NLRP7 Is Involved in the Differentiation of the Decidual Macrophages
Abstract
:1. Introduction
2. Results
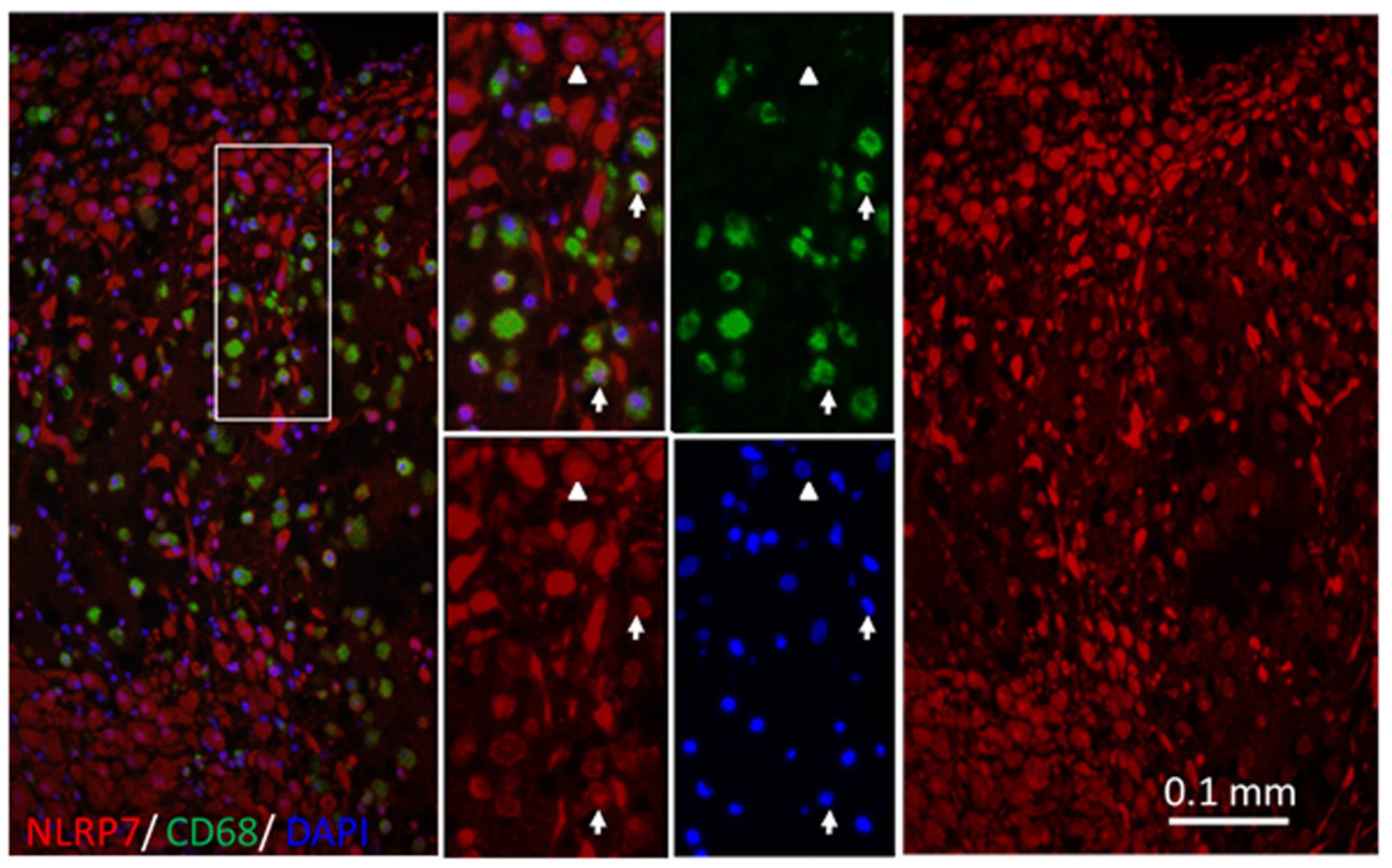
2.1. NLRP7 Expressed in Decidual Macrophages of the First-Trimester Pregnancy
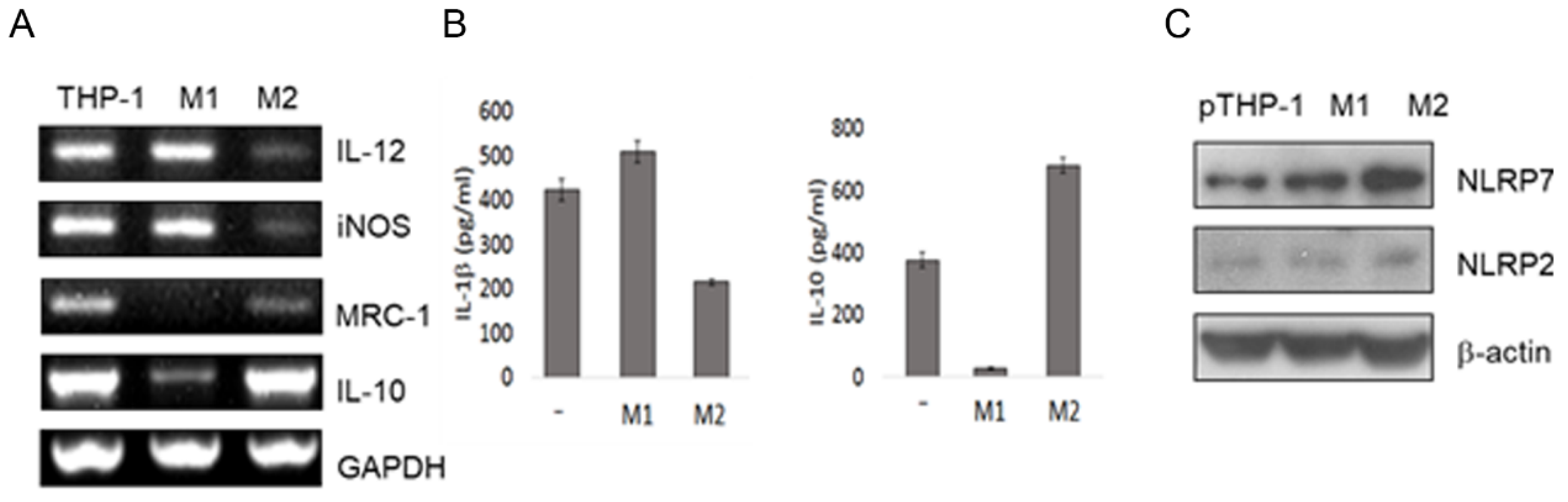
2.2. Macrophage Differentiation Increases NLRP7 Expression
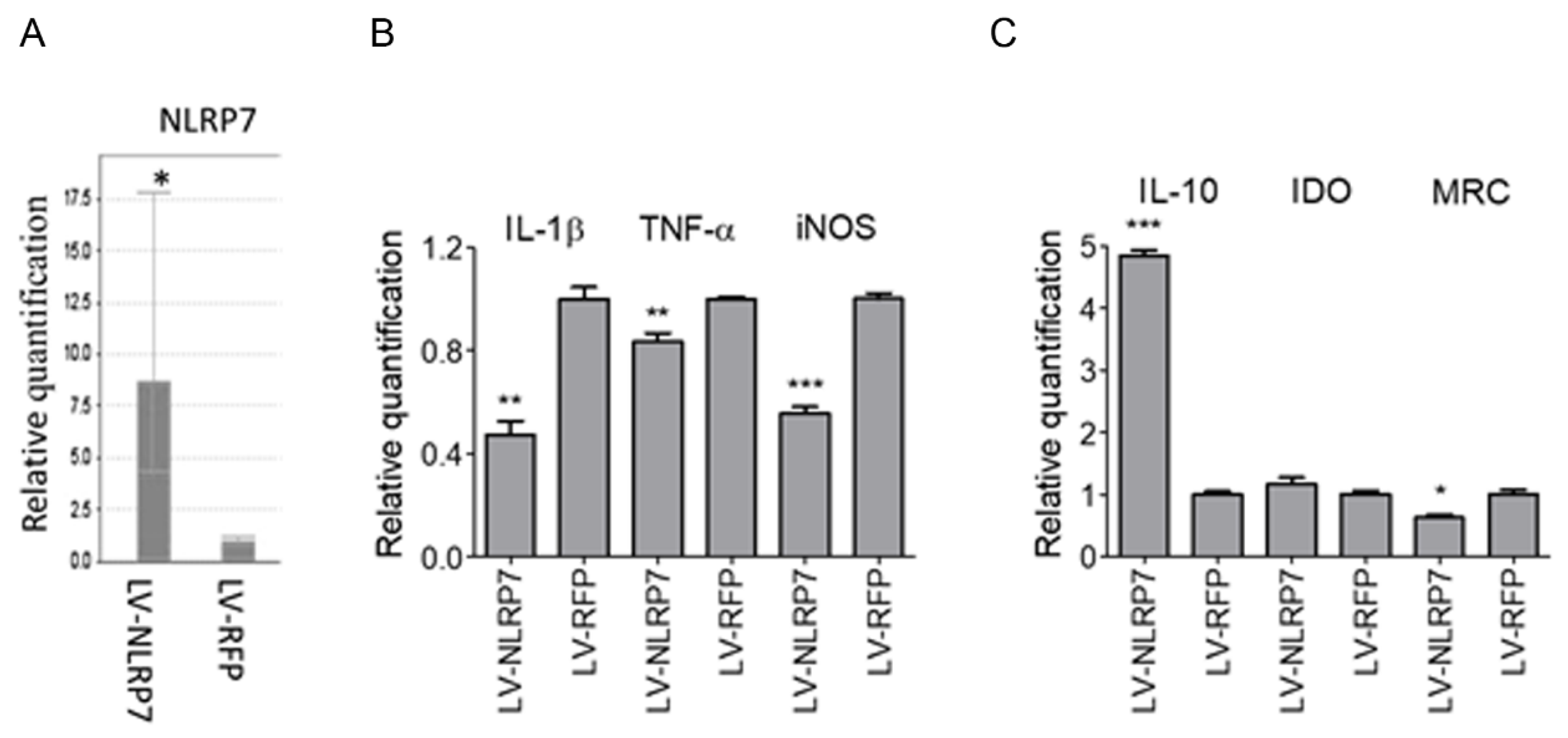
2.3. Ectopic Expression of NLRP7 in Macrophage Results in M2 Polarization
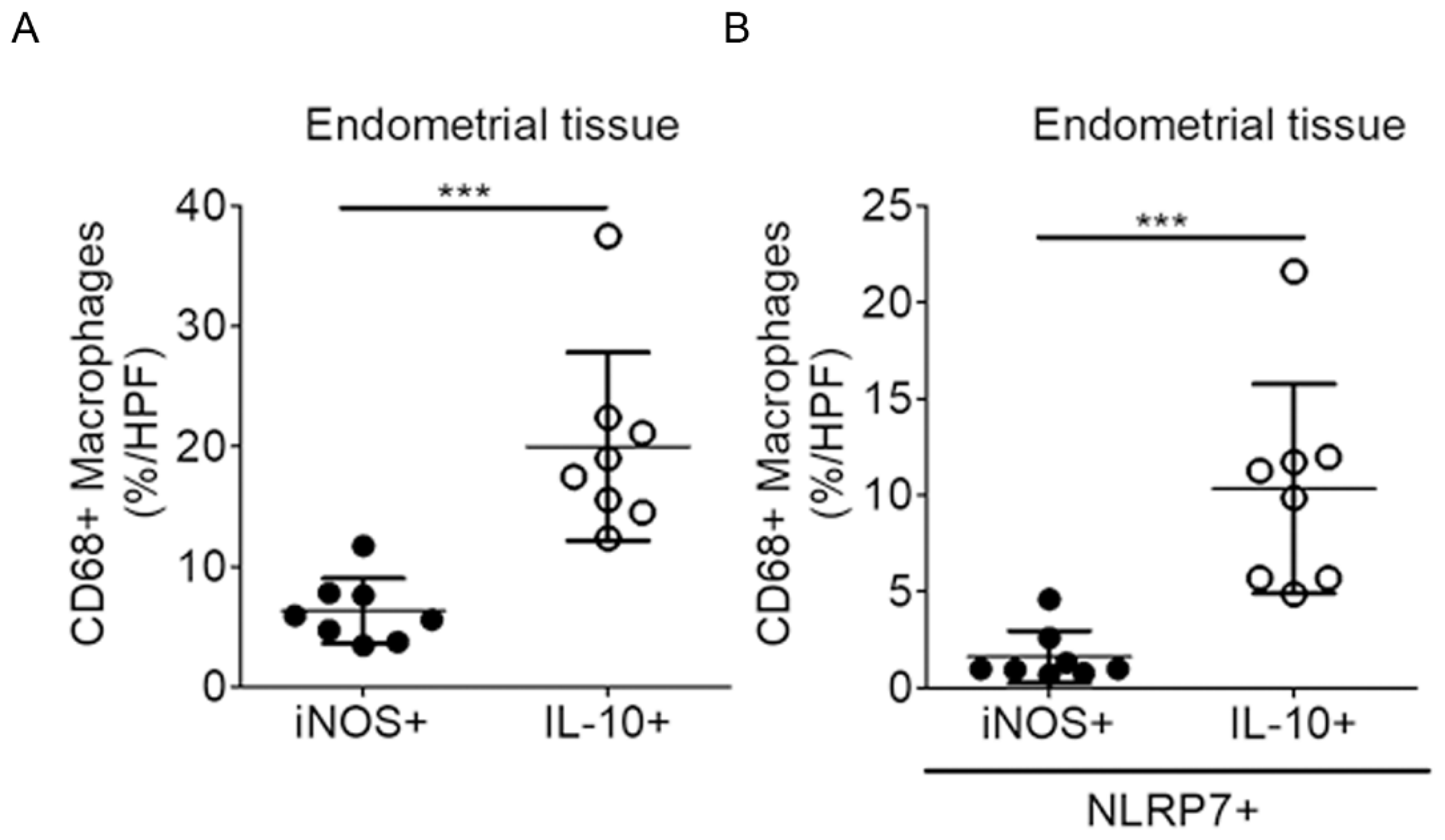
2.4. NLRP7 Are More Prevalent in the Decidual M2 Macrophages in the Human Endometrium
3. Discussion
4. Materials and Methods
4.1. Tissue Samples and Cells
4.2. Reagents
4.3. Lentivirus Preparation and Infection
4.4. M1 and M2 Macrophage Differentiation of THP-1 Cells
4.5. ELISA
4.6. RT-PCR and Quantitative Real-Time PCR
4.7. Western Blotting
4.8. Immunofluorescence Staining
4.9. Statistics
Reference
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Erlebacher, A. Immunology of the maternal-fetal interface. Annu. Rev. Immunol. 2013, 31, 387–411. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. Macrophage arginine metabolism to ornithine/urea or nitric oxide/citrulline: A life or death issue. Crit. Rev. Immunol. 2001, 21, 399–425. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Kincaid, K.; Alt, J.M.; Heilman, M.J.; Hill, A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000, 164, 6166–6173. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Schonkeren, D.; van der Hoorn, M.L.; Khedoe, P.; Swings, G.; van Beelen, E.; Claas, F.; van Kooten, C.; de Heer, E.; Scherjon, S. Differential distribution and phenotype of decidual macrophages in preeclamptic versus control pregnancies. Am. J. Pathol. 2011, 178, 709–717. [Google Scholar] [CrossRef]
- Prins, J.R.; Faas, M.M.; Melgert, B.N.; Huitema, S.; Timmer, A.; Hylkema, M.N.; Erwich, J.J. Altered expression of immune-associated genes in first-trimester human decidua of pregnancies later complicated with hypertension or foetal growth restriction. Placenta 2012, 33, 453–455. [Google Scholar] [CrossRef]
- Guenther, S.; Vrekoussis, T.; Heublein, S.; Bayer, B.; Anz, D.; Knabl, J.; Navrozoglou, I.; Dian, D.; Friese, K.; Makrigiannakis, A.; et al. Decidual macrophages are significantly increased in spontaneous miscarriages and over-express FasL: A potential role for macrophages in trophoblast apoptosis. Int. J. Mol. Sci. 2012, 13, 9069–9080. [Google Scholar] [CrossRef]
- Diamond, A.K.; Sweet, L.M.; Oppenheimer, K.H.; Bradley, D.F.; Phillippe, M. Modulation of monocyte chemotactic protein-1 expression during lipopolysaccharide-induced preterm delivery in the pregnant mouse. Reprod. Sci. 2007, 14, 548–559. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Q.; Liu, L.; Xu, X.; Chen, H.; Wang, H.; Kong, L.; Wang, W.; Zhang, A.; Cai, Y.; et al. Trophoblast apoptosis through polarization of macrophages induced by Chinese Toxoplasma gondii isolates with different virulence in pregnant mice. Parasitol. Res. 2013, 112, 3019–3027. [Google Scholar] [CrossRef]
- Tsao, F.Y.; Wu, M.Y.; Chang, Y.L.; Wu, C.T.; Ho, H.N. M1 macrophages decrease in the deciduae from normal pregnancies but not from spontaneous abortions or unexplained recurrent spontaneous abortions. J. Formos. Med. Assoc. 2018, 117, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.B.; von Chamier, M.; Allam, A.B.; Reyes, L. M1/M2 macrophage polarity in normal and complicated pregnancy. Front. Immunol. 2014, 5, 606. [Google Scholar] [CrossRef] [PubMed]
- Ting, J.P.; Willingham, S.B.; Bergstralh, D.T. NLRs at the intersection of cell death and immunity. Nat. Rev. Immunol. 2008, 8, 372–379. [Google Scholar] [CrossRef]
- Khare, S.; Dorfleutner, A.; Bryan, N.B.; Yun, C.; Radian, A.D.; de Almeida, L.; Rojanasakul, Y.; Stehlik, C. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 2012, 36, 464–476. [Google Scholar] [CrossRef]
- Zhou, Y.; Shah, S.Z.; Yang, L.; Zhang, Z.; Zhou, X.; Zhao, D. Virulent Mycobacterium bovis Beijing Strain Activates the NLRP7 Inflammasome in THP-1 Macrophages. PLoS ONE 2016, 11, e0152853. [Google Scholar] [CrossRef]
- Messaed, C.; Akoury, E.; Djuric, U.; Zeng, J.; Saleh, M.; Gilbert, L.; Seoud, M.; Qureshi, S.; Slim, R. NLRP7, a nucleotide oligomerization domain-like receptor protein, is required for normal cytokine secretion and co-localizes with Golgi and the microtubule-organizing center. J. Biol. Chem. 2011, 286, 43313–43323. [Google Scholar] [CrossRef]
- Huang, J.Y.; Yu, P.H.; Li, Y.C.; Kuo, P.L. NLRP7 contributes to in vitro decidualization of endometrial stromal cells. Reprod. Biol. Endocrinol. 2017, 15, 66. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Kinoshita, T.; Wang, Y.; Hasegawa, M.; Imamura, R.; Suda, T. PYPAF3, a PYRIN-containing APAF-1-like protein, is a feedback regulator of caspase-1-dependent interleukin-1beta secretion. J. Biol. Chem. 2005, 280, 21720–21725. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Yamabe, M.; Yamaguchi, Y.; Kobayashi, Y.; Konno, T.; Tada, K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int. J. Cancer 1980, 26, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Slim, R.; Wallace, E.P. NLRP7 and the Genetics of Hydatidiform Moles: Recent Advances and New Challenges. Front. Immunol. 2013, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.B.; Sharma, S. Interleukin-10: a pleiotropic regulator in pregnancy. Am. J. Reprod. Immunol. 2015, 73, 487–500. [Google Scholar] [CrossRef]
- Sharma, S.; Godbole, G.; Modi, D. Decidual Control of Trophoblast Invasion. Am. J. Reprod. Immunol. 2016, 75, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Amoushahi, M.; Sunde, L.; Lykke-Hartmann, K. The pivotal roles of the NOD-like receptors with a PYD domain, NLRPs, in oocytes and early embryo developmentdagger. Biol. Reprod. 2019, 101, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Duenez-Guzman, E.A.; Haig, D. The evolution of reproduction-related NLRP genes. J. Mol. Evol. 2014, 78, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Radian, A.D.; de Almeida, L.; Dorfleutner, A.; Stehlik, C. NLRP7 and related inflammasome activating pattern recognition receptors and their function in host defense and disease. Microbes Infect. 2013, 15, 630–639. [Google Scholar] [CrossRef]
- Radian, A.D.; Khare, S.; Chu, L.H.; Dorfleutner, A.; Stehlik, C. ATP binding by NLRP7 is required for inflammasome activation in response to bacterial lipopeptides. Mol. Immunol. 2015, 67, 294–302. [Google Scholar] [CrossRef]
- Murdoch, S.; Djuric, U.; Mazhar, B.; Seoud, M.; Khan, R.; Kuick, R.; Bagga, R.; Kircheisen, R.; Ao, A.; Ratti, B.; et al. Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nat. Genet. 2006, 38, 300–302. [Google Scholar] [CrossRef]
- Messaed, C.; Chebaro, W.; Di Roberto, R.B.; Rittore, C.; Cheung, A.; Arseneau, J.; Schneider, A.; Chen, M.F.; Bernishke, K.; Surti, U.; et al. NLRP7 in the spectrum of reproductive wastage: Rare non-synonymous variants confer genetic susceptibility to recurrent reproductive wastage. J. Med. Genet. 2011, 48, 540–548. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Zhang, L.; Reddy, R.; Dery, C.; Arseneau, J.; Cheung, A.; Surti, U.; Hoffner, L.; Seoud, M.; Zaatari, G.; et al. Comprehensive genotype-phenotype correlations between NLRP7 mutations and the balance between embryonic tissue differentiation and trophoblastic proliferation. J. Med. Genet. 2014, 51, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Delgado, M.; Martin-Trujillo, A.; Tayama, C.; Vidal, E.; Esteller, M.; Iglesias-Platas, I.; Deo, N.; Barney, O.; Maclean, K.; Hata, K.; et al. Absence of Maternal Methylation in Biparental Hydatidiform Moles from Women with NLRP7 Maternal-Effect Mutations Reveals Widespread Placenta-Specific Imprinting. PLoS Genet. 2015, 11, e1005644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soellner, L.; Kopp, K.M.; Mutze, S.; Meyer, R.; Begemann, M.; Rudnik, S.; Rath, W.; Eggermann, T.; Zerres, K. NLRP genes and their role in preeclampsia and multi-locus imprinting disorders. J. Perinat. Med. 2018, 46, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Begemann, M.; Rezwan, F.I.; Beygo, J.; Docherty, L.E.; Kolarova, J.; Schroeder, C.; Buiting, K.; Chokkalingam, K.; Degenhardt, F.; Wakeling, E.L.; et al. Maternal variants in NLRP and other maternal effect proteins are associated with multilocus imprinting disturbance in offspring. J. Med. Genet. 2018, 55, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Akoury, E.; Zhang, L.; Ao, A.; Slim, R. NLRP7 and KHDC3L, the two maternal-effect proteins responsible for recurrent hydatidiform moles, co-localize to the oocyte cytoskeleton. Hum. Reprod. 2015, 30, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Mahadevan, S.; Wen, S.; Wan, Y.W.; Peng, H.H.; Otta, S.; Liu, Z.; Iacovino, M.; Mahen, E.M.; Kyba, M.; Sadikovic, B.; et al. NLRP7 affects trophoblast lineage differentiation, binds to overexpressed YY1 and alters CpG methylation. Hum. Mol. Genet. 2014, 23, 706–716. [Google Scholar] [CrossRef] [Green Version]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef] [Green Version]
- Lombardelli, L.; Aguerre-Girr, M.; Logiodice, F.; Kullolli, O.; Casart, Y.; Polgar, B.; Berrebi, A.; Romagnani, S.; Maggi, E.; Le Bouteiller, P.; et al. HLA-G5 induces IL-4 secretion critical for successful pregnancy through differential expression of ILT2 receptor on decidual CD4(+) T cells and macrophages. J. Immunol. 2013, 191, 3651–3662. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.L.; Guo, Y.; So, K.H.; Vijayan, M.; Guo, Y.; Wong, V.H.; Yao, Y.; Lee, K.F.; Chiu, P.C.; Yeung, W.S. Soluble human leukocyte antigen G5 polarizes differentiation of macrophages toward a decidual macrophage-like phenotype. Hum. Reprod. 2015, 30, 2263–2274. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Piao, L.; Chen, C.P.; Wu, X.; Yeh, C.C.; Masch, R.; Chang, C.C.; Huang, S.J. Modulation of Decidual Macrophage Polarization by Macrophage Colony-Stimulating Factor Derived from First-Trimester Decidual Cells: Implication in Preeclampsia. Am. J. Pathol. 2016, 186, 1258–1266. [Google Scholar] [CrossRef] [Green Version]
- Sheng, Y.R.; Hu, W.T.; Wei, C.Y.; Tang, L.L.; Liu, Y.K.; Liu, Y.Y.; Qiu, J.P.; Li, D.J.; Zhu, X.Y. IL-33/ST2 axis affects the polarization and efferocytosis of decidual macrophages in early pregnancy. Am. J. Reprod. Immunol. 2018, 79, e12836. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Zhou, W.J.; Hou, X.X.; Fu, Q.; Li, D.J. Trophoblast-derived CXCL16 induces M2 macrophage polarization that in turn inactivates NK cells at the maternal-fetal interface. Cell. Mol. Immunol. 2018, 15, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Lindau, R.; Mehta, R.B.; Lash, G.E.; Papapavlou, G.; Boij, R.; Berg, G.; Jenmalm, M.C.; Ernerudh, J.; Svensson-Arvelund, J. Interleukin-34 is present at the fetal-maternal interface and induces immunoregulatory macrophages of a decidual phenotype in vitro. Hum. Reprod. 2018, 33, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef] [PubMed]
- Svensson-Arvelund, J.; Ernerudh, J. The Role of Macrophages in Promoting and Maintaining Homeostasis at the Fetal-Maternal Interface. Am. J. Reprod. Immunol. 2015, 74, 100–109. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, P.-Y.; Chen, K.-R.; Li, Y.-C.; Kuo, P.-L. NLRP7 Is Involved in the Differentiation of the Decidual Macrophages. Int. J. Mol. Sci. 2019, 20, 5994. https://doi.org/10.3390/ijms20235994
Tsai P-Y, Chen K-R, Li Y-C, Kuo P-L. NLRP7 Is Involved in the Differentiation of the Decidual Macrophages. International Journal of Molecular Sciences. 2019; 20(23):5994. https://doi.org/10.3390/ijms20235994
Chicago/Turabian StyleTsai, Pei-Yin, Kuan-Ru Chen, Yueh-Chun Li, and Pao-Lin Kuo. 2019. "NLRP7 Is Involved in the Differentiation of the Decidual Macrophages" International Journal of Molecular Sciences 20, no. 23: 5994. https://doi.org/10.3390/ijms20235994
APA StyleTsai, P.-Y., Chen, K.-R., Li, Y.-C., & Kuo, P.-L. (2019). NLRP7 Is Involved in the Differentiation of the Decidual Macrophages. International Journal of Molecular Sciences, 20(23), 5994. https://doi.org/10.3390/ijms20235994




