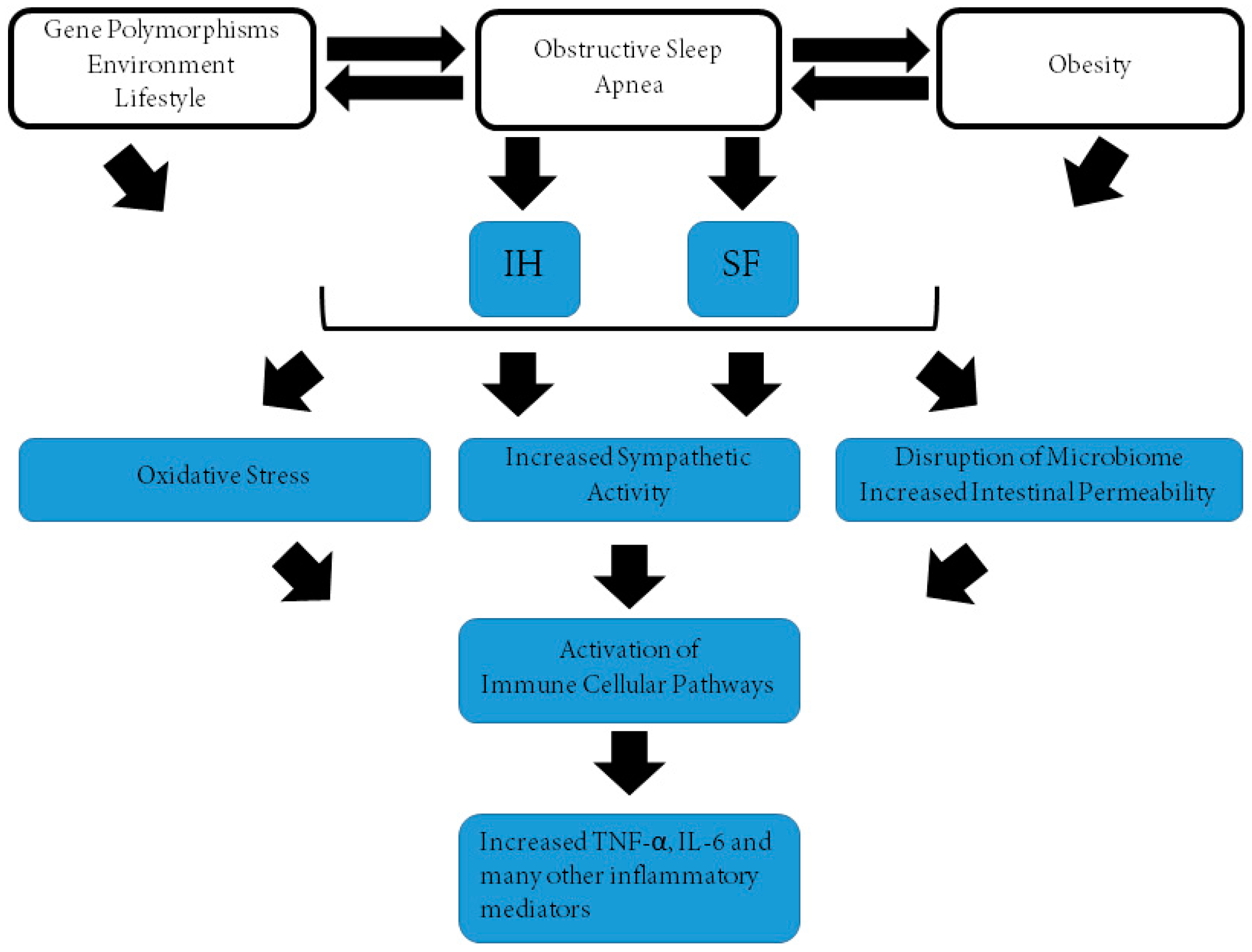
1. Obstructive Sleep Apnea Syndrome (OSAS) and Morbidity
2. Tumor Necrosis Factor-α
3. Interleukin 6
4. Conclusions
Funding
Conflicts of Interest
References
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Owens, R.L.; Malhotra, A.; Eckert, D.J.; White, D.P.; Jordan, A.S. The influence of end-expiratory lung volume on measurements of pharyngeal collapsibility. J. Appl. Physiol. 2010, 108, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Tauman, R.; Gozal, D. Obesity and obstructive sleep apnea in children. Paediatr. Respir. Rev. 2006, 7, 247–259. [Google Scholar] [CrossRef]
- Dayyat, E.; Kheirandish-Gozal, L.; Sans Capdevila, O.; Maarafeya, M.M.A.; Gozal, D. Obstructive sleep apnea in children: Relative contributions of body mass index and adenotonsillar hypertrophy. Chest 2009, 136, 137–144. [Google Scholar] [CrossRef]
- Brenner, R.; Kivity, S.; Peker, M.; Reinhorn, D.; Keinan-Boker, L.; Silverman, B.; Liphsitz, I.; Kolitz, T.; Levy, C.; Shlomi, D.; et al. Increased Risk for Cancer in Young Patients with Severe Obstructive Sleep Apnea. Respiration 2019, 97, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Sillah, A.; Watson, N.F.; Schwartz, S.M.; Gozal, D.; Phipps, A.I. Sleep apnea and subsequent cancer incidence. Cancer Causes Control 2018, 29, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Nagore, E.; Martorell, A.; Rodriguez-Peralto, J.L.; Riveiro-Falkenbach, E.; Hernandez, L.; Bañuls, J.; Arias, E.; Ortiz, P.; et al. Sleep-Disordered Breathing Is Independently Associated With Increased Aggressiveness of Cutaneous Melanoma: A Multicenter Observational Study in 443 Patients. Chest 2018, 154, 1348–1358. [Google Scholar] [CrossRef]
- Malhotra, R.K. Neurodegenerative disorders and sleep. Sleep Med. Clin. 2018, 13, 63–70. [Google Scholar] [CrossRef]
- Haba-Rubio, J.; Marti-Soler, H.; Tobback, N.; Andries, D.; Marques-Vidal, P.; Waeber, G.; Vollenweider, P.; von Gunten, A.; Preisig, M.; Castelao, E.; et al. Sleep characteristics and cognitive impairment in the general population: The HypnoLaus study. Neurology 2017, 88, 463–469. [Google Scholar] [CrossRef]
- Heinzer, R.; Vat, S.; Marques-Vidal, P.; Marti-Soler, H.; Andries, D.; Tobback, N.; Mooser, V.; Preisig, M.; Malhotra, A.; Waeber, G.; et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir. Med. 2015, 3, 310–318. [Google Scholar] [CrossRef]
- Drager, L.F.; McEvoy, R.D.; Barbe, F.; Lorenzi-Filho, G.; Redline, S.; INCOSACT Initiative (International Collaboration of Sleep Apnea Cardiovascular Trialists). Sleep apnea and cardiovascular disease: Lessons from recent trials and need for team science. Circulation 2017, 136, 1840–1850. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; McEvoy, C.T.; Allen, I.E.; Yaffe, K. Association of sleep-disordered breathing with cognitive function and risk of cognitive impairment: A systematic review and meta-analysis. JAMA Neurol. 2017, 74, 1237–1245. [Google Scholar] [CrossRef]
- Abuzaid, A.S.; Al Ashry, H.S.; Elbadawi, A.; Ld, H.; Saad, M.; Elgendy, I.Y.; Elgendy, A.; Mahmoud, A.N.; Mentias, A.; Barakat, A.; et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure therapy in patients with obstructive sleep apnea. Am. J. Cardiol. 2017, 120, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Mokhlesi, B. Obstructive sleep apnea and diabetes: A state of the art review. Chest 2017, 152, 1070–1086. [Google Scholar] [CrossRef]
- Ferini-Strambi, L.; Lombardi, G.E.; Marelli, S.; Galbiati, A. Neurological deficits in obstructive sleep apnea. Curr. Treat Options Neurol. 2017, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Ehsan, Z.; Ishman, S.L.; Kimball, T.R.; Zhang, N.; Zou, Y.; Amin, R.S. Longitudinal cardiovascular outcomes of sleep disordered breathing in children: A meta-analysis and systematic review. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Javaheri, S.; Barbe, F.; Campos-Rodriguez, F.; Dempsey, J.A.; Khayat, R.; Javaheri, S.; Malhotra, A.; Martinez-Garcia, M.A.; Mehra, R.; Pack, A.I.; et al. Sleep apnea: Types, mechanisms, and clinical cardiovascular consequences. J. Am. Coll. Cardiol. 2017, 69, 841–858. [Google Scholar] [CrossRef]
- Patinkin, Z.W.; Feinn, R.; Santos, M. Metabolic consequences of obstructive sleep apnea in adolescents with obesity: A systematic literature review and meta-analysis. Child. Obes. 2017, 13, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Zinchuk, A.V.; Gentry, M.J.; Concato, J.; Yaggi, H.K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med. Rev. 2017, 35, 113–123. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Pediatric OSA syndrome morbidity biomarkers: The hunt is finally on! Chest 2017, 151, 500–506. [Google Scholar] [CrossRef]
- Huon, L.K.; Liu, S.Y.; Camacho, M.; Guilleminault, C. The association between ophthalmologic diseases and obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2016, 20, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Gokdemir, Y.; Ersu, R. Sleep disordered breathing in childhood. Eur. Respir. Rev. 2016, 25, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Dumin, M.; Gozal, D. Role of sleep quality in the metabolic syndrome. Diabetes Metab. Syndr. Obes. 2016, 9, 281–310. [Google Scholar] [PubMed]
- Tan, H.L.; Gozal, D.; Kheirandish-Gozal, L. Obstructive sleep apnea in children: A critical update. Nat. Sci. Sleep 2013, 5, 109–123. [Google Scholar] [PubMed]
- Gozal, D.; Kheirandish-Gozal, L. The multiple challenges of obstructive sleep apnea in children: Morbidity and treatment. Curr. Opin. Pediatr. 2008, 20, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D. Obstructive sleep apnea in children: Implications for the developing central nervous system. Semin. Pediatr. Neurol. 2008, 15, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Marrone, O.; Bonsignore, M.R. Obstructive sleep apnea and chronic kidney disease: Open questions on a potential public health problem. J. Thorac. Dis. 2018, 10, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Hwu, D.W.; Lin, K.D.; Lin, K.C.; Lee, Y.J.; Chang, Y.H. The association of obstructive sleep apnea and renal outcomes-a systematic review and meta-analysis. BMC Nephrol. 2017, 18, 313. [Google Scholar] [CrossRef] [PubMed]
- Finamore, P.; Scarlata, S.; Laudisio, A.; Galdi, F.; Pipita, M.E.; Chiarella, I.; Giua, R.; Cortese, L.; Rivera, C.; Antonelli Incalzi, R. Occurrence of nocturia is not mediated by nocturnal hypoxia length and severity in patients with sleep-disordered breathing. Sleep Med. 2018, 45, 69–73. [Google Scholar] [CrossRef]
- Carra, M.C.; Bruni, O.; Huynh, N. Topical review: Sleep bruxism, headaches, and sleep-disordered breathing in children and adolescents. J. Orofac. Pain 2012, 26, 267–276. [Google Scholar]
- Campos-Juanatey, F.; Fernandez-Barriales, M.; Gonzalez, M.; Portillo-Martin, J.A. Effects of obstructive sleep apnea and its treatment over the erectile function: A systematic review. Asian J. Androl. 2017, 19, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Butler, J.P.; Owens, R.L.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; Smales, E.T.; et al. Identifying obstructive sleep apnea patients responsive to supplemental oxygen therapy. Eur. Respir. J. 2018, 1800674. [Google Scholar] [CrossRef] [PubMed]
- Sands, S.A.; Edwards, B.A.; Terrill, P.I.; Taranto-Montemurro, L.; Azarbarzin, A.; Marques, M.; Hess, L.B.; White, D.P.; Wellman, A. Phenotyping Pharyngeal Pathophysiology using Polysomnography in Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2018, 197, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Kaditis, A.G.; Alonso Alvarez, M.L.; Boudewyns, A.; Alexopoulos, E.I.; Ersu, R.; Joosten, K.; Larramona, H.; Miano, S.; Narang, I.; Trang, H.; et al. Obstructive sleep disordered breathing in 2- to 18-year-old children: Diagnosis and management. Eur. Respir. J. 2016, 47, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Gozal, D. Intermittent hypoxia and cancer: Undesirable bed partners? Respir. Physiol. Neurobiol. 2018, 256, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Farré, R.; Nieto, F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016, 27, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Kim, J.; Kheirandish-Gozal, L.; Gozal, D. Obesity and obstructive sleep apnea syndrome in children: A tale of inflammatory cascades. Pediatr. Pulmonol. 2011, 46, 313–323. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L.; Bhattacharjee, R.; Kim, J. C-reactive protein and obstructive sleep apnea syndrome in children. Front. Biosci. 2012, 4, 2410–2422. [Google Scholar] [CrossRef]
- Gozal, D.; Hakim, F.; Kheirandish-Gozal, L. Chemoreceptors, baroreceptors, and autonomic deregulation in children with obstructive sleep apnea. Respir. Physiol. Neurobiol. 2013, 185, 177–185. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Gozal, D. Genotype-phenotype interactions in pediatric obstructive sleep apnea. Respir. Physiol. Neurobiol. 2013, 189, 338–343. [Google Scholar] [CrossRef]
- Zhang, S.X.; Wang, Y.; Gozal, D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr. Physiol. 2012, 2, 1767–1777. [Google Scholar] [PubMed]
- Almendros, I.; Wang, Y.; Gozal, D. The polymorphic and contradictory aspects of intermittent hypoxia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L129–L140. [Google Scholar] [CrossRef] [PubMed]
- Khalyfa, A.; Kheirandish-Gozal, L.; Khalyfa, A.A.; Philby, M.F.; Alonso-Álvarez, M.L.; Mohammadi, M.; Bhattacharjee, R.; Terán-Santos, J.; Huang, L.; Andrade, J.; et al. Circulating plasma extracellular microvesicle microRNA cargo and endothelial dysfunction in children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2016, 194, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Philby, M.F.; Alonso-Álvarez, M.L.; Terán-Santos, J.; Gozal, D. Biomarkers of Alzheimer disease in children with obstructive sleep apnea: Effect of adenotonsillectomy. Sleep 2016, 39, 1225–1232. [Google Scholar] [CrossRef]
- Alkhouri, N.; Kheirandish-Gozal, L.; Matloob, A.; Alonso-Álvarez, M.L.; Khalyfa, A.; Terán-Santos, J.; Okwu, V.; Lopez, R.; Gileles-Hillel, A.; Dweik, R.; et al. Evaluation of circulating markers of hepatic apoptosis and inflammation in obese children with and without obstructive sleep apnea. Sleep Med. 2015, 16, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Kheirandish-Gozal, L.; Gileles-Hillel, A.; Alonso-Álvarez, M.L.; Peris, E.; Bhattacharjee, R.; Terán-Santos, J.; Duran-Cantolla, J.; Gozal, D. Effects of adenotonsillectomy on plasma inflammatory biomarkers in obese children with obstructive sleep apnea: A community-based study. Int. J. Obes. (Lond.) 2015, 39, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Kheirandish-Gozal, L.; Carreras, A.; Khalyfa, A.; Peris, E. Obstructive sleep apnea and obesity are associated with reduced GPR 120 plasma levels in children. Sleep 2014, 37, 935–941. [Google Scholar] [CrossRef]
- Kim, J.; Gozal, D.; Bhattacharjee, R.; Kheirandish-Gozal, L. TREM-1 and pentraxin-3 plasma levels and their association with obstructive sleep apnea, obesity, and endothelial function in children. Sleep 2013, 36, 923–931. [Google Scholar] [CrossRef]
- Kim, J.; Bhattacharjee, R.; Khalyfa, A.; Kheirandish-Gozal, L.; Capdevila, O.S.; Wang, Y.; Gozal, D. DNA methylation in inflammatory genes among children with obstructive sleep apnea. Am. J. Respir. Crit. Care Med. 2012, 185, 330–338. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Bhattacharjee, R.; Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Leukocyte telomere length and plasma catestatin and myeloid-related protein 8/14 concentrations in children with obstructive sleep apnea. Chest 2010, 138, 91–99. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Circulating exosomes in obstructive sleep apnea as phenotypic biomarkers and mechanistic messengers of end-organ morbidity. Respir. Physiol. Neurobiol. 2018, 256, 143–156. [Google Scholar] [CrossRef] [PubMed]
- De Luca Canto, G.; Pachêco-Pereira, C.; Aydinoz, S.; Major, P.W.; Flores-Mir, C.; Gozal, D. Biomarkers associated with obstructive sleep apnea and morbidities: A scoping review. Sleep Med. 2015, 16, 347–357. [Google Scholar] [CrossRef]
- Cortese, R.; Gileles-Hillel, A.; Khalyfa, A.; Almendros, I.; Akbarpour, M.; Khalyfa, A.A.; Qiao, Z.; Garcia, T.; Andrade, J.; Gozal, D. Aorta macrophage inflammatory and epigenetic changes in a murine model of obstructive sleep apnea: Potential role of CD36. Sci. Rep. 2017, 7, 43648. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Khalyfa, A.; Qiao, Z.; Almendros, I.; Farré, R. Temporal trajectories of novel object recognition performance in mice exposed to intermittent hypoxia. Eur. Respir. J. 2017, 50, 1701456. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, P.; He, Z.; Yang, J.; Liang, C.; Ren, Y.; Wu, Z. Obstructive sleep apnea combined dyslipidemia render additive effect on increasing atherosclerotic cardiovascular diseases prevalence. Lipids Health Dis. 2016, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Sethi, R.; Li, R.; Ho, H.H.; Hein, T.; Jim, M.H.; Loo, G.; Koo, C.Y.; Gao, X.F.; Chandra, S.; et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Mittleman, M.A.; Bravata, D.M.; Concato, J.; Ware, J.; Stoney, C.M.; Redline, S. Reducing cardiovascular risk through treatment of obstructive sleep apnea: Methodological approaches. Am. Heart J. 2016, 172, 135–143. [Google Scholar] [CrossRef]
- Monneret, D.; Tamisier, R.; Ducros, V.; Faure, P.; Halimi, S.; Baguet, J.P.; Lévy, P.; Pépin, J.L.; Borel, A.L. Glucose tolerance and cardiovascular risk biomarkers in non-diabetic non-obese obstructive sleep apnea patients: Effects of long-term continuous positive airway pressure. Respir. Med. 2016, 112, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Bauters, F.; Rietzschel, E.R.; Hertegonne, K.B.; Chirinos, J.A. The link between obstructive sleep apnea and cardiovascular disease. Curr. Atheroscler. Rep. 2016, 18, 1. [Google Scholar] [CrossRef]
- Kostopoulos, K.; Alhanatis, E.; Pampoukas, K.; Georgiopoulos, G.; Zourla, A.; Panoutsopoulos, A.; Kallianos, A.; Velentza, L.; Zarogoulidis, P.; Trakada, G. CPAP therapy induces favorable short-term changes in epicardial fat thickness and vascular and metabolic markers in apparently healthy subjects with obstructive sleep apnea-hypopnea syndrome (OSAHS). Sleep Breath. 2016, 20, 483–493. [Google Scholar] [CrossRef]
- Schwarz, E.I.; Puhan, M.A.; Schlatzer, C.; Stradling, J.R.; Kohler, M. Effect of CPAP therapy on endothelial function in obstructive sleep apnoea: A systematic review and meta-analysis. Respirology 2015, 20, 889–895. [Google Scholar] [CrossRef] [PubMed]
- De Araújo Freitas, I.G.; de Bruin, P.F.; Bittencourt, L.; de Bruin, V.M.; Tufik, S. What can blood biomarkers tell us about cardiovascular risk in obstructive sleep apnea? Sleep Breath. 2015, 19, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Gouveris, H.; Bahr, K.; Jahn, C.; Matthias, C.; Simon, P. The Apnea-Hypopnea Index Underestimates Systemic Inflammation in Women with Sleep-Disordered Breathing. J. Womens Health (Larchmt.) 2018, 27, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Horvath, P.; Tarnoki, D.L.; Tarnoki, A.D.; Karlinger, K.; Lazar, Z.; Losonczy, G.; Kunos, L.; Bikov, A. Complement system activation in obstructive sleep apnea. J. Sleep Res. 2018, 27, e12674. [Google Scholar] [CrossRef] [PubMed]
- Perrini, S.; Cignarelli, A.; Quaranta, V.N.; Falcone, V.A.; Kounaki, S.; Porro, S.; Ciavarella, A.; Ficarella, R.; Barbaro, M.; Genchi, V.A.; et al. Correction of intermittent hypoxia reduces inflammation in obese subjects with obstructive sleep apnea. JCI Insight. 2017, 2, 94379. [Google Scholar] [CrossRef] [PubMed]
- Bouloukaki, I.; Mermigkis, C.; Tzanakis, N.; Kallergis, E.; Moniaki, V.; Mauroudi, E.; Schiza, S.E. Evaluation of Inflammatory Markers in a Large Sample of Obstructive Sleep Apnea Patients without Comorbidities. Mediators Inflamm. 2017, 2017, 4573756. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Lin, H.C.; Lu, C.H.; Chen, P.C.; Huang, C.C.; Chou, K.H.; Su, M.C.; Friedman, M.; Chen, Y.W.; Lin, W.C. Systemic inflammation and alterations to cerebral blood flow in obstructive sleep apnea. J. Sleep Res. 2017, 26, 789–798. [Google Scholar] [CrossRef]
- Zychowski, K.E.; Sanchez, B.; Pedrosa, R.P.; Lorenzi-Filho, G.; Drager, L.F.; Polotsky, V.Y.; Campen, M.J. Serum from obstructive sleep apnea patients induces inflammatory responses in coronary artery endothelial cells. Atherosclerosis 2016, 254, 59–66. [Google Scholar] [CrossRef]
- Vicente, E.; Marin, J.M.; Carrizo, S.J.; Osuna, C.S.; González, R.; Marin-Oto, M.; Forner, M.; Vicente, P.; Cubero, P.; Gil, A.V.; et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur. Respir. J. 2016, 48, 1108–1117. [Google Scholar] [CrossRef]
- DeMartino, T.; Ghoul, R.E.; Wang, L.; Bena, J.; Hazen, S.L.; Tracy, R.; Patel, S.R.; Auckley, D.; Mehra, R. Oxidative Stress and Inflammation Differentially Elevated in Objective Versus Habitual Subjective Reduced Sleep Duration in Obstructive Sleep Apnea. Sleep 2016, 39, 1361–1369. [Google Scholar] [CrossRef]
- Trzepizur, W.; Cortese, R.; Gozal, D. Murine models of sleep apnea: Functional implications of altered macrophage polarity and epigenetic modifications in adipose and vascular tissues. Metabolism 2018, 84, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Churchill, L.; Rector, D.M.; Yasuda, K.; Fix, C.; Rojas, M.J.; Yasuda, T.; Krueger, J.M. Tumor necrosis factor alpha: Activity dependent expression and promotion of cortical column sleep in rats. Neuroscience 2008, 156, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Rockstrom, M.D.; Chen, L.; Taishi, P.; Nguyen, J.T.; Gibbons, C.M.; Veasey, S.C.; Krueger, J.M. Tumor necrosis factor alpha in sleep regulation. Sleep Med. Rev. 2018, 40, 69–78. [Google Scholar] [CrossRef]
- Krueger, J.M.; Opp, M.R. Sleep and Microbes. Int. Rev. Neurobiol. 2016, 131, 207–225. [Google Scholar] [PubMed]
- Jewett, K.A.; Taishi, P.; Sengupta, P.; Roy, S.; Davis, C.J.; Krueger, J.M. Tumor necrosis factor enhances the sleep-like state and electrical stimulation induces a wake-like state in co-cultures of neurons and glia. Eur. J. Neurosci. 2015, 42, 2078–2090. [Google Scholar] [CrossRef]
- Ramesh, V.; Thatte, H.S.; McCarley, R.W.; Basheer, R. Adenosine and sleep deprivation promote NF-kappaB nuclear translocation in cholinergic basal forebrain. J. Neurochem. 2007, 100, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, N.; Ramesh, V.; Gozal, D. TNF-α and temporal changes in sleep architecture in mice exposed to sleep fragmentation. PLoS ONE 2012, 7, e45610. [Google Scholar] [CrossRef]
- Ramesh, V.; Nair, D.; Zhang, S.X.; Hakim, F.; Kaushal, N.; Kayali, F.; Wang, Y.; Li, R.C.; Carreras, A.; Gozal, D. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J. Neuroinflamm. 2012, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Kapur, V.K. Sleep-disordered breathing and excessive daytime sleepiness. Sleep Med. Clin. 2017, 12, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Kheirandish-Gozal, L. Obesity and excessive daytime sleepiness in prepubertal children with obstructive sleep apnea. Pediatrics 2009, 123, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Tauman, R.; O’Brien, L.M.; Holbrook, C.R.; Gozal, D. Sleep pressure score: A new index of sleep disruption in snoring children. Sleep 2004, 27, 274–278. [Google Scholar] [CrossRef]
- Gozal, D.; Wang, M.; Pope, D.W., Jr. Objective sleepiness measures in pediatric obstructive sleep apnea. Pediatrics 2001, 108, 693–697. [Google Scholar] [CrossRef]
- Strohl, K.P. Tumor necrosis factor and sleep apnea. Am. J. Respir. Crit. Care Med. 1996, 153, 893. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Álvarez, M.; Gea, J.; Barreiro, E. Inflammatory Events and Oxidant Production in the Diaphragm, Gastrocnemius, and Blood of Rats Exposed to Chronic Intermittent Hypoxia: Therapeutic Strategies. J. Cell Physiol. 2017, 232, 1165–1175. [Google Scholar] [CrossRef]
- Nácher, M.; Farré, R.; Montserrat, J.M.; Torres, M.; Navajas, D.; Bulbena, O.; Serrano-Mollar, A. Biological consequences of oxygen desaturation and respiratory effort in an acute animal model of obstructive sleep apnea (OSA). Sleep Med. 2009, 10, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Kent, B.D.; Crinion, S.J.; McNicholas, W.T.; Ryan, S. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem. Biophys. Re.s Commun. 2014, 447, 660–665. [Google Scholar] [CrossRef]
- Da Rosa, D.P.; Forgiarini, L.F.; Baronio, D.; Feijó, C.A.; Martinez, D.; Marroni, N.P. Simulating sleep apnea by exposure to intermittent hypoxia induces inflammation in the lung and liver. Mediators Inflamm. 2012, 2012, 879419. [Google Scholar] [CrossRef]
- Song, D.; Fang, G.; Mao, S.Z.; Ye, X.; Liu, G.; Gong, Y.; Liu, S.F. Chronic intermittent hypoxia induces atherosclerosis by NF-κB-dependent mechanisms. Biochim. Biophys. Acta 2012, 1822, 1650–1659. [Google Scholar] [CrossRef]
- Dyugovskaya, L.; Polyakov, A.; Ginsberg, D.; Lavie, P.; Lavie, L. Molecular pathways of spontaneous and TNF-{alpha}-mediated neutrophil apoptosis under intermittent hypoxia. Am. J. Respir. Cell Mol. Biol. 2011, 45, 154–162. [Google Scholar] [CrossRef]
- Ryan, S.; Taylor, C.T.; McNicholas, W.T. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am. J. Respir. Crit. Care Med. 2006, 174, 824–830. [Google Scholar] [CrossRef]
- Poulain, L.; Richard, V.; Lévy, P.; Dematteis, M.; Arnaud, C. Toll-like receptor-4 mediated inflammation is involved in the cardiometabolic alterations induced by intermittent hypoxia. Mediators Inflamm. 2015, 2015, 620258. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L.; Polotsky, V. Cardiovascular aspects in obstructive sleep apnea syndrome—molecular issues, hypoxia and cytokine profiles. Respiration 2009, 78, 361–370. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, K.D.; Lewis, P.; McDonald, F. Sex, stress and sleep apnoea: Decreased susceptibility to upper airway muscle dysfunction following intermittent hypoxia in females. Respir. Physiol. Neurobiol. 2017, 245, 76–82. [Google Scholar] [CrossRef] [PubMed]
- O’Halloran, K.D. Chronic intermittent hypoxia creates the perfect storm with calamitous consequences for respiratory control. Respir. Physiol. Neurobiol. 2016, 226, 63–67. [Google Scholar] [CrossRef] [PubMed]
- McDonald, F.B.; Williams, R.; Sheehan, D.; O’Halloran, K.D. Early life exposure to chronic intermittent hypoxia causes upper airway dilator muscle weakness, which persists into young adulthood. Exp. Physiol. 2015, 100, 947–966. [Google Scholar] [CrossRef] [PubMed]
- Edge, D.; O’Halloran, K.D. Chronic Intermittent Hypoxia Blunts the Expression of Ventilatory Long Term Facilitation in Sleeping Rats. Adv. Exp. Med. Biol. 2015, 860, 335–342. [Google Scholar] [PubMed]
- Edge, D.; McDonald, F.B.; Jones, J.F.; Bradford, A.; O’Halloran, K.D. Effect of chronic intermittent hypoxia on the reflex recruitment of the genioglossus during airway obstruction in the anesthetized rat. Prog. Brain Res. 2014, 209, 147–168. [Google Scholar]
- Shortt, C.M.; Fredsted, A.; Chow, H.B.; Williams, R.; Skelly, J.R.; Edge, D.; Bradford, A.; O’Halloran, K.D. Reactive oxygen species mediated diaphragm fatigue in a rat model of chronic intermittent hypoxia. Exp. Physiol. 2014, 99, 688–700. [Google Scholar] [CrossRef]
- Reid, M.B.; Lannergren, J.; Westerblad, H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: Involvement of muscle myofilaments. Am. J. Respir. Crit. Care Med. 2002, 166, 479–484. [Google Scholar] [CrossRef]
- Wilcox, P.; Milliken, C.; Bressler, B. High-dose tumor necrosis factor alpha produces an impairment of hamster diaphragm contractility. Attenuation with a prostaglandin inhibitor. Am. J. Respir. Crit. Care Med. 1996, 153, 1611–1615. [Google Scholar] [CrossRef]
- Li, X.; Moody, M.R.; Engel, D.; Walker, S.; Clubb, F.J., Jr.; Sivasubramanian, N.; Mann, D.L.; Reid, M.B. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 2000, 102, 1690–1696. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M. The role of cytokines in sleep regulation. Curr. Pharm. Des. 2008, 14, 3408–3416. [Google Scholar] [CrossRef] [PubMed]
- Terao, A.; Matsumura, H.; Yoneda, H.; Saito, M. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport 1998, 9, 3791–3796. [Google Scholar] [CrossRef]
- Shoham, S.; Davenne, D.; Cady, A.B.; Dinarello, C.A.; Krueger, J.M. Recombinant tumor necrosis factor and interleukin 1 enhance slow-wave sleep. Am. J. Physiol. 1987, 253, R142–R149. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Pan, L.; Ren, D.; Du, C.; Guo, Y. Effects of continuous positive airway pressure therapy on systemic inflammation in obstructive sleep apnea: A meta-analysis. Sleep Med. 2013, 14, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Riha, R.L.; Brander, P.; Vennelle, M.; McArdle, N.; Kerr, S.M.; Anderson, N.H.; Douglas, N.J. Tumour necrosis factor-alpha (-308) gene polymorphism in obstructive sleep apnoea-hypopnoea syndrome. Eur. Respir. J. 2005, 26, 673–678. [Google Scholar] [CrossRef]
- Bhushan, B.; Guleria, R.; Misra, A.; Luthra, K.; Vikram, N.K. TNF-alpha gene polymorphism and TNF-alpha levels in obese Asian Indians with obstructive sleep apnea. Respir. Med. 2009, 103, 386–392. [Google Scholar] [CrossRef]
- Popko, K.; Gorska, E.; Potapinska, O.; Wasik, M.; Stoklosa, A.; Plywaczewski, R.; Winiarska, M.; Gorecka, D.; Sliwinski, P.; Popko, M.; et al. Frequency of distribution of inflammatory cytokines IL-1, IL-6 and TNF-alpha gene polymorphism in patients with obstructive sleep apnea. J. Physiol. Pharmacol. 2008, 59 (Suppl. 6), 607–614. [Google Scholar]
- Khalyfa, A.; Serpero, L.D.; Kheirandish-Gozal, L.; Capdevila, O.S.; Gozal, D. TNF-α gene polymorphisms and excessive daytime sleepiness in pediatric obstructive sleep apnea. J. Pediatr. 2011, 158, 77–82. [Google Scholar] [CrossRef]
- Lan, F.; Cao, C.; Liu, J.; Li, W. Obstructive sleep apnea syndrome susceptible genes in the Chinese population: A meta-analysis of 21 case-control studies. Sleep Breath. 2015, 19, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Almpanidou, P.; Hadjigeorgiou, G.; Gourgoulianis, K.; Papadimitriou, A. Association of tumor necrosis factor-α gene polymorphism (-308) and obstructive sleep apnea-hypopnea syndrome. Hippokratia 2012, 16, 217–220. [Google Scholar] [PubMed]
- Vgontzas, A.N.; Zoumakis, E.; Lin, H.M.; Bixler, E.O.; Trakada, G.; Chrousos, G.P. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J. Clin. Endocrinol. Metab. 2004, 89, 4409–4413. [Google Scholar] [CrossRef] [PubMed]
- Bozic, J.; Borovac, J.A.; Galic, T.; Kurir, T.T.; Supe-Domic, D.; Dogas, Z. Adropin and Inflammation Biomarker Levels in Male Patients with Obstructive Sleep Apnea: A Link with Glucose Metabolism and Sleep Parameters. J. Clin. Sleep Med. 2018, 14, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.Q.; Xue, J.S.; Xu, J.; Zhou, Z.X.; Ji, Y.L. Efficacy of continuous positive airway pressure treatment in treating obstructive sleep apnea hypopnea syndrome associated with carotid arteriosclerosis. Exp. Ther. Med. 2017, 14, 6176–6182. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Julián, E.; Pérez-Carbonell, T.; Marco, R.; Pellicer, V.; Rodriguez-Borja, E.; Marco, J. Impact of an oral appliance on obstructive sleep apnea severity, quality of life, and biomarkers. Laryngoscope 2017. [Google Scholar] [CrossRef]
- Jin, F.; Liu, J.; Zhang, X.; Cai, W.; Zhang, Y.; Zhang, W.; Yang, J.; Lu, G.; Zhang, X. Effect of continuous positive airway pressure therapy on inflammatory cytokines and atherosclerosis in patients with obstructive sleep apnea syndrome. Mol. Med. Rep. 2017, 16, 6334–6339. [Google Scholar] [CrossRef]
- Mutlu, M.; Vuralkan, E.; Akin, I.; Firat, H.; Ardic, S.; Akaydin, S.; Miser, E. Alteration of serum levels of inflammatory cytokines and polysomnographic indices after uvulopalatal flap surgery in obstructive sleep apnea. Ear Nose Throat J. 2017, 96, 65–68. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, X. Tumor necrosis factor alpha is a promising circulating biomarker for the development of obstructive sleep apnea syndrome: A meta-analysis. Oncotarget 2017, 8, 27616–27626. [Google Scholar] [CrossRef]
- Hirotsu, C.; Albuquerque, R.G.; Nogueira, H.; Hachul, H.; Bittencourt, L.; Tufik, S.; Andersen, M.L. The relationship between sleep apnea, metabolic dysfunction and inflammation: The gender influence. Brain Behav. Immun. 2017, 59, 211–218. [Google Scholar] [CrossRef]
- De Santis, S.; Cambi, J.; Tatti, P.; Bellussi, L.; Passali, D. Changes in ghrelin, leptin and pro-inflammatory cytokines after therapy in Obstructive Sleep Apnea Syndrome (OSAS) patients. Otolaryngol. Pol. 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Leon-Cabrera, S.; Arana-Lechuga, Y.; Esqueda-León, E.; Terán-Pérez, G.; Gonzalez-Chavez, A.; Escobedo, G.; Velázquez Moctezuma, J. Reduced systemic levels of IL-10 are associated with the severity of obstructive sleep apnea and insulin resistance in morbidly obese humans. Mediators Inflamm. 2015, 2015, 493409. [Google Scholar] [CrossRef]
- Ciccone, M.M.; Scicchitano, P.; Zito, A.; Cortese, F.; Boninfante, B.; Falcone, V.A.; Quaranta, V.N.; Ventura, V.A.; Zucano, A.; Di Serio, F.; et al. Correlation between inflammatory markers of atherosclerosis and carotid intima-media thickness in Obstructive Sleep Apnea. Molecules 2014, 19, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Yamauchi, M.; Fujita, Y.; Koyama, N.; Fukuoka, A.; Tamaki, S.; Yamamoto, Y.; Tomoda, K.; Kimura, H. The impact of obstructive sleep apnea and nasal CPAP on circulating adiponectin levels. Lung 2014, 192, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Matos, G.; Hirotsu, C.; Alvarenga, T.A.; Cintra, F.; Bittencourt, L.; Tufik, S.; Andersen, M.L. The association between TNF-α and erectile dysfunction complaints. Andrology 2013, 1, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Oyama, J.; Yamamoto, H.; Maeda, T.; Ito, A.; Node, K.; Makino, N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin. Cardiol. 2012, 35, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Svensson, M.; Venge, P.; Janson, C.; Lindberg, E. Relationship between sleep- disordered breathing and markers of systemic inflammation in women from the general population. J. Sleep Res. 2012, 21, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hegglin, A.; Schoch, O.D.; Korte, W.; Hahn, K.; Hürny, C.; Münzer, T. Eight months of continuous positive airway pressure (CPAP) decrease tumor necrosis factor alpha (TNFA) in men with obstructive sleep apnea syndrome. Sleep Breath. 2012, 16, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Eun, Y.G.; Kim, M.G.; Kwon, K.H.; Shin, S.Y.; Cho, J.S.; Kim, S.W. Short-term effect of multilevel surgery on adipokines and pro-inflammatory cytokines in patients with obstructive sleep apnea. Acta Otolaryngol. 2010, 130, 1394–1398. [Google Scholar] [CrossRef] [PubMed]
- Li, N.F.; Yao, X.G.; Zhu, J.; Yang, J.; Liu, K.J.; Wang, Y.C.; Wang, X.L.; Zu, F.Y. Higher levels of plasma TNF-alpha and neuropeptide Y in hypertensive patients with obstructive sleep apnea syndrome. Clin. Exp. Hypertens. 2010, 32, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sahlman, J.; Miettinen, K.; Peuhkurinen, K.; Seppä, J.; Peltonen, M.; Herder, C.; Punnonen, K.; Vanninen, E.; Gylling, H.; Partinen, M.; et al. The activation of the inflammatory cytokines in overweight patients with mild obstructive sleep apnoea. J. Sleep Res. 2010, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, S.; Yamauchi, M.; Fukuoka, A.; Makinodan, K.; Koyama, N.; Tomoda, K.; Yoshikawa, M.; Kimura, H. Production of inflammatory mediators by monocytes in patients with obstructive sleep apnea syndrome. Intern. Med. 2009, 48, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Constantinidis, J.; Ereliadis, S.; Angouridakis, N.; Konstantinidis, I.; Vital, V.; Angouridaki, C. Cytokine changes after surgical treatment of obstructive sleep apnoea syndrome. Eur. Arch. Otorhinolaryngol. 2008, 265, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Minoguchi, K.; Tazaki, T.; Yokoe, T.; Minoguchi, H.; Watanabe, Y.; Yamamoto, M.; Adachi, M. Elevated production of tumor necrosis factor-alpha by monocytes in patients with obstructive sleep apnea syndrome. Chest 2004, 126, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Steiropoulos, P.; Kotsianidis, I.; Nena, E.; Tsara, V.; Gounari, E.; Hatzizisi, O.; Kyriazis, G.; Christaki, P.; Froudarakis, M.; Bouros, D. Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep 2009, 32, 537–543. [Google Scholar] [CrossRef]
- Dorkova, Z.; Petrasova, D.; Molcanyiova, A.; Popovnakova, M.; Tkacova, R. Effects of continuous positive airway pressure on cardiovascular risk profile in patients with severe obstructive sleep apnea and metabolic syndrome. Chest 2008, 134, 686–692. [Google Scholar] [CrossRef]
- Kanbay, A.; Kokturk, O.; Ciftci, T.U.; Tavil, Y.; Bukan, N. Comparison of serum adiponectin and tumor necrosis factor-alpha levels between patients with and without obstructive sleep apnea syndrome. Respiration 2008, 76, 324–330. [Google Scholar] [CrossRef]
- Bravo Mde, L.; Serpero, L.D.; Barceló, A.; Barbé, F.; Agustí, A.; Gozal, D. Inflammatory proteins in patients with obstructive sleep apnea with and without daytime sleepiness. Sleep Breath. 2007, 11, 177–185. [Google Scholar] [CrossRef]
- Kataoka, T.; Enomoto, F.; Kim, R.; Yokoi, H.; Fujimori, M.; Sakai, Y.; Ando, I.; Ichikawa, G.; Ikeda, K. The effect of surgical treatment of obstructive sleep apnea syndrome on the plasma TNF-alpha levels. Tohoku J. Exp. Med. 2004, 204, 267–272. [Google Scholar] [CrossRef]
- Ciftci, T.U.; Kokturk, O.; Bukan, N.; Bilgihan, A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine 2004, 28, 87–91. [Google Scholar] [CrossRef]
- Alberti, A.; Sarchielli, P.; Gallinella, E.; Floridi, A.; Floridi, A.; Mazzotta, G.; Gallai, V. Plasma cytokine levels in patients with obstructive sleep apnea syndrome: A preliminary study. J. Sleep Res. 2003, 12, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Heizati, M.; Li, N.; Shao, L.; Yao, X.; Wang, Y.; Hong, J.; Zhou, L.; Zhang, D.; Chang, G.; Abulikemu, S. Does increased serum d-lactate mean subclinical hyperpermeability of intestinal barrier in middle-aged nonobese males with OSA? Medicine (Baltimore) 2017, 96, e9144. [Google Scholar] [CrossRef] [PubMed]
- Thunström, E.; Glantz, H.; Yucel-Lindberg, T.; Lindberg, K.; Saygin, M.; Peker, Y. CPAP does not reduce inflammatory biomarkers in patients with coronary artery disease and nonsleepy obstructive sleep apnea: A randomized controlled trial. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Binar, M.; Akcam, T.M.; Karakoc, O.; Sagkan, R.I.; Musabak, U.; Gerek, M. Effect of modern surgical treatment on the inflammatory/anti-inflammatory balance in patients withobstructive sleep apnoea. J. Laryngol. Otol. 2017, 131, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Stradling, J.R.; Craig, S.E.; Kohler, M.; Nicoll, D.; Ayers, L.; Nunn, A.J.; Bratton, D.J. Markers of inflammation: Data from the MOSAIC randomised trial of CPAP for minimally symptomatic OSA. Thorax 2015, 70, 181–182. [Google Scholar] [CrossRef] [PubMed]
- Salord, N.; Gasa, M.; Mayos, M.; Fortuna-Gutierrez, A.M.; Montserrat, J.M.; Sánchez-de-la-Torre, M.; Barceló, A.; Barbé, F.; Vilarrasa, N.; Monasterio, C. Impact of OSA on biological markers in morbid obesity and metabolic syndrome. J. Clin. Sleep Med. 2014, 10, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Karamanlı, H.; Özol, D.; Ugur, K.S.; Yıldırım, Z.; Armutçu, F.; Bozkurt, B.; Yigitoglu, R. Influence of CPAP treatment on airway and systemic inflammation in OSAS patients. Sleep Breath. 2014, 18, 251–256. [Google Scholar] [CrossRef]
- Aihara, K.; Oga, T.; Chihara, Y.; Harada, Y.; Tanizawa, K.; Handa, T.; Hitomi, T.; Uno, K.; Mishima, M.; Chin, K. Analysis of systemic and airway inflammation in obstructive sleep apnea. Sleep Breath. 2013, 17, 597–604. [Google Scholar] [CrossRef]
- Imagawa, S.; Yamaguchi, Y.; Ogawa, K.; Obara, N.; Suzuki, N.; Yamamoto, M.; Nagasawa, T. Interleukin-6 and tumor necrosis factor-alpha in patients with obstructive sleep apnea-hypopnea syndrome. Respiration 2004, 71, 24–29. [Google Scholar] [CrossRef]
- Qian, X.; Yin, T.; Li, T.; Kang, C.; Guo, R.; Sun, B.; Liu, C. High levels of inflammation and insulin resistance in obstructive sleep apnea patients with hypertension. Inflammation 2012, 35, 1507–1511. [Google Scholar] [CrossRef]
- Kontos, A.; Willoughby, S.; van den Heuvel, C.; Kennedy, D.; Martin, J.; Hodge, G.; Worthley, M.; Chin, A.K.; Nelson, A.; Teo, K.; et al. Ascending aortic blood flow velocity is increased in children with primary snoring/mild sleep-disordered breathing and associated with an increase in CD8 (+) T cells expressing TNFα and IFNγ. Heart Vessels 2017. [Google Scholar] [CrossRef]
- Mutlu, M.; Vuralkan, E.; Yardim Akaydin, S.; Akin, I.; Miser, E. Effects of adenoid/tonsillectomy on inflammatory response in snoring children with witnessed apnoea. Clin. Otolaryngol. 2014, 39, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Serpero, L.D.; Kheirandish-Gozal, L.; Capdevila, O.S.; Khalyfa, A.; Tauman, R. Sleep measures and morning plasma TNF-alpha levels in children with sleep-disordered breathing. Sleep 2010, 33, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Van Eyck, A.; Van Hoorenbeeck, K.; De Winter, B.Y.; Van Gaal, L.; De Backer, W.; Verhulst, S.L. Sleep-disordered breathing, systemic adipokine secretion, and metabolic dysregulation in overweight and obese children and adolescents. Sleep Med. 2017, 30, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Hossain, M.M.; Hura, A.; Huang, G.; McConnell, K.; Ishman, S.L.; Amin, R.S. Inflammatory milieu and cardiovascular homeostasis in children with obstructive sleep apnea. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Calhoun, S.L.; He, F.; Liao, D.; Sawyer, M.D.; Bixler, E.O. Inflammation mediates the association between visceral adiposity and obstructive sleep apnea in adolescents. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E851–E858. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, E.I.; Theologi, V.; Malakasioti, G.; Maragozidis, P.; Tsilioni, I.; Chrousos, G.; Gourgoulianis, K.; Kaditis, A.G. Obstructive sleep apnea, excessive daytime sleepiness, and morning plasma TNF-α levels in Greek children. Sleep 2013, 36, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Li, Q. The evaluation of adenotonsillectomy on TNF-α and IL-6 levels in obese children with obstructive sleep apnea. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 690–694. [Google Scholar] [CrossRef]
- Baessler, A.; Nadeem, R.; Harvey, M.; Madbouly, E.; Younus, A.; Sajid, H.; Naseem, J.; Asif, A.; Bawaadam, H. Treatment for sleep apnea by continuous positive airway pressure improves levels of inflammatory markers—A meta-analysis. J. Inflamm. (Lond.) 2013, 10, 13. [Google Scholar] [CrossRef]
- Li, A.M.; Lam, H.S.; Chan, M.H.; So, H.K.; Ng, S.K.; Chan, I.H.; Lam, C.W.; Wing, Y.K.V. Inflammatory cytokines and childhood obstructive sleep apnoea. Ann. Acad. Med. Singapore 2008, 37, 649–654. [Google Scholar]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and its receptors: A highly regulated and dynamic system. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Gileles-Hillel, A.; Cortese, R.; Li, Y.; Almendros, I.; Qiao, Z.; Khalyfa, A.A.; Andrade, J.; Khalyfa, A. Visceral white adipose tissue after chronic intermittent and sustained hypoxia in mice. Am. J. Respir. Cell Mol. Biol. 2017, 56, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Poroyko, V.A.; Carreras, A.; Khalyfa, A.; Khalyfa, A.A.; Leone, V.; Peris, E.; Almendros, I.; Gileles-Hillel, A.; Qiao, Z.; Hubert, N.; et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 2016, 6, 35405. [Google Scholar] [CrossRef] [PubMed]
- Gileles-Hillel, A.; Kheirandish-Gozal, L.; Gozal, D. Biological plausibility linking sleep apnoea and metabolic dysfunction. Nat. Rev. Endocrinol. 2016, 12, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.X.; Khalyfa, A.; Wang, Y.; Carreras, A.; Hakim, F.; Neel, B.A.; Brady, M.J.; Qiao, Z.; Hirotsu, C.; Gozal, D. Sleep fragmentation promotes NADPH oxidase 2-mediated adipose tissue inflammation leading to insulin resistance in mice. Int. J. Obes. (Lond.) 2014, 38, 619–624. [Google Scholar] [CrossRef]
- Gharib, S.A.; Khalyfa, A.; Abdelkarim, A.; Bhushan, B.; Gozal, D. Integrative miRNA-mRNA profiling of adipose tissue unravels transcriptional circuits induced by sleep fragmentation. PLoS ONE 2012, 7, e37669. [Google Scholar] [CrossRef] [PubMed]
- Gharib, S.A.; Khalyfa, A.; Abdelkarim, A.; Ramesh, V.; Buazza, M.; Kaushal, N.; Bhushan, B.; Gozal, D. Intermittent hypoxia activates temporally coordinated transcriptional programs in visceral adipose tissue. J. Mol. Med. (Berl.) 2012, 90, 435–445. [Google Scholar] [CrossRef]
- Lee, M.Y.; Wang, Y.; Mak, J.C.; Ip, M.S. Intermittent hypoxia induces NF-κB-dependent endothelial activation via adipocyte-derived mediators. Am. J. Physiol. Cell Physiol. 2016, 310, C446–C455. [Google Scholar] [CrossRef]
- Lee, W.Y.; Allison, M.A.; Kim, D.J.; Song, C.H.; Barrett-Connor, E. Association of interleukin-6 and C-reactive protein with subclinical carotid atherosclerosis (the Rancho Bernardo Study). Am. J. Cardiol. 2007, 99, 99–102. [Google Scholar] [CrossRef]
- Esteve, E.; Castro, A.; López-Bermejo, A.; Vendrell, J.; Ricart, W.; Fernández-Real, J.M. Serum interleukin-6 correlates with endothelial dysfunction in healthy men independently of insulin sensitivity. Diabetes Care 2007, 30, 939–945. [Google Scholar] [CrossRef]
- Schaefer, E.; Wu, W.; Mark, C.; Yang, A.; DiGiacomo, E.; Carlton-Smith, C.; Salloum, S.; Brisac, C.; Lin, W.; Corey, K.E.; et al. Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol. Commun. 2017, 1, 326–337. [Google Scholar] [CrossRef]
- Li, Y.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Kritikou, I.; Basta, M.; Pejovic, S.; Gaines, J.; Bixler, E.O. Objective, but not subjective, sleepiness is associated with inflammation in sleep apnea. Sleep 2017, 40. [Google Scholar] [CrossRef]
- Huang, Y.S.; Guilleminault, C.; Hwang, F.M.; Cheng, C.; Lin, C.H.; Li, H.Y.; Lee, L.A. Inflammatory cytokines in pediatric obstructive sleep apnea. Medicine (Baltimore) 2016, 95, e4944. [Google Scholar] [CrossRef]
- Kaditis, A.G.; Gozal, D.; Khalyfa, A.; Kheirandish-Gozal, L.; Capdevila, O.S.; Gourgoulianis, K.; Alexopoulos, E.I.; Chaidas, K.; Bhattacharjee, R.; Kim, J.; et al. Variants in C-reactive protein and IL-6 genes and susceptibility to obstructive sleep apnea in children: A candidate-gene association study in European American and Southeast European populations. Sleep Med. 2014, 15, 228–235. [Google Scholar] [CrossRef]
- Zhong, A.; Xiong, X.; Shi, M.; Xu, H. Roles of interleukin (IL)-6 gene polymorphisms, serum IL-6 levels, and treatment in obstructive sleep apnea: A meta-analysis. Sleep Breath. 2016, 20, 719–731. [Google Scholar] [CrossRef]
- Kong, D.; Qin, Z.; Wang, W.; Kang, J. Effect of obstructive sleep apnea on carotid artery intima media thickness related to inflammation. Clin. Investig. Med. 2017, 40, E25–E33. [Google Scholar] [CrossRef]
| Reference (First Author, Year) | Number of Subjects | Association With | TNF-α Levels Associated With | Effect of Treatment (Tx) | Comments | TNF-α Levels Are Increased in OSA Yes/No/Equivocal |
|---|---|---|---|---|---|---|
| ADULTS | ||||||
| [114] | 50 OSA and 50 controls | OSAS severity Insulin resistance | No Tx | YES | ||
| [115] | 120 OSA; 40 controls | Carotid atherosclerosis | OSA severity cIMT | CPAP reduced TNF-α levels | YES | |
| [116] | 30 OSA 10 controls | MAD reduced TNF-α levels | YES | |||
| [117] | 100 OSA 50 controls | Atherosclerosis cIMT; pulse wave velocity | No | CPAP for 3 months reduced TNF-α levels | YES | |
| [118] | 25 OSA undergoing uvulopalatal flap (UPF) surgery | No | UPF surgery reduced TNF-α levels | YES | ||
| [119] | Meta-analysis of 2857 OSA and 2115 controls | OSA severity | Yes; in mild, mild-to-moderate, moderate, moderate-to-severe, and severe OSAS, circulating TNF-alpha was higher than controls by 0.99, 1.48. 7.79, 10.08, and 8.85 pg/mL, with significant heterogeneity (I2: 91.2%, 74.5%, 97.6%, 99.0% and 98.1%) | No Tx | YES | |
| [120] | 1042 subjects from community | OSA severity Metabolic syndrome | Positive association in women and negative in men | No Tx | YES | |
| [121] | 20 obese OSA 6 non-obese OSA | Reduced cytokines after 6 months CPAP or surgery | YES | |||
| [122] | 52 subjects (10 controls, 42 obese OSA) | Insulin resistance | Higher TNF-α | No Tx | Inverse relationship between IL-10, but not TNF-α and insulin resistance | YES |
| [66] | 31 OSA and erectile dysfunction (ED) 15 OSA and no ED | Higher TNF-α plasma levels when ED present | YES | |||
| [123] | 80 OSA 40 controls | cIMT | Higher TNF-α plasma levels associated with higher cIMT | No Tx | YES | |
| [124] | 22 OSA | Association with apnea-hypopnea index | Higher TNF-α plasma levels | CPAP for 3 months reduced TNF-α plasma levels | YES | |
| [125] | 363 men | ED | Higher TNF-α plasma levels when ED and OSA present | No Tx | YES | |
| [126] | 32 OSA and metabolic syndrome | Endothelial function | CPAP for 3 months reduced TNF-α plasma levels | YES | ||
| [127] | 230 habitually snoring women and 170 controls | AHI ODI3% | Significant association between TNF-α levels and ODI3% | YES | ||
| [128] | 66 OSA | CPAP 8 months reduced TNF-α plasma levels in men but not in women | YES | |||
| [129] | 51 OSA | EDS | Upper airway surgery with 4-week follow-up showed significant reductions in TNF-α plasma levels and EDS | YES | ||
| [130] | OSA (n = 113) Hypertensive without OSA (n = 73) Hypertensive with OSA (n = 134) Controls (n = 97) | OSA patients have higher TNF-α levels | No Tx arm | YES | ||
| [131] | 84 mild OSA 40 controls | OSA patients have higher TNF-α levels | No Tx arm | YES | ||
| Monocyte production of TNF-α levels | Circulating monocytes in OSA patients have higher TNF-α levels | No Tx arm | YES | |||
| [132] | 33 OSA 13 controls | |||||
| [133] | 24 OSA 12 non-obese and 15 obese controls | Surgery decreased monocyte TNF-α production | ||||
| [134] | 24 OSA 27 controls | CPAP for 1 month decreased monocyte production of TNF-α | ||||
| [135] | 52 OSA | CPAP for 6 months (n = 32 with good adherence and 20 non-adherent). Good adherence reduced TNF-α plasma levels | YES | |||
| [136] | 32 severe OSA and metabolic syndrome | CPAP adherence for 8 weeks (n = 16) reduced TNF-α plasma levels but no changes if non-adherent (<4 h/night) | YES | |||
| [137] | 106 OSA 32 controls | OSA patients, particularly if concurrent obesity, have higher TNF-α levels | No Tx arm | YES | ||
| [138] | 50 OSA 20 controls | EDS | OSA have higher TNF-α levels unrelated to EDS | No Tx arm | YES | |
| [139] | 27 OSA 11 controls | Higher TNF-α levels in OSA | No Tx arm | YES | ||
| [140] | 43 OSA 22 controls | BMI | OSA have higher TNF-α levels unrelated to BMI | No Tx arm | YES | |
| [141] | 18 OSA 20 controls | OSA have higher TNF-α levels | No Tx arm | YES | ||
| [142] | 159 OSA and no-OSA, obese and non-obese | Serum d-lactate Intestinal permeability | No significant associations | No Tx arm | NO | |
| [143] | 220 non-sleepy OSA | Coronary artery disease | No | Randomization to CPAP or no CPAP for 1 year had no effect on TNF-α levels | NO | |
| [144] | 28 OSA on CPAP 29 OSA undergoing upper airway surgery | No | No effects of either Tx on TNF-α levels | NO | ||
| [145] | 391 OSA | No differences before and after treatment | CPAP for 6 months | NO | ||
| [146] | 52 OSA and no-OSA obese | Metabolic syndrome | No differences in OSA with or without metabolic syndrome | No Tx arm | NO | |
| [147] | 35 OSA | CPAP for 3 months – no changes in TNF-α plasma levels | NO | |||
| [148] | 43 OSA | Serum and induced sputum | Sputum TNF-α levels, but not serum levels, correlated with OSA severity | No Tx | NO | |
| [149] | 110 OSA 45 controls | No differences in TNF-α levels | No Tx arm | NO | ||
| [69] | 89 OSA; 28 snorers; 26 controls | Pharyngeal lavage and plasma | Higher cytokines including TNF-α in pharyngeal lavage but not in plasma | 1-year follow up CPAP—improvements in TNF-α in pharyngeal lavage | Equivocal | |
| [150] | 70 severe OSA | Hypertension | Higher TNF-α plasma levels associated with hypertension | No Tx arm | Equivocal | |
| CHILDREN | ||||||
| [151] | 19 children | Cardiac magnetic resonance imaging (aortic blood flow velocity and left and right ventricular systolic function) | - | No Tx arm | Intra-cellular TNF-α in CD8+T cells | YES |
| [152] | 35 children OSA | None | None | T&A reduced TNF-α at 6 months follow up | YES | |
| [153] | 298 snoring children | EDS | TNF-α significantly higher with more severe OSA and when EDS present | T&A and 3 months follow-up showed significant reductions in TNF-α | YES | |
| [154] | 164 overweight and obese children (111 controls, 28 mild OSA, 25 moderate-to-severe OSA) | OSA severity | None | No Tx arm | NO | |
| [155] | 90 controls 65 OSA | Pulse transit time (PTT) | Shorter PTT | No Tx arm | NO | |
| [156] | 392 adolescents with no OSA, mild, moderate and severe OSA | Visceral adipose tissue | None | No Tx arm | NO | |
| [157] | 24 moderate to severe OSA 22 mild OSA 22 controls | EDS | No differences in TNF-α across 3 groups; no association with EDS | No Tx arm | NO | |
| [158] | 90 obese children with OSA | T&A and 6-month follow-up showed no changes in TNF-α or IL-6 | NO | |||
| [159] | 47 non-obese OSA 32 controls | Cognitive function | Association with general cognitive function | No Tx arm | Equivocal | |
| [160] | 142 snoring children | TNF-α not higher in OSA but IL-6 and IL-8 elevated | No Tx arm | Equivocal | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheirandish-Gozal, L.; Gozal, D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. Int. J. Mol. Sci. 2019, 20, 459. https://doi.org/10.3390/ijms20030459
Kheirandish-Gozal L, Gozal D. Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. International Journal of Molecular Sciences. 2019; 20(3):459. https://doi.org/10.3390/ijms20030459
Chicago/Turabian StyleKheirandish-Gozal, Leila, and David Gozal. 2019. "Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines" International Journal of Molecular Sciences 20, no. 3: 459. https://doi.org/10.3390/ijms20030459
APA StyleKheirandish-Gozal, L., & Gozal, D. (2019). Obstructive Sleep Apnea and Inflammation: Proof of Concept Based on Two Illustrative Cytokines. International Journal of Molecular Sciences, 20(3), 459. https://doi.org/10.3390/ijms20030459






