Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease
Abstract
1. Introduction
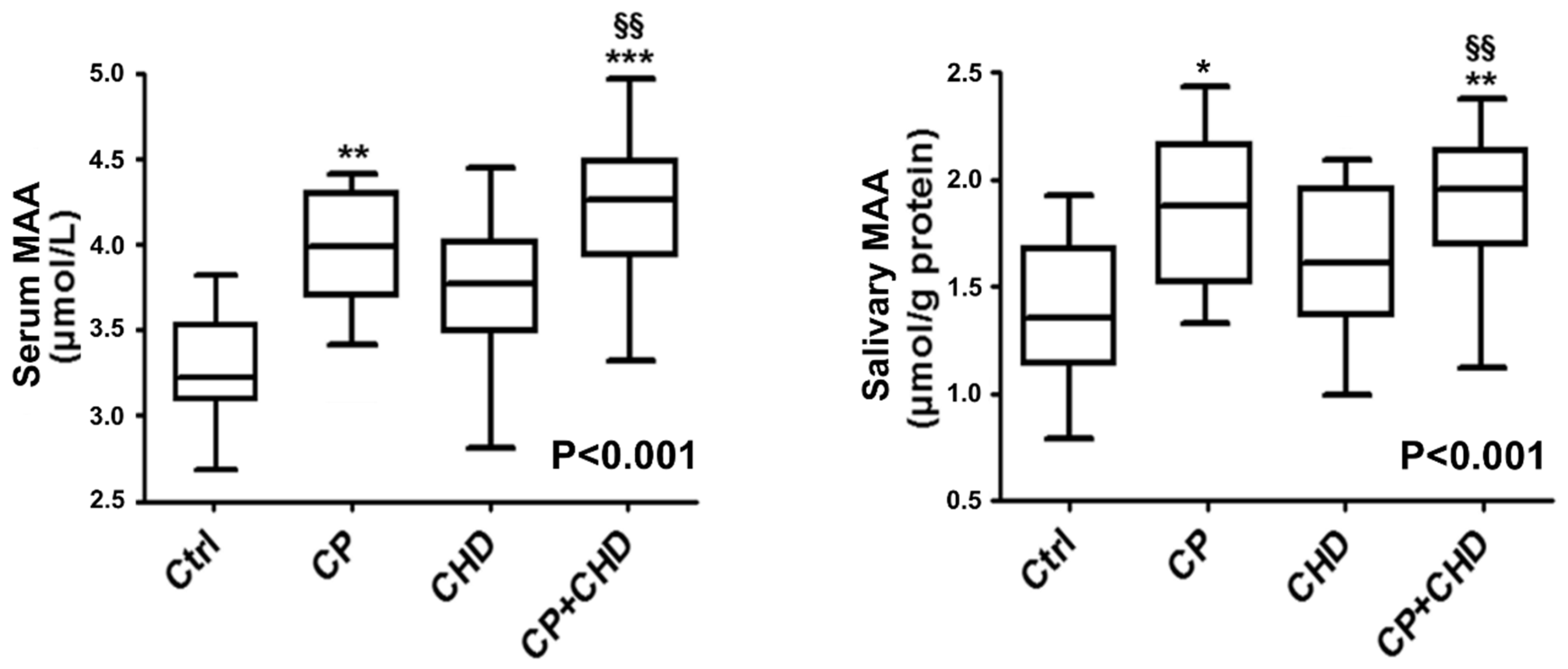
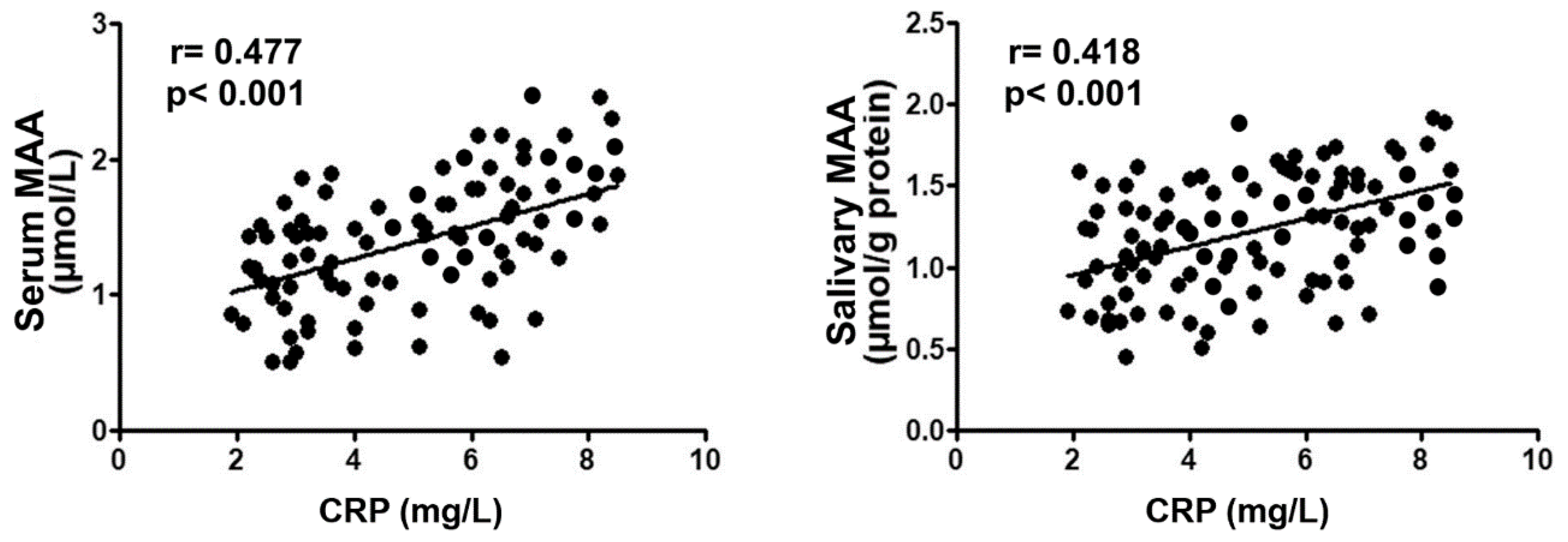
2. Results
Study Participant
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Serum and Salivary MAA Measures
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kornman, K.S.; Giannobile, W.V.; Duff, G.W. Quo vadis: What is the future of periodontics? How will we get there? Periodontol. 2000 2017, 75, 353–371. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabe, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Wei, L.; Thornton-Evans, G.O.; Borrell, L.N.; Borgnakke, W.S.; Dye, B.; Genco, R.J. Risk Indicators for Periodontitis in US Adults: NHANES 2009 to 2012. J. Periodontol. 2016, 87, 1174–1185. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: Periodontitis and atherosclerotic cardiovascular disease. J. Periodontol. 2009, 80, 1021–1032. [Google Scholar]
- Isola, G.; Matarese, G.; Ramaglia, L.; Pedullà, E.; Rapisarda, E.; Iorio-Siciliano, V. Association between periodontitis and glycosylated hemoglobin before diabetes onset: A cross-sectional study. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef] [PubMed]
- Holmlund, A.; Holm, G.; Lind, L. Number of teeth as a predictor of cardiovascular mortality in a cohort of 7,674 subjects followed for 12 years. J. Periodontol. 2010, 81, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Vedin, O.; Hagström, E.; Budaj, A.; Denchev, S.; Harrington, R.A.; Koenig, W.; Soffer, J.; Sritara, P.; Stebbins, A.; Stewart, R.H.; et al. Tooth loss is independently associated with poor outcomes in stable coronary heart disease. Eur. J. Prev. Cardiol. 2016, 23, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lv, Z.; Shi, Z.; Zhu, Y.; Wu, Y.; Li, L.; Iheozor-Ejiofor, Z. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst Rev. 2017, 11, CD009197. [Google Scholar] [CrossRef]
- Mercanoglu, F.; Oflaz, H.; Oz, O.; Gökbuget, A.Y.; Genchellac, H.; Sezer, M.; Nişanci, Y.; Umman, S. Endothelial dysfunction in patients with chronic periodontitis and its improvement after initial periodontal therapy. J. Periodontol. 2004, 75, 1694–1700. [Google Scholar] [CrossRef]
- Martinez-Herrera, M.; López-Domènech, S.; Silvestre, F.J.; Silvestre-Rangil, J.; Bañuls, C.; Victor, V.M.; Rocha, M. Chronic periodontitis impairs polymorphonuclear leucocyte-endothelium cell interactions and oxidative stress in humans. J. Clin. Periodontol. 2018, 45, 1429–1439. [Google Scholar] [CrossRef]
- Hampton, T.G.; Amende, I.; Fong, J.; Laubach, V.E.; Li, J.; Metais, C.; Simons, M. Basic FGF reduces stunning via a NOS2-dependent pathway in coronary perfused mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H260–H268. [Google Scholar] [CrossRef]
- Gistera, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Piancino, M.G.; Isola, G.; Cannavale, R.; Cutroneo, G.; Vermiglio, G.; Bracco, P.; Anastasi, G.P. From periodontal mechanoreceptors to chewing motor control: A systematic review. Arch. Oral Biol. 2017, 78, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.; Thiele, G.M.; Manavis, J.; Mikuls, T.R.; Payne, J.B.; Bartold, P.M. Gingival tissue, an extrasynovial source of malondialdehyde-acetaldehyde adducts, citrullinated and carbamylated proteins. J. Periodontal Res. 2018, 53, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, D.T.; Duryee, M.J.; Mikuls, T.R.; Thiele, G.M.; Anderson, D.R. Aldehyde-modified proteins as mediators of early inflammation in atherosclerotic disease. Free Radic Biol. Med. 2015, 89, 409–418. [Google Scholar] [CrossRef]
- Wang, C.; Turunen, S.P.; Kummu, O.; Veneskoski, M.; Lehtimäki, J.; Nissinen, A.E.; Hörkkö, S. Natural antibodies of newborns recognize oxidative stress-related malondialdehyde acetaldehyde adducts on apoptotic cells and atherosclerotic plaques. Int. Immunol. 2013, 25, 575–587. [Google Scholar] [CrossRef]
- Anderson, D.R.; Duryee, M.J.; Shurmur, S.W.; Um, J.Y.; Bussey, W.D.; Hunter, C.D.; Garvin, R.P.; Sayles, H.R.; Mikuls, T.R.; Klassen, L.W.; et al. Unique antibody responses to malondialdehyde-acetaldehyde (MAA)-protein adducts predict coronary artery disease. PLoS ONE 2014, 9, e107440. [Google Scholar] [CrossRef]
- Karvonen, J.; Päivänsalo, M.; Kesäniemi, Y.A.; Hörkkö, S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation 2003, 108, 2107–2112. [Google Scholar] [CrossRef]
- Liljestrand, J.M.; Paju, S.; Pietiäinen, M.; Buhlin, K.; Persson, G.R.; Nieminen, M.S.; Sinisalo, J.; Mäntylä, P.; Pussinen, P.J. Immunologic burden links periodontitis to acute coronary syndrome. Atherosclerosis 2018, 268, 177–184. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Hofmann, M.A.; Bucciarelli, L.; Jerud, A.P.; Tucker, S.; Lu, Y.; Papapanou, P.N.; Schmidt, A.M. Oral infection with a periodontal pathogen accelerates early atherosclerosis in apolipoprotein E-null mice. Arterioscler Thromb Vasc. Biol. 2003, 23, 1405–1411. [Google Scholar] [CrossRef]
- Turunen, S.P.; Kummu, O.; Harila, K.; Veneskoski, M.; Soliymani, R.; Baumann, M.; Pussinen, P.J.; Hörkkö, S. Recognition of Porphyromonas gingivalis gingipain epitopes by natural IgM binding to malondialdehyde modified low-density lipoprotein. PLoS ONE 2012, 7, e34910. [Google Scholar] [CrossRef][Green Version]
- Holtfreter, B.; Empen, K.; Glaser, S.; Gläser, S.; Lorbeer, R.; Völzke, H.; Ewert, R.; Kocher, T.; Dörr, M. Periodontitis is associated with endothelial dysfunction in a general population: A cross-sectional study. PLoS ONE 2013, 8, e84603. [Google Scholar] [CrossRef] [PubMed]
- Seinost, G.; Wimmer, G.; Skerget, M.; Thaller, E.; Brodmann, M.; Gasser, R.; Bratschko, R.O.; Pilger, E. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am. Heart J. 2005, 149, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; D’ Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of periodontitis and endothelial function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Kocher, T. Evaluation of salivary and serum ADMA levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2019, in press. [Google Scholar]
- Gurav, A.N. The implication of periodontitis in vascular endothelial dysfunction. Eur. J. Clin. Investig. 2014, 44, 1000–1009. [Google Scholar] [CrossRef]
- Yilmaz Avci, A.; Akkucuk, M.H.; Torun, E.; Arikan, S.; Can, U.; Tekindal, M.A. Migraine and subclinical atherosclerosis: Endothelial dysfunction biomarkers and carotid intima-media thickness: A case-control study. Neurol. Sci. 2019, 40, 703–711. [Google Scholar] [CrossRef]
- Kendall, H.K.; Marshall, R.I.; Bartold, P.M. Nitric oxide and tissue destruction. Oral Dis. 2001, 7, 2–10. [Google Scholar] [CrossRef]
- Aurer, A.; Aleksic, J.; Ivic-Kardum, M.; Aurer, J.; Culo, F. Nitric oxide synthesis is decreased in periodontitis. J. Clin. Periodontol. 2001, 28, 565–568. [Google Scholar] [CrossRef]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Tomofuji, T.; Azuma, T.; Kusano, H.; Sanbe, T.; Ekuni, D.; Tamaki, N.; Yamamoto, T.; Watanabe, T. Oxidative damage of periodontal tissue in the rat periodontitis model: Effects of a high-cholesterol diet. FEBS Lett. 2006, 580, 3601–3604. [Google Scholar] [CrossRef]
- Kyrklund, M.; Kummu, O.; Kankaanpää, J.; Akhi, R.; Nissinen, A.; Turunen, S.P.; Pussinen, P.; Wang, C.; Hörkkö, S. Immunization with gingipain A hemagglutinin domain of Porphyromonas gingivalis induces IgM antibodies binding to malondialdehyde-acetaldehyde modified low-density lipoprotein. PLoS ONE 2018, 13, e0191216. [Google Scholar] [CrossRef] [PubMed]
- Önder, C.; Kurgan, Ş.; Altıngöz, S.M.; Bağış, N.; Uyanık, M.; Serdar, M.A.; Kantarcı, A.; Günhan, M. Impact of non-surgical periodontal therapy on saliva and serum levels of markers of oxidative stress. Clin. Oral Investig. 2017, 21, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Pink, C.; Kocher, T.; Meisel, P.; Dörr, M.; Markus, M.R.; Jablonowski, L.; Grotevendt, A.; Nauck, M.; Holtfreter, B. Longitudinal effects of systemic inflammation markers on periodontitis. J. Clin. Periodontol. 2015, 42, 988–997. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.V.; Ambati, M.; Koduganti, R. Systemic lycopene as an adjunct to scaling and root planing in chronic periodontitis patients with type 2 diabetes mellitus. J. Int. Soc. Prev. Community Dent. 2015, 5, 25–31. [Google Scholar]
- Andrukhov, O.; Haririan, H.; Bertl, K.; Rausch, W.D.; Bantleon, H.P.; Moritz, A.; Rausch-Fan, X. Nitric oxide production, systemic inflammation and lipid metabolism in periodontitis patients: Possible gender aspect. J. Clin. Periodontol. 2013, 40, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Lo Giudice, A.; Barbato, E.; Cosentino, L.; Ferraro, C.M.; Leonardi, R. Alveolar bone changes after rapid maxillary expansion with tooth-born appliances: A systematic review. Eur. J. Orthod. 2018, 40, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Caccianiga, G.; Paiusco, A.; Perillo, L.; Nucera, R.; Pinsino, A.; Maddalone, M.; Cordasco, G.; Lo Giudice, A. Does low-level laser therapy enhance the efficiency of orthodontic dental alignment? Results from a randomized pilot study. Photomed Laser Surg. 2017, 35, 421–426. [Google Scholar] [CrossRef]
- Gullu, C.; Ozmeric, N.; Tokman, B.; Elgun, S.; Balos, K. Effectiveness of scaling and root planing versus modified Widman flap on nitric oxide synthase and arginase activity in patients with chronic periodontitis. J. Period. Res. 2005, 40, 168–175. [Google Scholar] [CrossRef]
- Ozer, L.; Elgun, S.; Ozdemir, B.; Pervane, B.; Ozmeric, N. Arginine-nitric oxide-polyamine metabolism in periodontal disease. J. Periodontol. 2011, 82, 320–328. [Google Scholar] [CrossRef]
- Bodis, S.; Haregewoin, A. Significantly reduced salivary nitric oxide levels in smokers. Ann. Oncol. 1994, 5, 371–372. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Ramaglia, L.; Cicciù, M.; Matarese, G. Evaluation of the efficacy of celecoxib and ibuprofen on postoperative pain, swelling, and mouth opening after surgical removal of impacted third molars: A randomized, controlled clinical trial. Int. J. Oral Maxillofac Surg. 2019, 48, 1348–1354. [Google Scholar] [CrossRef] [PubMed]
- Patini, R.; Staderini, E.; Gallenzi, P. Multidisciplinary surgical management of Cowden syndrome: Report of a case. J. Clin. Exp. Dent. 2016, 8, e472–e474. [Google Scholar] [CrossRef] [PubMed]
- Amar, S.; Gokce, N.; Morgan, S.; Loukideli, M.; van Dyke, T.E.; Vita, J.A. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc. Biol. 2003, 23, 1245–1249. [Google Scholar] [CrossRef] [PubMed]
- Facciolo, M.T.; Riva, F.; Gallenzi, P.; Patini, R.; Gaglioti, D. A rare case of oral multisystem Langerhans cell histiocytosis. J. Clin. Exp. Dent. 2017, 9, e820–e824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staderini, E.; Patini, R.; De Luca, M.; Gallenzi, P. Three-dimensional stereophotogrammetric analysis of nasolabial soft tissue effects of rapid maxillary expansion: A systematic review of clinical trials. Acta Otorhinolaryngol. Ital. 2018, 38, 399–408. [Google Scholar] [PubMed]
- Higashi, Y.; Goto, C.; Jitsuiki, D.; Umemura, T.; Nishioka, K.; Hidaka, T.; Takemoto, H.; Nakamura, S.; Soga, J.; Chayama, K.; et al. Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension 2008, 51, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Periodontol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, M.; Ramaglia, L.; Iorio-Siciliano, V.; Cordasco, G.; Matarese, G. Efficacy of a drug composed of herbal extracts on postoperative discomfort after surgical removal of impacted mandibular third molar: A randomized, triple-blind, controlled clinical trial. Clin. Oral Investig. 2019, 23, 2443–2453. [Google Scholar] [CrossRef]
- Bassand, J.P.; Hamm, C.W.; Ardissino, D.; Boersma, E.; Budaj, A.; Fernandez-Aviles, F.; Fox, K.A.; Hasdai, D.; Ohman, E.M.; Wallentin, L.; et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes: The Task Force for the Diagnosis and Treatment of Non-ST-Segment Elevation Acute Coronary Syndromes of the European Society of Cardiology. Eur. Heart J. 2007, 28, 1598–1660. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, G.; Alibrandi, A.; Dalessandri, D.; Migliorati, M.; Pedullà, E.; Rapisarda, E. Comparison of Effectiveness of Etoricoxib and Diclofenac on Pain and Perioperative Sequelae After Surgical Avulsion of Mandibular Third Molars: A Randomized, Controlled, Clinical Trial. Clin. J. Pain. 2019, 35, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Akhi, R.; Wang, C.; Kyrklund, M.; Kummu, O.; Turunen, S.P.; Hyvärinen, K.; Kullaa, A.; Salo, T.; Pussinen, P.J.; Hörkkö, S. Cross-reactive saliva IgA antibodies to oxidized LDL and periodontal pathogens in humans. J. Clin. Periodontol. 2017, 44, 682–691. [Google Scholar] [CrossRef] [PubMed]
| Clinical Features | Controls (n = 32) | Periodontitis (n = 34) | CHD (n = 33) | Periodontitis + CHD (n = 34) |
|---|---|---|---|---|
| Age (years) | 58 (54; 60) | 57 (52; 59) | 55 (49; 58) | 56 (50; 59) |
| Gender (male/female) | 16/16 | 16/18 | 17/15 | 17/17 |
| Education level | ||||
| Primary school, n (%) | 11 (34.3) | 12 (35.3) | 11 (33.3) | 13 (38.3) |
| High school, n (%) | 13 (40.6) | 14 (41.2) | 15 (45.5) | 13 (38.3) |
| College/university, n (%) | 8 (25) | 8 (23.5) | 7 (21.2) | 8 (23.5) |
| Body mass index (kg/m2) | 27.2 (25.6; 28.7) | 24.5 (23.4; 28.4) | 26.4 (23.2; 27.8) | 22.8 (20.6; 26.3) |
| Fasting glucose (mg/dL) | 89.1 (86.9; 91.8) | 89.6 (81.8; 129.3) | 89.1 (86.9; 132.4) | 91.3 (87.8; 128.6) |
| Current smokers, n (%) | 2 (6.2) | 3 (8.8) | 3 (9) | 7 (8.8) |
| Comorbidities | ||||
| Diabetes, n (%) | - | 4 (11.8) ** | 5 (15.1) ** | 4 (11.8) ** |
| Previous CVD | ||||
| Atrial fibrillation, n (%) | - | - | 6 (18.1)**,§§ | 10 (29.4) **,§§ |
| Angina pectoris, n (%) | - | - | 17 (51.5)**,§§ | 18 (53) **,§§ |
| Stroke, n (%) | - | - | 9 (27.3) **,§§ | 11 (32.3) **,§§ |
| Heart failure, n (%) | - | - | 10 (30.3) **,§§ | 11 (32.3) **,§§ |
| Drug treatment of CVD | ||||
| Antihypertensive, n (%) | - | - | 14 (42.4) **,§§ | 15 (44.1) **,§§ |
| Statins, n (%) | - | - | 13 (39.4) **,§§ | 13 (38.2) **,§§ |
| Low-dose aspirin, n (%) | - | - | 12 (36.4) **,§§ | 12 (35.3) **,§§ |
| Beta blockers, n (%) | - | - | 13 (39.4) **,§§ | 14 (41.2) **,§§ |
| hs-CRP (mg/L) | 2.7 (2.3; 3.0) | 3.5 (3.1; 4.1) * | 6.1 (5.4; 6.3) ** | 6.8 (6.1; 8) **,§§,# |
| Total cholesterol (mg/dL) | 161 (125; 186) | 171 (144; 197) | 177 (153; 198) | 174 (155; 201) |
| Triglycerids (mg/dL) | 137 (107; 145) | 114 (59; 122) | 141 (112; 168) | 143 (111; 159) |
| Periodontal Parameters | Controls (n = 32) | Periodontitis (n = 34) | CHD (n = 33) | Periodontitis + CHD (n = 34) |
|---|---|---|---|---|
| N of teeth | 25 (24; 27) | 17 (15; 19) ** | 22 (21; 24) **,§§ | 18 (15; 19) **,## |
| CAL (mm) | 1.2 (1; 1.3) | 3.8 (3.2; 4.2) ** | 2.3 (2.2; 2.6) **,§§ | 3.8 (3.7; 4.6) **,## |
| CAL 4–5 mm (% sites) | - | 39.1 (37.3; 41.8) ** | - | 42.2 (37.4; 49.1) **,## |
| CAL ≥6 mm (% sites) | - | 18.2 (18.7; 21.4) ** | - | 18.8 (16.4; 24.5) **,## |
| PD (mm) | 1.6 (1.2; 2.1) | 4.3 (4.1; 4.8) ** | 2.1 (1.7; 2.3) **,§§ | 4.1 (4.0; 4.8) **,## |
| PD 4–5 mm (% sites) | - | 41.8 (40.7; 44.6) ** | - | 43.8 (40.4; 55.7) **,## |
| PD ≥6 mm (% sites) | - | 21.5 (18.9; 23.6) ** | - | 25.3 (21.4; 27.5) **,§§,## |
| BOP (%) | 9.9 (8.8; 11.4) | 45.9 (45.7; 51.8) ** | 11.5 (14.5; 18.6) **,§§ | 47.5 (45.6; 55.2) **,§§,## |
| PI (%) | 10.1 (9.6; 11.2) | 35.6 (33.5; 36.2) ** | 15.2 (14.5; 16.7) **,§§ | 33.5 (32.5; 32.9) **,## |
| Univariate | Multivariate | ||||||
|---|---|---|---|---|---|---|---|
| Variable | B | 95% CI | p | B | 95% CI | p | |
| Serum MAA Levels | CHD | 0.384 | 0.221; 0.569 | <0.001 | 0.075 | −0.356; 0.531 | 0.665 |
| Periodontitis | 0.221 | 0.015; 0.412 | 0.041 | 0.161 | −0.039; 0.355 | 0.133 | |
| Hs-CRP | 0.115 | 0.071; 0.156 | <0.001 | 0.133 | 0.056; 0.178 | <0.001 | |
| Age (years) | −0.022 | −0.35; 0.004 | 0.088 | −0.031 | −0.208; 0.204 | 0.416 | |
| Female gender | 0.156 | −0.035; 0.358 | 0.156 | 0.145 | −0.121; 0.356 | 0.065 | |
| Education SES | −0.071 | −0.202; 0.054 | 0.289 | −0.075 | −0.156; 0.31 | 0.132 | |
| Salivary MAA Levels | CHD | 0.281 | 0.134; 0.431 | <0.001 | −0.051 | −0.431; 0.356 | 0.769 |
| Periodontitis | 0.073 | −0.089; 0.232 | 0.456 | 0.004 | −0.145; 0.181 | 0.782 | |
| Hs-CRP | 0.089 | 0.043; 0.131 | <0.001 | 0.096 | 0.041; 0.156 | <0.001 | |
| Age (years) | −0.012 | −0.021; 0.007 | 0.346 | 0.004 | −0.012; 0.023 | 0.754 | |
| Female gender | 0.047 | −0.131; 0.223 | 0.462 | 0.089 | −0.089; 0.312 | 0.278 | |
| Education SES | −0.095 | −0.183; −0.004 | 0.048 | −0.89 | −0.056; 0.332 | 0.039 | |
| Serum MAA | 0.166 | −0.031; 0.338 | 0.079 | −0.045 | −0.234; 0.136 | 0.599 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Ferlito, S. Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. Int. J. Mol. Sci. 2019, 20, 6061. https://doi.org/10.3390/ijms20236061
Isola G, Polizzi A, Santonocito S, Alibrandi A, Ferlito S. Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. International Journal of Molecular Sciences. 2019; 20(23):6061. https://doi.org/10.3390/ijms20236061
Chicago/Turabian StyleIsola, Gaetano, Alessandro Polizzi, Simona Santonocito, Angela Alibrandi, and Sebastiano Ferlito. 2019. "Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease" International Journal of Molecular Sciences 20, no. 23: 6061. https://doi.org/10.3390/ijms20236061
APA StyleIsola, G., Polizzi, A., Santonocito, S., Alibrandi, A., & Ferlito, S. (2019). Expression of Salivary and Serum Malondialdehyde and Lipid Profile of Patients with Periodontitis and Coronary Heart Disease. International Journal of Molecular Sciences, 20(23), 6061. https://doi.org/10.3390/ijms20236061






