A Potential Lock-Type Mechanism for Unconventional Secretion in Fungi
Abstract
1. Introduction
2. Unconventional Secretion in Fungi
3. Unconventional Secretion of Chitinase Cts1 in Ustilago maydis
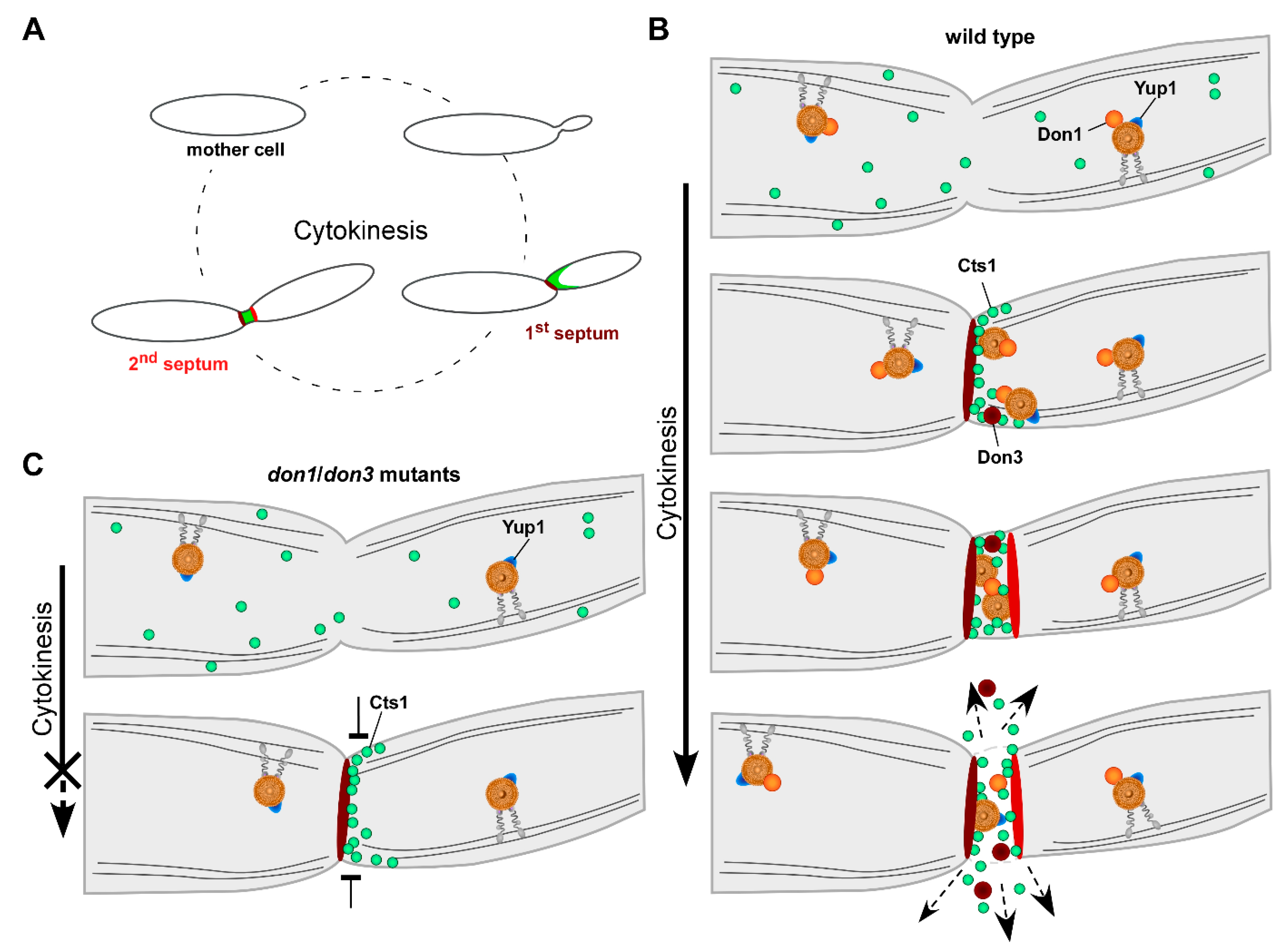
4. Insights into the Mechanism of Unconventional Cts1 Secretion
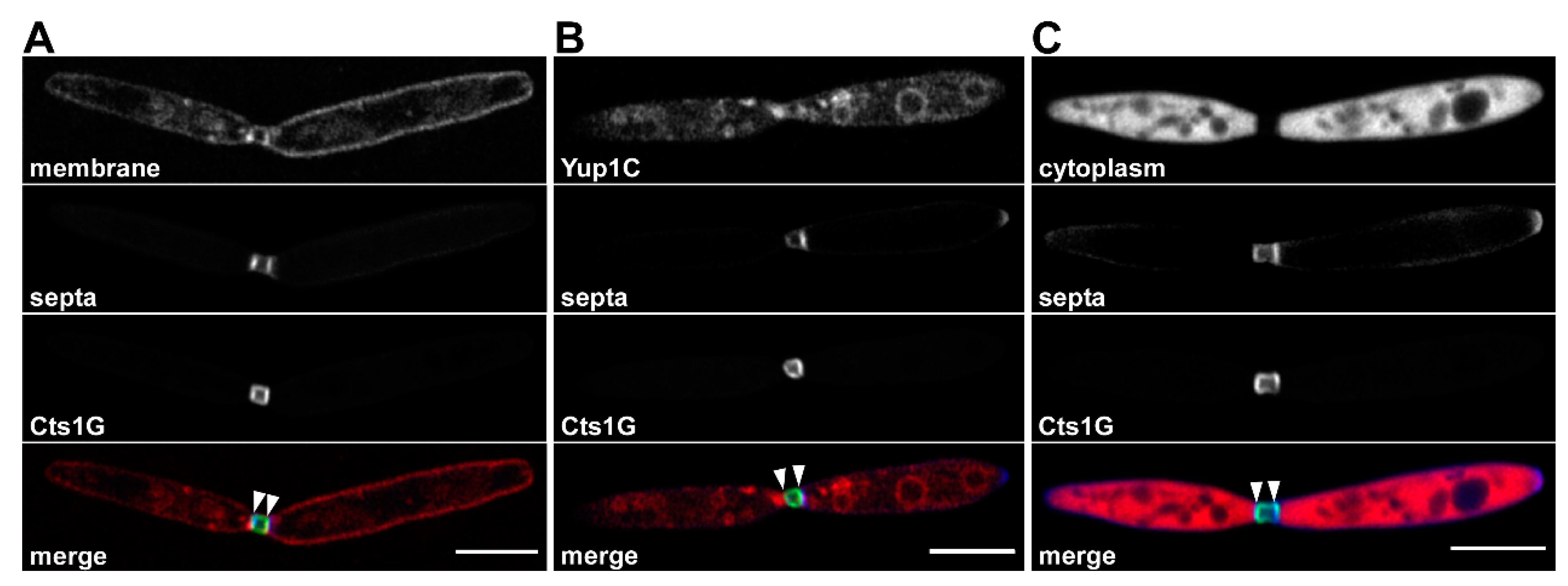
5. The Fragmentation Zone as a Potential Novel Site of Lock-Type Protein Exit
6. Conservation of Unconventional Secretion via the Fragmentation Zone
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BiC | biotrophic interfacial complexes |
| CUPS | compartments for unconventional protein secretion |
| eGfp | enhanced green fluorescent protein |
| ER | endoplasmic reticulum |
| ESCRT | endosomal sorting complex required for transport |
| GEF | guanine nucleotide exchange factor |
| GRASP | Golgi reassembly-stacking protein |
| MVB | multivesicular body |
| t-SNARE | target-soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
References
- Viotti, C. ER to Golgi-dependent protein secretion: The conventional pathway. Methods Mol. Biol. 2016, 1459, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Benham, A.M. Protein secretion and the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2012, 4, a012872. [Google Scholar] [CrossRef] [PubMed]
- Breitling, J.; Aebi, M. N-linked protein glycosylation in the endoplasmic reticulum. Cold Spring Harb. Perspect. Biol. 2013, 5, a013359. [Google Scholar] [CrossRef] [PubMed]
- Rabouille, C. Pathways of unconventional protein secretion. Trends Cell Biol. 2017, 27, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Schekman, R. Cell biology. Unconventional secretion, unconventional solutions. Science 2013, 340, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Dimou, E.; Nickel, W. Unconventional mechanisms of eukaryotic protein secretion. Curr. Biol. 2018, 28, R406–R410. [Google Scholar] [CrossRef]
- Rabouille, C.; Malhotra, V.; Nickel, W. Diversity in unconventional protein secretion. J. Cell Sci. 2012, 125, 5251–5255. [Google Scholar] [CrossRef]
- Nickel, W.; Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 2009, 10, 148–155. [Google Scholar] [CrossRef]
- Steringer, J.P.; Nickel, W. The molecular mechanism underlying unconventional secretion of Fibroblast Growth Factor 2 from tumour cells. Biol. Cell 2017, 109, 375–380. [Google Scholar] [CrossRef]
- Steringer, J.P.; Nickel, W. A direct gateway into the extracellular space: Unconventional secretion of FGF2 through self-sustained plasma membrane pores. Semin. Cell Dev. Biol. 2018. [Google Scholar] [CrossRef]
- Brough, D.; Pelegrin, P.; Nickel, W. An emerging case for membrane pore formation as a common mechanism for the unconventional secretion of FGF2 and IL-1beta. J. Cell Sci. 2017, 130, 3197–3202. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, M.; Steringer, J.P.; Muller, H.M.; Mayer, M.P.; Nickel, W. HIV-Tat protein forms phosphoinositide-dependent membrane pores implicated in unconventional protein secretion. J. Biol. Chem. 2015, 290, 21976–21984. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H. Predicting secretory proteins with SignalP. Methods Mol. Biol. 2017, 1611, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Ueda, M. Evaluation of unconventional protein secretion by Saccharomyces cerevisiae and other fungi. Cells 2018, 7, 128. [Google Scholar] [CrossRef] [PubMed]
- Nombela, C.; Gil, C.; Chaffin, W.L. Non-conventional protein secretion in yeast. Trends Microbiol. 2006, 14, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Kawaguchi, K.; Kikuma, T.; Takegawa, K.; Kitamoto, K.; Higuchi, Y. Analysis of an acyl-CoA binding protein in Aspergillus oryzae that undergoes unconventional secretion. Biochem. Biophys. Res. Comm. 2017, 493, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Y.; Kirkman, E.; Lin, X. Secreted Acb1 Contributes to the Yeast-to-Hypha Transition in Cryptococcus neoformans. Appl. Env. Microbiol. 2016, 82, 1069–1079. [Google Scholar] [CrossRef]
- Cruz-Garcia, D.; Malhotra, V.; Curwin, A.J. Unconventional protein secretion triggered by nutrient starvation. Semin. Cell Dev. Biol. 2018, 83, 22–28. [Google Scholar] [CrossRef]
- Anjard, C.; Loomis, W.F. Peptide signaling during terminal differentiation of Dictyostelium. Proc. Nat. Acad. Sci. USA 2005, 102, 7607–7611. [Google Scholar] [CrossRef]
- Duran, J.M.; Anjard, C.; Stefan, C.; Loomis, W.F.; Malhotra, V. Unconventional secretion of Acb1 is mediated by autophagosomes. J. Cell Biol. 2010, 188, 527–536. [Google Scholar] [CrossRef]
- Bruns, C.; McCaffery, J.M.; Curwin, A.J.; Duran, J.M.; Malhotra, V. Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J. Cell Biol. 2011, 195, 979–992. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, V. Unconventional protein secretion: An evolving mechanism. EMBO J. 2013, 32, 1660–1664. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Garcia, D.; Brouwers, N.; Duran, J.M.; Mora, G.; Curwin, A.J.; Malhotra, V. A diacidic motif determines unconventional secretion of wild-type and ALS-linked mutant SOD1. J. Cell Biol. 2017. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, W.; Sims, A.H.; Zhao, C.; Wang, A.; Tang, G.; Qin, J.; Wang, H. Isolation of four pepsin-like protease genes from Aspergillus niger and analysis of the effect of disruptions on heterologous laccase expression. Fungal Gen. Biol. 2008, 45, 17–27. [Google Scholar] [CrossRef]
- Burggraaf, A.M.; Punt, P.J.; Ram, A.F. The unconventional secretion of PepN is independent of a functional autophagy machinery in the filamentous fungus Aspergillus niger. FEMS Microbiol. Lett. 2016, 363. [Google Scholar] [CrossRef] [PubMed]
- Krombach, S.; Reissmann, S.; Kreibich, S.; Bochen, F.; Kahmann, R. Virulence function of the Ustilago maydis sterol carrier protein 2. New Phytol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1996. [Google Scholar] [CrossRef]
- Liu, T.; Song, T.; Zhang, X.; Yuan, H.; Su, L.; Li, W.; Xu, J.; Liu, S.; Chen, L.; Chen, T.; et al. Unconventionally secreted effectors of two filamentous pathogens target plant salicylate biosynthesis. Nat. Commun. 2014, 5, 4686. [Google Scholar] [CrossRef]
- Koeck, M.; Hardham, A.R.; Dodds, P.N. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell. Microbiol. 2011, 13, 1849–1857. [Google Scholar] [CrossRef]
- Lo Presti, L.; Kahmann, R. How filamentous plant pathogen effectors are translocated to host cells. Curr. Opin. Plant Biol. 2017, 38, 19–24. [Google Scholar] [CrossRef]
- Ridout, C.J.; Skamnioti, P.; Porritt, O.; Sacristan, S.; Jones, J.D.; Brown, J.K. Multiple avirulence paralogues in cereal powdery mildew fungi may contribute to parasite fitness and defeat of plant resistance. Plant Cell 2006, 18, 2402–2414. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.Y.; Noh, S.H.; Tang, B.L.; Kim, K.H.; Lee, M.G. Rescue of DeltaF508-CFTR trafficking via a GRASP-dependent unconventional secretion pathway. Cell 2011, 146, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Feldbrügge, M.; Kellner, R.; Schipper, K. The biotechnological use and potential of plant pathogenic smut fungi. Appl. Microbiol. Biotechnol. 2013, 97, 3253–3265. [Google Scholar] [CrossRef] [PubMed]
- Kämper, J.; Kahmann, R.; Bölker, M.; Ma, L.J.; Brefort, T.; Saville, B.J.; Banuett, F.; Kronstad, J.W.; Gold, S.E.; Müller, O.; et al. Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 2006, 444, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Matei, A.; Doehlemann, G. Cell biology of corn smut disease-Ustilago maydis as a model for biotrophic interactions. Curr. Opin. Microbiol. 2016, 34, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Niessing, D.; Jansen, R.P.; Pohlmann, T.; Feldbrügge, M. mRNA transport in fungal top models. Wiley Interdiscip. Rev. RNA 2018, 9. [Google Scholar] [CrossRef]
- Heimel, K.; Freitag, J.; Hampel, M.; Ast, J.; Bölker, M.; Kämper, J. Crosstalk between the unfolded protein response and pathways that regulate pathogenic development in Ustilago maydis. Plant Cell 2013, 25, 4262–4277. [Google Scholar] [CrossRef] [PubMed]
- Lanver, D.; Tollot, M.; Schweizer, G.; Lo Presti, L.; Reissmann, S.; Ma, L.S.; Schuster, M.; Tanaka, S.; Liang, L.; Ludwig, N.; et al. Ustilago maydis effectors and their impact on virulence. Nat. Rev. Microbiol. 2017, 15, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Geiser, E.; Przybilla, S.K.; Friedrich, A.; Buckel, W.; Wierckx, N.; Blank, L.M.; Bölker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microbial Biotechnol. 2015. [Google Scholar] [CrossRef]
- Geiser, E.; Reindl, M.; Blank, L.M.; Feldbrügge, M.; Wierckx, N.; Schipper, K. Activating intrinsic carbohydrate-active enzymes of the smut fungus Ustilago maydis for the degradation of plant cell wall components. Appl. Environ. Microbiol. 2016, 82, 5174–5185. [Google Scholar] [CrossRef]
- Langner, T.; Özturk, M.; Hartmann, S.; Cord-Landwehr, S.; Moerschbacher, B.; Walton, J.D.; Göhre, V. Chitinases are essential for cell separation in Ustilago maydis. Euk. Cell 2015, 14, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, G.; Leveleki, L.; Hassel, A.; Kost, G.; Wanner, G.; Bölker, M. Regulation of cell separation in the dimorphic fungus Ustilago maydis. Mol. Microbiol. 2002, 45, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Roncero, C. The genetic complexity of chitin synthesis in fungi. Curr. Gen. 2002, 41, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hartl, L.; Zach, S.; Seidl-Seiboth, V. Fungal chitinases: Diversity, mechanistic properties and biotechnological potential. Appl. Microbiol. Biotechnol. 2012, 93, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Langner, T.; Göhre, V. Fungal chitinases: Function, regulation, and potential roles in plant/pathogen interactions. Curr. Gen. 2016, 62, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Aschenbroich, J.; Hussnaetter, K.P.; Stoffels, P.; Langner, T.; Zander, S.; Sandrock, B.; Bölker, M.; Feldbrügge, M.; Schipper, K. The germinal centre kinase Don3 is crucial for unconventional secretion of chitinase Cts1 in Ustilago maydis. Biochim. Biophys. Acta Proteins Proteom. 2018. [Google Scholar] [CrossRef] [PubMed]
- Huff, J. The Airyscan detector from ZEISS: Confocal imaging with improved signal-to-noise ratio and super-resolution. Nat. Meth. 2015, 12, 1205. [Google Scholar] [CrossRef]
- Karthik, N.; Akanksha, K.; Pandey, A. Production, purification and properties of fungal chitinase—A review. Indian J. Exp. Biol. 2014, 52, 1025–1035. [Google Scholar]
- Koepke, J.; Kaffarnik, F.; Haag, C.; Zarnack, K.; Luscombe, N.M.; König, J.; Ule, J.; Kellner, R.; Begerow, D.; Feldbrügge, M. The RNA-binding protein Rrm4 is essential for efficient secretion of endochitinase Cts1. Mol. Cell. Proteom. 2011, 10. [Google Scholar] [CrossRef]
- Stock, J.; Sarkari, P.; Kreibich, S.; Brefort, T.; Feldbrügge, M.; Schipper, K. Applying unconventional secretion of the endochitinase Cts1 to export heterologous proteins in Ustilago maydis. J. Biotechnol. 2012, 161, 80–91. [Google Scholar] [CrossRef]
- Stock, J.; Terfrüchte, M.; Schipper, K. A reporter system to study unconventional secretion of proteins avoiding N-glycosylation in Ustilago maydis. In Unconventional Protein Secretion: Methods and Protocols; Humana Press: New York, NY, USA, 2016; Volume 1459, pp. 149–160. [Google Scholar]
- Iturriaga, G.; Jefferson, R.A.; Bevan, M.W. Endoplasmic reticulum targeting and glycosylation of hybrid proteins in transgenic tobacco. Plant Cell 1989, 1, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Terfrüchte, M.; Reindl, M.; Jankowski, S.; Sarkari, P.; Feldbrügge, M.; Schipper, K. Applying unconventional secretion in Ustilago maydis for the export of functional nanobodies. Int. J. Mol. Sci. 2017, 18, 937. [Google Scholar] [CrossRef] [PubMed]
- Sarkari, P.; Reindl, M.; Stock, J.; Müller, O.; Kahmann, R.; Feldbrügge, M.; Schipper, K. Improved expression of single-chain antibodies in Ustilago maydis. J. Biotechnol. 2014, 191, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, C.; Ripp, C.; Bölker, M. The germinal centre kinase Don3 triggers the dynamic rearrangement of higher-order septin structures during cytokinesis in Ustilago maydis. Mol. Microbiol. 2009, 74, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Böhmer, C.; Böhmer, M.; Bölker, M.; Sandrock, B. Cdc42 and the Ste20-like kinase Don3 act independently in triggering cytokinesis in Ustilago maydis. J. Cell Sci. 2008, 121, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Göhre, V.; Vollmeister, E.; Bölker, M.; Feldbrügge, M. Microtubule-dependent membrane dynamics in Ustilago maydis: Trafficking and function of Rab5a-positive endosomes. Commun. Int. Biol. 2012, 5, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Hlubek, A.; Schink, K.O.; Mahlert, M.; Sandrock, B.; Bölker, M. Selective activation by the guanine nucleotide exchange factor Don1 is a main determinant of Cdc42 signalling specificity in Ustilago maydis. Mol. Microbiol. 2008, 68, 615–623. [Google Scholar] [CrossRef]
- Schink, K.O.; Bölker, M. Coordination of cytokinesis and cell separation by endosomal targeting of a Cdc42-specific guanine nucleotide exchange factor in Ustilago maydis. Mol. Biol. Cell 2009, 20, 1081–1088. [Google Scholar] [CrossRef]
- Zander, S.; Baumann, S.; Weidtkamp-Peters, S.; Feldbrügge, M. Endosomal assembly and transport of heteromeric septin complexes promote septin cytoskeleton formation. J. Cell Sci. 2016, 129, 2778–2792. [Google Scholar] [CrossRef]
- Sandrock, B.; Böhmer, C.; Bölker, M. Dual function of the germinal centre kinase Don3 during mitosis and cytokinesis in Ustilago maydis. Mol. Microbiol. 2006, 62, 655–666. [Google Scholar] [CrossRef]
- Bethune, J.; Jansen, R.P.; Feldbrügge, M.; Zarnack, K. Membrane-associated RNA-binding proteins orchestrate organelle-coupled translation. Trends Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Haag, C.; Steuten, B.; Feldbrügge, M. Membrane-coupled mRNA trafficking in fungi. Ann. Rev. Microbiol. 2015, 69, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, S.C.; Schuster, M.; Bielska, E.; Dagdas, G.; Kilaru, S.; Meadows, B.R.; Schrader, M.; Steinberg, G. Peroxisomes, lipid droplets, and endoplasmic reticulum “hitchhike” on motile early endosomes. J. Cell Biol. 2015, 211, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Francisco, T.; Rodrigues, T.A.; Dias, A.F.; Barros-Barbosa, A.; Bicho, D.; Azevedo, J.E. Protein transport into peroxisomes: Knowns and unknowns. BioEssays 2017, 39, 1700047. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Ann. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef] [PubMed]
- Ökmen, B.; Kemmerich, B.; Hilbig, D.; Wemhöner, R.; Aschenbroich, J.; Perrar, A.; Huesgen, P.F.; Schipper, K.; Doehlemann, G. Dual function of a secreted fungalysin metalloprotease in Ustilago maydis. New Phytol. 2018. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Ishikawa, E.; Shoji, J.Y.; Nakano, H.; Kitamoto, K. Septum-directed secretion in the filamentous fungus Aspergillus oryzae. Mol. Microbiol. 2011, 81, 40–55. [Google Scholar] [CrossRef]
- Cabib, E. The septation apparatus, a chitin-requiring machine in budding yeast. Arch. Biochem. Biophys. 2004, 426, 201–207. [Google Scholar] [CrossRef]
- Dünkler, A.; Jorde, S.; Wendland, J. An Ashbya gossypii cts2 mutant deficient in a sporulation-specific chitinase can be complemented by Candida albicans CHT4. Microbiol. Res. 2008, 163, 701–710. [Google Scholar] [CrossRef]
- Kuranda, M.J.; Robbins, P.W. Chitinase is required for cell separation during growth of Saccharomyces cerevisiae. J. Biol. Chem. 1991, 266, 19758–19767. [Google Scholar]
- Bhavsar-Jog, Y.P.; Bi, E. Mechanics and regulation of cytokinesis in budding yeast. Semin. Cell Dev. Biol. 2017, 66, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Colman-Lerner, A.; Chin, T.E.; Brent, R. Yeast Cbk1 and Mob2 activate daughter-specific genetic programs to induce asymmetric cell fates. Cell 2001, 107, 739–750. [Google Scholar] [CrossRef]
- Gee, H.Y.; Kim, J.Y.; Lee, M.G. Analysis of conventional and unconventional trafficking of CFTR and other membrane proteins. Methods Mol. Biol. 2015, 1270, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Terfrüchte, M.; Wewetzer, S.; Sarkari, P.; Stollewerk, D.; Franz-Wachtel, M.; Macek, B.; Schlepütz, T.; Feldbrügge, M.; Büchs, J.; Schipper, K. Tackling destructive proteolysis of unconventionally secreted heterologous proteins in Ustilago maydis. J. Biotechnol. 2018, 284, 37–51. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reindl, M.; Hänsch, S.; Weidtkamp-Peters, S.; Schipper, K. A Potential Lock-Type Mechanism for Unconventional Secretion in Fungi. Int. J. Mol. Sci. 2019, 20, 460. https://doi.org/10.3390/ijms20030460
Reindl M, Hänsch S, Weidtkamp-Peters S, Schipper K. A Potential Lock-Type Mechanism for Unconventional Secretion in Fungi. International Journal of Molecular Sciences. 2019; 20(3):460. https://doi.org/10.3390/ijms20030460
Chicago/Turabian StyleReindl, Michèle, Sebastian Hänsch, Stefanie Weidtkamp-Peters, and Kerstin Schipper. 2019. "A Potential Lock-Type Mechanism for Unconventional Secretion in Fungi" International Journal of Molecular Sciences 20, no. 3: 460. https://doi.org/10.3390/ijms20030460
APA StyleReindl, M., Hänsch, S., Weidtkamp-Peters, S., & Schipper, K. (2019). A Potential Lock-Type Mechanism for Unconventional Secretion in Fungi. International Journal of Molecular Sciences, 20(3), 460. https://doi.org/10.3390/ijms20030460



