Glucose Tolerance-Improving Activity of Helichrysoside in Mice and Its Structural Requirements for Promoting Glucose and Lipid Metabolism
Abstract
1. Introduction
2. Results and Discussion
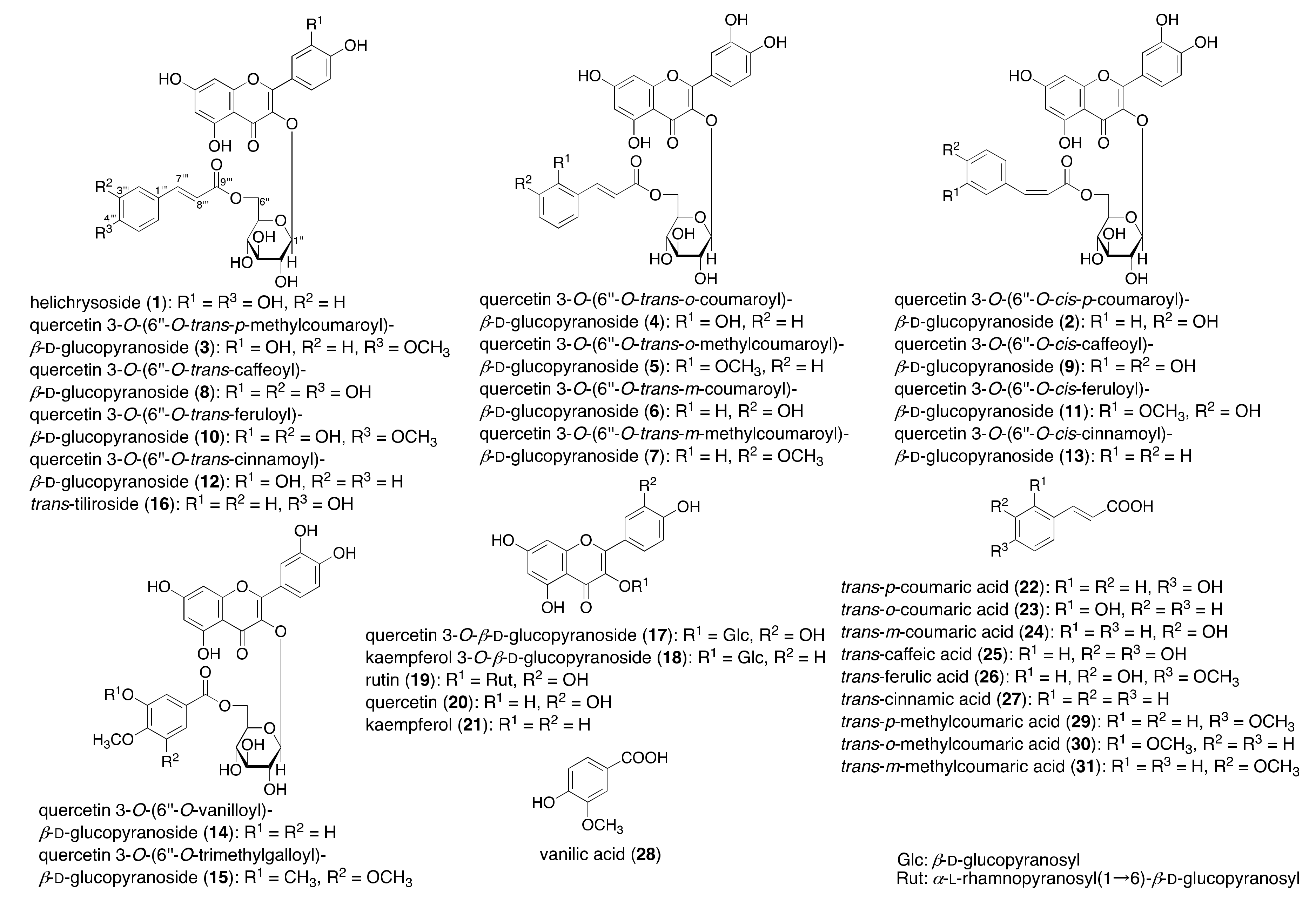
2.1. Synthesis of Acylated Flavonol Glycosides (1–15)
2.2. Effect of Helichrysoside (1) on the Liver Triglyceride (TG) Content and Glucose Tolerance Test after 14 Days of Administration in Mice
2.3. Effects on Glucose Consumption in HepG2 Cells
2.4. Effects on Lipid Metabolism in HepG2 Cells
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. General Experimental Procedures
3.3. Enzymatic Hydrolysis of Rutin (19) Monitored by HPLC
3.4. Practical Derivation from Rutin (19) to Quercetin 3-O-β-d-glucopyranoside (17) by Naringinase
3.5. Synthesis of Helichrysoside (1)
3.6. Synthesis of 3–8, 10, 12, 14, and 15
3.7. Isomerization of 1, 8, 10, and 12
3.8. Animals
3.9. Effects on the Glucose Tolerance Test in Mice
3.10. Cell Culture
3.11. Effects on Glucose Consumption in HepG2 Cells
3.12. Effects on High Glucose-Induced TG Accumulation in HepG2 Cells
3.13. Effects on TG contents in High Glucose-Pretreated HepG2 Cells
3.14. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Symonowicz, M.; Kolanek, M. Flavonoids and their properties to form chelate complexes. Biotechnol. Food Sci. 2012, 76, 35–41. [Google Scholar]
- Santos-Buelga, C.; Feliciano, A.S. Flavonoids: From structure to health issues. Molecules 2017, 22, 477. [Google Scholar] [CrossRef] [PubMed]
- Usman, H.; Abdulrahman, F.I.; Kaita, H.A.; Khan, I.Z.; Tijjani, M.A. Flavonoids: The bioactive phytochemical agent—A review. Chem. Res. J. 2017, 2, 59–72. [Google Scholar]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef]
- Teplova, V.V.; Isakova, E.P.; Klein, O.I.; Dergachova, D.I.; Gessler, N.N.; Deryabina, Y.I. Natural polyphenols: Biological activity, pharmacological potential, means of metabolic engineering (review). Appl. Biochem. Microbiol. 2018, 54, 221–237. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health—A review of emerging biologic pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Fraga, C.G. Research trends in flavonoids and health. Arch. Biochem. Biophys. 2018, 646, 107–112. [Google Scholar] [CrossRef]
- Harnly, J. Importance of accurate measurements in nutrition research: Dietary flavonoids as a case study. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Ishiwada, T.; Morikawa, T.; Kagawa, M.; Higashi, Y.; Matsuda, H. New flavonol oligoglycosides and polyacylated sucroases with inhibitory effects on aldose reductase and platelet aggregation from the flowers of Prunus mume. J. Nat. Prod. 2002, 65, 1151–1155. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Yoshikawa, M. Antidiabetogenic constituents from several natural medicines. Pure Appl. Chem. 2002, 74, 1301–1308. [Google Scholar] [CrossRef]
- Morikawa, T.; Xie, H.; Wang, T.; Matsuda, H.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XXXII. Aminopeptidase N and aldose reductase inhibitors from Sinocrassula indica: Structures of sinocrassosides B4, B5, C1, and D1–D3. Chem. Pharm. Bull. 2008, 56, 1438–1444. [Google Scholar] [CrossRef]
- Morikawa, T. Search for bioactive constituents from several medicinal foods: Hepatoprotective, antidiabetic, and antiallergic activities. J. Nat. Med. 2007, 61, 112–126. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2002, 10, 3123–3128. [Google Scholar]
- Matsuda, H.; Sugimoto, S.; Morikawa, T.; Matsuhira, K.; Mizoguchi, E.; Nakamura, S.; Yoshikawa, M. Bioactive constituents from Chinese natural medicines. XX. Inhibitors of antigen-induced degranulation in RBL-2H3 cells from the seeds of Psoralea corylifolia. Chem. Pharm. Bull. 2007, 55, 106–110. [Google Scholar] [CrossRef]
- Matsuda, H.; Morikawa, T.; Ando, S.; Toguchida, I.; Yoshikawa, M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg. Med. Chem. 2003, 11, 1995–2000. [Google Scholar] [CrossRef]
- Morikawa, T.; Xu, F.; Matsuda, H.; Yoshikawa, M. Structures of new flavonoids, erycibenins D, E, and F, and NO production inhibitors from Erycibe expansa originationg in Thailand. Chem. Pharm. Bull. 2006, 54, 1530–1534. [Google Scholar] [CrossRef][Green Version]
- Morikawa, T.; Funakoshi, K.; Ninomiya, K.; Yasuda, D.; Miyagawa, K.; Matsuda, H.; Yoshikawa, M. Medicinal foodstuffs. XXXIV. Structures of new prenylchalcones and prenylflavonones with TNF-α and aminopeptidase N inhibitory activities from Boesenbergia rotunda. Chem. Pharm. Bull. 2008, 56, 956–962. [Google Scholar] [CrossRef]
- Morikawa, T.; Wang, L.-B.; Nakamura, S.; Ninomiya, K.; Yokoyama, E.; Matsuda, H.; Muraoka, O.; Wu, L.-J.; Yoshikawa, M. Medicinal flowers. XXVII. New flavanone and chalcone glycosides, arenariumosides I, II, III, and IV, and tumor necrosis factor-α inhibitors from everlasting, flowers of Helichrysum arenarium. Chem. Pharm. Bull. 2009, 57, 361–367. [Google Scholar] [CrossRef]
- Morikawa, T.; Xie, H.; Wang, T.; Matsuda, H.; Yoshikawa, M. Acylated flavonol bisdesmosides, sinocrassosides A3–A7 and B3, with aminopeptidase N inhibitory activity from Sinocrassula indica. Chem. Biodivers. 2009, 6, 411–420. [Google Scholar] [CrossRef]
- Chaipech, S.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O. Structures of two new phenolic glycosides, kaempferiaosides A and B, and hepatoprotective constituents from the rhizomes of Kaempferia parviflora. Chem. Pharm. Bull. 2012, 60, 62–69. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Funakoshi, K.; Ochi, M.; Pongpiriyadacha, Y.; Matsuda, H. Medicinal foodstuffs. XXXIII. Gastroprotective principles from Boesenbergia rotunda (Zingiberaceae)—absolute stereostructures of Diels-Alder type addition prenylchalcone. Heterocycles 2008, 75, 1639–1650. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsumoto, T.; Chaipech, S.; Miyake, S.; Katsuyama, Y.; Tsuboyama, A.; Pongpiriyadacha, Y.; Hayakawa, T.; Muraoka, O.; Morikawa, T. Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J. Nat. Med. 2016, 70, 179–189. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Akaki, J.; Kakihara, N.; Kuramoto, H.; Matsumoto, Y.; Hayakawa, T.; Muraoka, O.; Wang, L.-B.; Nakamura, S.; et al. Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum arenarium. J. Nat. Med. 2015, 69, 494–506. [Google Scholar] [CrossRef]
- Candy, H.A.; Laing, M.; Weeks, C.M. The crystal and molecular structure of helichrysoside, a new acylated flavonoid glycoside from Helichrysum kraussii. Tetrahedron Lett. 1975, 14, 1211–1214. [Google Scholar] [CrossRef]
- Lavault, M.; Richomme, P. Constituents of Helichrysum stoechas variety olonnense. Chem. Nat. Compd. 2004, 40, 118–121. [Google Scholar] [CrossRef]
- Ninomiya, K.; Matsuda, H.; Kubo, M.; Morikawa, T.; Nishida, N.; Yoshikawa, M. Potent anti-obese principle from Rosa canina: Structural requirements and mode of action of trans-tiliroside. Bioorg. Med. Chem. Lett. 2007, 17, 3059–3064. [Google Scholar] [CrossRef]
- Fernandes, A.A.H.; Novelli, E.L.B.; Okoshi, K.; Okoshi, M.P.; Di Muzio, B.P.; Guimarães, J.F.C.; Junior, A.F. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Arumugam, T.V.; Brown, L. Rutin attenuates metabolic changes, nonalcoholic steatohepatitis, and cardiovascular remodeling in high-carbohydrate, high-fat diet-fed rats. J. Nutr. 2011, 141, 1062–1069. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef]
- Chua, L.S. A review on plant-based rutin extraction methods and its pharmacological activities. J. Ethnopharmacol. 2013, 150, 805–817. [Google Scholar] [CrossRef]
- Heřmánková-Vavříková, E.; Křenková, A.; Petrásková, L.; Chambers, C.S.; Zápal, J.; Kuzma, M.; Valentová, K.; Křen, V. Synthesis and antiradical activity of isoquercitrin esters with aromatic acids and their homologues. Int. J. Mol. Sci. 2017, 18, 1074–1087. [Google Scholar] [CrossRef]
- Ren, X.; Shen, L.L.; Muraoka, O.; Cheng, M. Synthesis of quercetin 3-O-[6′′-O-(trans-p-coumaroyl)]-β-d-glucopyranoside. J. Carbohydr. Chem. 2011, 30, 119–131. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakajima, N. Structural aspects of acylated plant pigments: Stabilization of flavonoid glucosides and interpretation of their functions. J. Mol. Catal. B Enzym. 2003, 23, 411–417. [Google Scholar] [CrossRef]
- Calzada, F.; Cedillo-Rivera, R.; Mata, R. Antiprotozoal activity of the constituents of Conyza filaginoides. J. Nat. Prod. 2001, 64, 671–673. [Google Scholar] [CrossRef]
- Danieli, B.; Bertario, A. Chemo-enzymatic synthesis of 6′′-O-(3-arylprop-2-enoyl) derivatives of the flavonol glucoside isoquercitrin. Helv. Chim. Acta 1993, 76, 2981–2991. [Google Scholar] [CrossRef]
- Gao, C.; Mayon, P.; MacManus, D.A.; Vulfson, E.N. Novel enzymatic approach to the synthesis of flavonoid glycosides and their esters. Biothech. Bioeng. 2001, 71, 235–243. [Google Scholar] [CrossRef]
- Jung, U.J.; Choi, M.-S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014, 15, 6184–6223. [Google Scholar] [CrossRef]
- Rodriguez-Araujo, G.; Nakagami, H. Pathophysiology of cardiovascular disease in diabetes mellitus. Cardiovasc Endocrinol. Metab. 2018, 7, 4–9. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Wang, T.; Morikawa, T.; Xie, H.; Matsuda, H. Bioactive constituents from Chinese natural medicines. XXIV. Hypoglycemic effects of Sinocrassula indica in sugar-loaded rats and genetically diabetic KK-Ay mice and structures of new acylated flavonol glycosides, sinocrassosides A1, A2, B1, and B2. Chem. Pharm. Bull. 2007, 55, 1308–1315. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Xu, F.; Morikawa, T.; Pongpiriyadacha, Y.; Nakamura, S.; Asao, Y.; Kumahara, A.; Matsuda, H. Medicinal flowers. XII. New spirostane-type steroid saponins with antidiabetogenic activity from Borassus flabellifer. Chem. Pharm. Bull. 2007, 55, 308–316. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Matsuda, H.; Tanabe, G.; Muraoka, O. Absolute stereostructure of potent α-glucosidase inhibitor, salacinol, with unique thiosugar sulfonium sulfate inner salt structure from Salacia reticulata. Bioorg. Med. Chem. 2002, 10, 1547–1554. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Pongpiriyadacha, Y.; Kishi, A.; Kageura, T.; Wang, T.; Morikawa, T.; Matsuda, H. Biological activities of Salacia chinensis originating in Thailand: The quality evaluation guided by α-glucosidase inhibitory activity. Yakugaku Zasshi 2003, 123, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Yoshikawa, M.; Morikawa, T.; Tanabe, G.; Muraoka, O. Antidiabetogenic constituents from Salacia species. J. Tradit. Med. 2005, 22 (Suppl. 1), 145–153. [Google Scholar]
- Kobayashi, M.; Akaki, J.; Yamashita, K.; Morikawa, T.; Ninomiya, K.; Yoshikawa, M.; Muraoka, O. Suippressive effect of the tablet containing Salacia chinensis extract on postprandial blood glucose. Jpn. Pharmacol. Ther. 2010, 38, 545–550. [Google Scholar]
- Morikawa, T.; Akaki, J.; Ninomiya, K.; Kinouchi, E.; Tanabe, G.; Pongpiriyadacha, Y.; Yoshikawa, M.; Muraoka, O. Salacinol and related analogs: New leads for type 2 diabetes therapeutic candidates from the Thai traditional natural medicine Salacia chinensis. Nutrients 2015, 7, 1480–1493. [Google Scholar] [CrossRef] [PubMed]
- Kabayashi, M.; Akaki, J.; Yamaguchi, Y.; Yamasaki, H.; Ninomiya, K.; Pongpiriyadacha, Y.; Yoshikawa, M.; Muraoka, O.; Morikawa, M. Salacia chinensis stem extract and its thiosugar sulfonium constituents, neokotalanol, improves HbA1c levels in ob/ob mice. J. Nat. Med. 2019, 73, 584–588. [Google Scholar] [CrossRef]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef]
- Huang, X.-L.; He, Y.; Ji, L.-L.; Wang, K.-Y.; Wang, Y.-L.; Chen, D.-F.; Geng, Y.; OuYang, P.; Lai, W.-M. Hepatoprotective potential of isoquercitrin against type 2 diabetes-induced hepatic injury in rats. Oncotarget 2017, 8, 101545–101559. [Google Scholar] [CrossRef]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar]
- Alkhalidy, H.; Moore, W.; Zhang, Y.; McMillan, R.; Wang, A.; Ali, M.; Suh, K.S.; Zhen, W.; Cheng, Z.; Jia, Z.; et al. Small molecule kaempferol promotes insulin sensitivity and preserved pancreatic β-cell mass in middle-aged obese diabetic mice. J. Diabetes. Res. 2015, 2015, 532984. [Google Scholar] [CrossRef]
- Bellentani, S.; Tiribelli, C.; Saccoccio, G.; Sodde, M.; Fratti, N.; De Martin, C.; Cristianini, G. Prevalence of chronic liver disease in the general population of northern Italy: The dionysos study. Hepatology 1994, 20, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- El-Hassan, A.Y.; Ibrahim, E.M.; Al-Mulnim, F.A.; Nabhan, A.A.; Chammas, M.Y. Fatty infiltration of the liver: Analysis of prevalence, radiological and clinical features and influence on patient management. Br. J. Radiol. 1992, 65, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Marceau, P.; Biron, S.; Hould, F.S.; Marceau, S.; Simard, S.; Thung, S.N.; Kral, J.G. Liver pathology and the metabolic syndrome X in severe obesity. J. Clin. Endocrinol. Metab. 1999, 84, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Brizi, M.; Morselli-Labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef]
- Yuk, T.; Kim, Y.; Yang, J.; Sung, J.; Jeong, H.S.; Lee, J. Nobiletin inhibits hepatic lipogenesis via activation of AMP-activated protein kinase. Evid. Based Complement. Alternat. Med. 2018, 2018, 7420265. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Kim, H.G.; Khanal, T.; Song, G.Y.; Nam, M.S.; Lee, H.-S.; Chung, Y.C.; Lee, Y.C.; Jeong, H.G. Saponins, especially platycodin D, from Platycodon grandiflorum modulate hepatic lipogenesis in high-fat diet-fed rats and high glucose-exposed HepG2 cells. Toxicol. Appl. Pharmacol. 2013, 267, 174–183. [Google Scholar] [CrossRef]
- Kawser Hossain, M.; Abdal Dayem, A.; Han, J.; Yin, Y.; Kim, K.; Kumar Saha, S.; Yang, G.-M.; Choi, H.Y.; Cho, S.-G. Molecular mechanisms of the anti-obesity and anti-diabetic properties of flavonoids. Int. J. Mol. Sci. 2016, 17, 569–600. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Miyake, S.; Miki, Y.; Okamoto, M.; Yoshikawa, M.; Muraoka, O. Flavonol glycosides with lipid accumulation inhibitory activity and simultaneous quantitative analysis of 15 polyphenols and caffeine in the flower buds of Camellia sinensis from different regions by LCMS. Food Chem. 2013, 140, 353–360. [Google Scholar] [CrossRef]
- Muraoka, O.; Morikawa, T.; Zhang, Y.; Ninomiya, K.; Nakamura, S.; Matsuda, H.; Yoshikawa, M. Novel megastigmanes with lipid accumulation inhibitory and lipid metabolism-promoting activities in HepG2 cells from Sedum sarmentosum. Tetrahedron 2009, 65, 4142–4148. [Google Scholar] [CrossRef]
- Morikawa, T.; Ninomiya, K.; Xu, F.; Okumura, N.; Matsuda, H.; Muraoka, O.; Hayakawa, T.; Yoshikawa, M. Acylated dolabellane-type diterpenes from Nigella sativa seeds with triglyceride metabolism-promoting activity in high glucose-pretreated HepG2 cells. Phytochem. Lett. 2013, 6, 198–204. [Google Scholar] [CrossRef]
- Inoue, T.; Matsui, Y.; Kikuchi, T.; Yamada, T.; In, Y.; Muraoka, O.; Sakai, C.; Ninomiya, K.; Morikawa, T.; Tanaka, R. Carapanolides M–S from seeds of andiroba (Carapa guianensis, Meliaceae) and triglyceride metabolism-promoting activity in high glucose-pretreated HepG2 cells. Tetrahedron 2015, 71, 2753–2760. [Google Scholar] [CrossRef]
- Gorgani-Firuzjaee, S.; Meshkani, R. SH2 domain-containing inositol 5-phosphatase (SHIP2) inhibition ameliorates high glucose-induced de-novo lipogenesis and VLDL production through regulating AMPK/mTOR/SREBP1 pathway and ROS production in HepG2 cells. Free Radic. Biol. Med. 2015, 89, 679–689. [Google Scholar] [CrossRef]
- Gan, C.-C.; Ni, T.-W.; Yu, Y.; Qin, N.; Chen, Y.; Jin, M.-N.; Duan, H.-Q. Flavonoid derivative (Fla-CN) inhibited adipocyte differentiation via activating AMPK and up-regulating microRNA-27 in 3T3-L1 cells. Eur. J. Pharmacol. 2017, 797, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Qin, N.; Hu, L.; Liu, L.; Duan, H.; Niu, W. Tiliroside-derivatives enhance GLUT4 translocation via AMPK in muscle cells. Diabetes Res. Clin. Pract. 2011, 92, e41–e46. [Google Scholar] [CrossRef] [PubMed]


| Reaction Time | Peak Area (%) | ||
|---|---|---|---|
| Rutin (19) | Quercetin 3-O-Glc (17) | Quercetin (20) | |
| 0 min | 99.1 | 0.0 | 0.0 |
| 5 min | 96.6 | 1.6 | 0.7 |
| 30 min | 60.8 | 30.7 | 7.5 |
| 1 h | 29.7 | 46.3 | 22.9 |
| 1.5 h | 16.4 | 50.1 | 32.6 |
| 2 h | 6.3 | 49.6 | 43.2 |
| 3 h | 0.0 | 40.8 | 57.7 |
| 4.5 h | 0.0 | 25.9 | 72.5 |
| 8 h | 0.0 | 9.0 | 89.4 |
| 24 h | 0.0 | 1.5 | 95.5 |
| Treatment | Glucose in the Medium (% of Control)(Protein (% of Control)) | ||||
|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| Helichrysoside (1) | 100.0 ± 2.6 (100.0 ± 5.4) | 94.7 ± 1.1 ** (96.6 ± 2.0) | 86.7 ± 1.2 ** (98.3 ± 2.0) | 89.3 ± 1.1 ** (102.9 ± 1.5) | 82.9 ± 1.5 ** (105.2 ± 1.4) |
| Trans-tiliroside (16) | 100.0 ± 6.4 (100.0 ± 4.7) | 84.6 ± 0.9 ** (105.4 ± 1.5) | 90.1 ± 0.6 ** (102.3 ± 2.7) | 96.8 ± 1.7 (103.4 ± 1.9) | 79.8 ± 1.0 ** (110.0 ± 1.0) |
| Quercetin 3-O-Glc (17) | 100.0 ± 1.1 (100.0 ± 1.3) | 100.9± 1.4 (104.0 ± 1.0) | 100.6 ± 1.3 (104.1 ± 0.9) | 241.7 ± 3.8 ** (47.2 ± 0.6 **) | 269.4 ± 5.2 ** (23.1± 1.4**) |
| Kaempferol 3-O-Glc (18) | 100.0 ± 3.0 (100.0 ± 3.5) | 95.9 ± 0.7 (96.4 ± 2.0) | 98.9 ± 2.7 (94.2 ± 1.4 *) | 93.4 ± 0.4 ** (100.1 ± 0.4) | 92.8 ± 1.1 ** (98.7 ± 1.4) |
| Quercetin (20) | 100.0 ± 2.0 (100.0 ± 0.5) | 98.6 ± 0.4 (102.0 ± 0.9) | 97.2 ± 1.7 (103.4 ± 0.8 **) | 105.6 ± 2.0 (98.9 ± 0.6) | 249.2 ± 3.9 ** (41.7 ± 0.4 **) |
| Kaempferol (21) | 100.0 ± 3.1 (100.0 ± 0.7) | 98.4 ± 2.2 (101.0 ± 0.8) | 95.2 ± 1.5 (100.7 ± 0.5) | 98.1 ± 0.9 (100.3 ± 1.2) | 224.4 ± 3.1 ** (55.4 ± 0.5 **) |
| Trans-p-coumaric acid (22) | 100.0 ± 3.6 (100.0 ± 3.7) | 96.9 ± 1.5 (99.7 ± 0.6) | 96.5 ± 0.5 (98.2 ± 1.7) | 96.8 ± 1.7 (98.3 ± 0.4) | 97.7 ± 1.8 (105.8 ± 1.8 *) |
| 0 µM | 62.5 µM | 125 µM | 250 µM | 500 µM | |
| Metformin | 100.0 ± 1.5 (100.0 ± 1.2) | 98.9 ± 1.9 (102.0 ± 1.8) | 95.1 ± 0.7 (100.9 ± 1.7) | 97.7 ± 1.8 (100.5 ± 2.1) | 87.5 ± 4.8 * (100.6 ± 2.3) |
| Treatment | TG/Protein (% of Control) | ||||
|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| Helichrysoside (1) | 100.0 ± 1.6 | 100.9 ± 7.1 | 96.0 ± 1.5 | 90.2 ± 2.2 | 76.9 ± 2.3 ** |
| Quercetin 3-O-(6′′-O-cis-p-coumaroyl)-Glc (2) | 100.0 ± 0.7 | 102.0 ± 2.1 | 97.3 ± 2.1 | 93.2 ± 1.2 * | 80.4 ± 1.2 ** |
| Quercetin 3-O-(6′′-O-trans-p-methylcoumaroyl)-Glc (3) | 100.0 ± 4.8 | 99.8 ± 10.2 | 107.4 ± 2.3 | 112.9 ± 2.6 | 58.2 ± 7.5 ** |
| Quercetin 3-O-(6′′-O-trans-o-coumaroyl)-Glc (4) | 100.0 ± 2.6 | 104.6 ± 4.7 | 98.2 ± 3.0 | 98.3 ± 2.4 | 93.4 ± 1.2 |
| Quercetin 3-O-(6′′-O-trans-o-methylcoumaroyl)-Glc (5) | 100.0 ± 6.7 | 84.4 ± 11.7 | 73.5 ± 12.2 | 107.6 ± 13.8 | 125.9 ± 3.6 |
| Quercetin 3-O-(6′′-O-trans-m-coumaroyl)-Glc (6) | 100.0 ± 3.7 | 97.4 ± 10.9 | 99.0 ± 2.7 | 96.4 ± 5.6 | 106.0 ± 4.8 |
| Quercetin 3-O-(6′′-O-trans-m-methylcoumaroyl)-Glc (7) | 100.0 ± 3.4 | 108.0 ± 4.5 | 80.5 ± 10.6 | 114.2 ± 8.5 | 92.2 ± 8.5 |
| Quercetin 3-O-(6′′-O-trans-caffeoyl)-Glc (8) | 100.0 ± 0.7 | 108.4 ± 1.6 * | 107.0 ± 1.7 * | 103.6 ± 2.3 | 98.1 ± 1.7 |
| Quercetin 3-O-(6′′-O-cis-caffeoyl)-Glc (9) | 100.0 ± 2.2 | 102.4 ± 1.8 | 102.2 ± 1.6 | 100.7 ± 1.7 | 93.8 ± 2.2 |
| Quercetin 3-O-(6′′-O-trans-feruloyl)-Glc (10) | 100.0 ± 3.2 | 104.7 ± 5.3 | 110.6 ± 2.4 | 108.4 ± 4.2 | 114.9 ± 3.6 |
| Quercetin 3-O-(6′′-O-cis-feruloyl)-Glc (11) | 100.0 ± 7.0 | 104.9 ± 3.3 | 101.6 ± 2.7 | 100.3 ± 2.5 | 92.9 ± 5.2 |
| Quercetin 3-O-(6′′-O-trans-cinnamoyl)-Glc (12) | 100.0 ± 1.3 | 106.8 ± 3.5 | 100.6 ± 2.0 | 102.8 ± 0.9 | 115.6 ± 2.9 ** |
| Quercetin 3-O-(6′′-O-cis-cinnamoyl)-Glc (13) | 100.0 ± 4.0 | 106.3 ± 4.1 | 100.2 ± 3.9 | 107.8 ± 3.2 | 121.0 ± 3.1 ** |
| Quercetin 3-O-(6′′-O-vanilloyl)-Glc (14) | 100.0 ± 3.0 | 96.3 ± 4.5 | 101.7 ± 2.7 | 105.9 ± 3.2 | 104.5 ± 1.3 |
| Quercetin 3-O-(6′′-O-trimethylgalloyl)-Glc (15) | 100.0 ± 2.9 | 99.7 ± 0.7 | 96.4 ± 1.1 | 92.3 ± 3.4 | 85.8 ± 4.1 ** |
| Trans-tiliroside (16) | 100.0 ± 2.0 | 99.1 ± 1.2 | 95.5 ± 2.0 | 109.6 ± 3.8 | 82.3 ± 3.0 ** |
| Quercetin 3-O-Glc (17) | 100.0 ± 1.1 | 97.1 ± 1.9 | 124.5 ± 1.3 ** | 96.0 ± 3.1 | 94.3 ± 5.2 |
| Kaempferol 3-O-Glc (18) | 100.0 ± 3.5 | 96.8 ± 3.2 | 95.5 ± 2.0 | 99.8 ± 1.3 | 91.9 ± 2.1 |
| Rutin (19) | 100.0 ± 4.1 | 102.1 ± 7.6 | 108.4 ± 3.4 | 103.9 ± 2.5 | 103.1 ± 2.6 |
| Quercetin (20) | 100.0 ± 0.9 | 99.8 ± 1.1 | 93.8 ± 0.4 ** | 82.6 ± 2.4 ** | 45.7 ± 0.4 ** |
| Kaempferol (21) | 100.0 ± 1.2 | 94.2 ± 1.2 | 100.0 ± 2.4 | 90.2 ± 2.4 ** | 25.5 ± 1.4 ** |
| Trans-p-coumaric acid (22) | 100.0 ± 0.8 | 98.4 ± 2.9 | 97.6 ± 3.4 | 99.6 ± 1.7 | 102.7 ± 2.1 |
| Trans-o-coumaric acid (24) | 100.0 ± 1.1 | 99.1 ± 0.7 | 97.4 ± 1.7 | 97.1 ± 1.5 | 96.2 ± 2.2 |
| Trans-m-coumaric acid (26) | 100.0 ± 1.1 | 99.2 ± 0.6 | 97.7 ± 2.1 | 97.6 ± 1.9 | 99.6 ± 1.9 |
| Trans-caffeic acid (28) | 100.0 ± 3.8 | 97.2 ± 1.9 | 105.1 ± 4.3 | 99.9 ± 3.3 | 89.5 ± 4.7 |
| Trans-ferulic acid (29) | 100.0 ± 3.4 | 95.0 ± 1.8 | 105.3 ± 4.5 | 101.9 ± 3.4 | 102.0 ± 2.6 |
| Trans-cinnamic acid (30) | 100.0 ± 4.7 | 103.4 ± 2.7 | 107.1 ± 1.3 | 100.0 ± 2.5 | 103.2 ± 5.6 |
| Vanillic acid (31) | 100.0 ± 2.4 | 97.2 ± 1.5 | 100.6 ± 0.7 | 99.3 ± 2.3 | 101.3 ± 1.4 |
| 0 mM | 0.125 mM | 0.25 mM | 0.5 mM | 1 mM | |
| Metformin | 100.0 ± 0.4 | 86.8 ± 1.5 ** | 75.6 ± 1.5 ** | 64.8 ± 1.0 ** | 61.1 ± 2.8 ** |
| Treatment | TG/Protein (% of Control) | ||||
|---|---|---|---|---|---|
| 0 µM | 3 µM | 10 µM | 30 µM | 100 µM | |
| Helichrysoside (1) | 100.0 ± 3.5 | 90.5 ± 0.6 | 82.9 ± 1.3* | 83.3 ± 4.6* | 62.1 ± 3.6** |
| Quercetin 3-O-(6′′-O-cis-p-coumaroyl)-Glc (2) | 100.0 ± 3.7 | 86.0 ± 0.8 * | 86.0 ± 3.2 * | 81.6 ± 1.4 ** | 74.6 ± 2.3 ** |
| Quercetin 3-O-(6′′-O-trans-p-methylcoumaroyl)-Glc (3) | 100.0 ± 2.2 | 90.3 ± 1.0 ** | 87.4 ± 0.9 ** | 83.3 ± 1.5 ** | 66.4 ± 1.4 ** |
| Quercetin 3-O-(6′′-O-trans-o-coumaroyl)-Glc (4) | 100.0 ± 2.3 | 93.3 ± 1.5 | 89.5 ± 2.6** | 79.6 ± 1.6** | 64.5 ± 1.8 ** |
| Quercetin 3-O-(6′′-O-trans-o-methylcoumaroyl)-Glc (5) | 100.0 ± 3.0 | 94.5 ± 2.7 | 93.4 ± 1.6 | 86.4 ± 1.2 ** | 64.6 ± 2.0 ** |
| Quercetin 3-O-(6′′-O-trans-m-coumaroyl)-Glc (6) | 100.0 ± 1.3 | 91.3 ± 3.6 | 87.5 ± 4.2 * | 83.7 ± 3.1 ** | 69.8 ± 3.2 ** |
| Quercetin 3-O-(6′′-O-trans-m-methylcoumaroyl)-Glc (7) | 100.0 ± 2.0 | 91.2 ± 1.9 * | 88.4 ± 2.0 ** | 83.8 ± 1.5 ** | 76.2 ± 1.5 ** |
| Quercetin 3-O-(6′′-O-trans-caffeoyl)-Glc (8) | 100.0 ± 13.8 | 91.1 ± 6.0 | 87.0 ± 5.2 | 84.2 ± 6.3 | 65.8 ± 5.4 ** |
| Quercetin 3-O-(6′′-O-cis-caffeoyl)-Glc (9) | 100.0 ± 2.9 | 95.6 ± 1.4 | 91.8 ± 2.0 | 87.4 ± 4.2 * | 87.4 ± 4.6 * |
| Quercetin 3-O-(6′′-O-trans-feruloyl)-Glc (10) | 100.0 ± 7.9 | 89.2 ± 3.9 | 94.0 ± 4.3 | 74.3 ± 5.8 ** | 75.8 ± 3.7 ** |
| Quercetin 3-O-(6′′-O-cis-feruloyl)-Glc (11) | 100.0 ± 10.6 | 86.8 ± 4.7 | 90.7 ± 2.2 | 82.4 ± 4.8 * | 77.3 ± 3.5 ** |
| Quercetin 3-O-(6′′-O-trans-cinnamoyl)-Glc (12) | 100.0 ± 3.4 | 91.4 ± 2.6 | 87.2 ± 2.1 * | 80.9 ± 2.1 ** | 68.7 ± 1.3 ** |
| Quercetin 3-O-(6′′-O-cis-cinnamoyl)-Glc (13) | 100.0 ± 2.1 | 93.2 ± 0.8 | 88.9 ± 1.8 ** | 89.9 ± 0.5 ** | 82.5 ± 1.0 ** |
| Quercetin 3-O-(6′′-O-vanilloyl)-Glc (14) | 100.0 ± 9.7 | 102.5 ± 7.4 | 75.0 ± 7.3 ** | 70.2 ± 5.2 ** | 60.8 ± 6.1 ** |
| Quercetin 3-O-(6′′-O-trimethylgalloyl)-Glc (15) | 100.0 ± 5.7 | 92.2 ± 2.0 | 93.1 ± 1.0 | 83.7 ± 1.3 ** | 81.7 ± 3.9 ** |
| Trans-tiliroside (16) | 100.0 ± 1.7 | 98.9 ± 2.9 | 96.2 ± 1.2 | 86.1 ± 3.1 ** | 72.8 ± 3.1 ** |
| Quercetin 3-O-Glc (17) | 100.0 ± 2.1 | 100.9 ± 2.6 | 100.5 ± 2.5 | 108.3 ± 1.2 | 108.0 ± 1.4 |
| Kaempferol 3-O-Glc (18) | 100.0 ± 3.2 | 98.6 ± 3.3 | 96.0 ± 2.3 | 88.8 ± 3.3 | 92.4 ± 5.1 |
| Rutin (19) | 100.0 ± 9.2 | 95.3 ± 4.0 | 91.8 ± 5.8 | 99.3 ± 2.7 | 94.9 ± 7.6 |
| Quercetin (20) | 100.0 ± 2.4 | 92.5 ± 1.1 | 92.1 ± 3.5 | 71.5 ± 3.3 ** | 63.2 ± 0.7 ** |
| Kaempferol (21) | 100.0 ± 2.4 | 103.4 ± 2.8 | 95.0 ± 4.6 | 82.8 ± 1.5 ** | 56.1 ± 3.6 ** |
| Trans-p-coumaric acid (22) | 100.0 ± 3.3 | 95.1 ± 4.5 | 99.8 ± 4.9 | 103.7 ± 3.3 | 118.6 ± 6.2 * |
| Trans-o-coumaric acid (24) | 100.0 ± 2.2 | 97.2 ± 0.9 | 93.8 ± 3.3 | 97.5 ± 4.1 | 102.4 ± 4.2 |
| Trans-m-coumaric acid (26) | 100.0 ± 2.3 | 93.7 ± 1.0 | 97.1 ± 3.4 | 92.1 ± 0.8 | 94.4 ± 1.7 |
| Trans-caffeic acid (28) | 100.0 ± 13.2 | 89.9 ± 6.6 | 89.6 ± 0.6 | 91.1 ± 2.6 | 97.6 ± 1.4 |
| Trans-ferulic acid (29) | 100.0 ± 11.2 | 88.6 ± 6.3 | 97.4 ± 3.1 | 100.5 ± 4.7 | 104.3 ± 6.6 |
| Trans-cinnamic acid (30) | 100.0 ± 3.0 | 102.8 ± 2.7 | 99.2 ± 7.0 | 98.5 ± 2.9 | 102.0 ± 5.3 |
| Vanillic acid (31) | 100.0 ± 9.2 | 92.9 ± 4.7 | 98.4 ± 2.8 | 95.1 ± 8.4 | 106.7 ± 2.5 |
| 0 µM | 0.125 mM | 0.25 mM | 0.5 mM | 1 mM | |
| Metformin | 100.0 ± 1.2 | 92.1 ± 1.4 * | 88.6 ± 1.5 ** | 87.8 ± 2.2 ** | 81.4 ± 1.4 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morikawa, T.; Nagatomo, A.; Oka, T.; Miki, Y.; Taira, N.; Shibano-Kitahara, M.; Hori, Y.; Muraoka, O.; Ninomiya, K. Glucose Tolerance-Improving Activity of Helichrysoside in Mice and Its Structural Requirements for Promoting Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2019, 20, 6322. https://doi.org/10.3390/ijms20246322
Morikawa T, Nagatomo A, Oka T, Miki Y, Taira N, Shibano-Kitahara M, Hori Y, Muraoka O, Ninomiya K. Glucose Tolerance-Improving Activity of Helichrysoside in Mice and Its Structural Requirements for Promoting Glucose and Lipid Metabolism. International Journal of Molecular Sciences. 2019; 20(24):6322. https://doi.org/10.3390/ijms20246322
Chicago/Turabian StyleMorikawa, Toshio, Akifumi Nagatomo, Takahiro Oka, Yoshinobu Miki, Norihisa Taira, Megumi Shibano-Kitahara, Yuichiro Hori, Osamu Muraoka, and Kiyofumi Ninomiya. 2019. "Glucose Tolerance-Improving Activity of Helichrysoside in Mice and Its Structural Requirements for Promoting Glucose and Lipid Metabolism" International Journal of Molecular Sciences 20, no. 24: 6322. https://doi.org/10.3390/ijms20246322
APA StyleMorikawa, T., Nagatomo, A., Oka, T., Miki, Y., Taira, N., Shibano-Kitahara, M., Hori, Y., Muraoka, O., & Ninomiya, K. (2019). Glucose Tolerance-Improving Activity of Helichrysoside in Mice and Its Structural Requirements for Promoting Glucose and Lipid Metabolism. International Journal of Molecular Sciences, 20(24), 6322. https://doi.org/10.3390/ijms20246322






