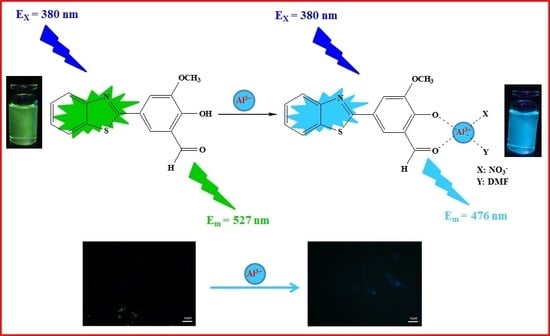
A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+ and Its Application in Water Samples and Cell Imaging
Abstract
:1. Introduction
2. Results and Discussion
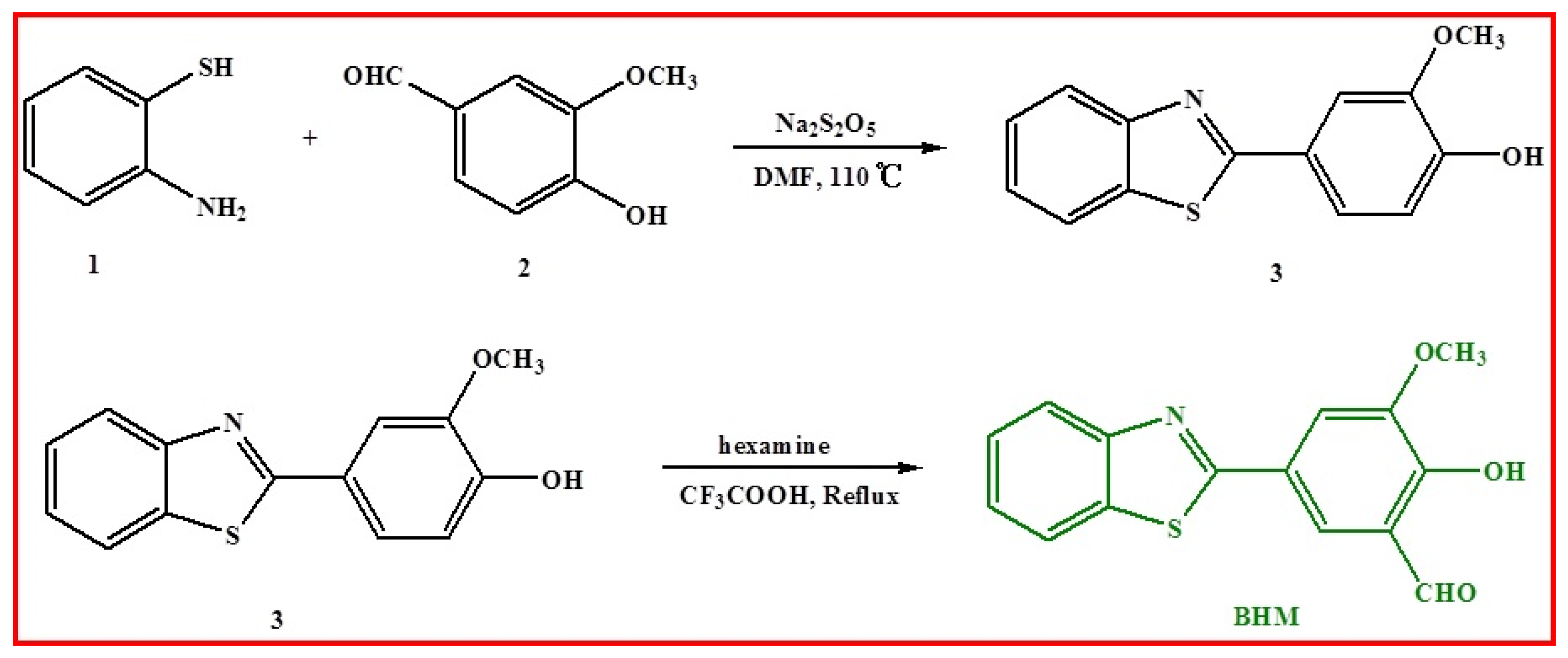
2.1. Synthesis of BHM
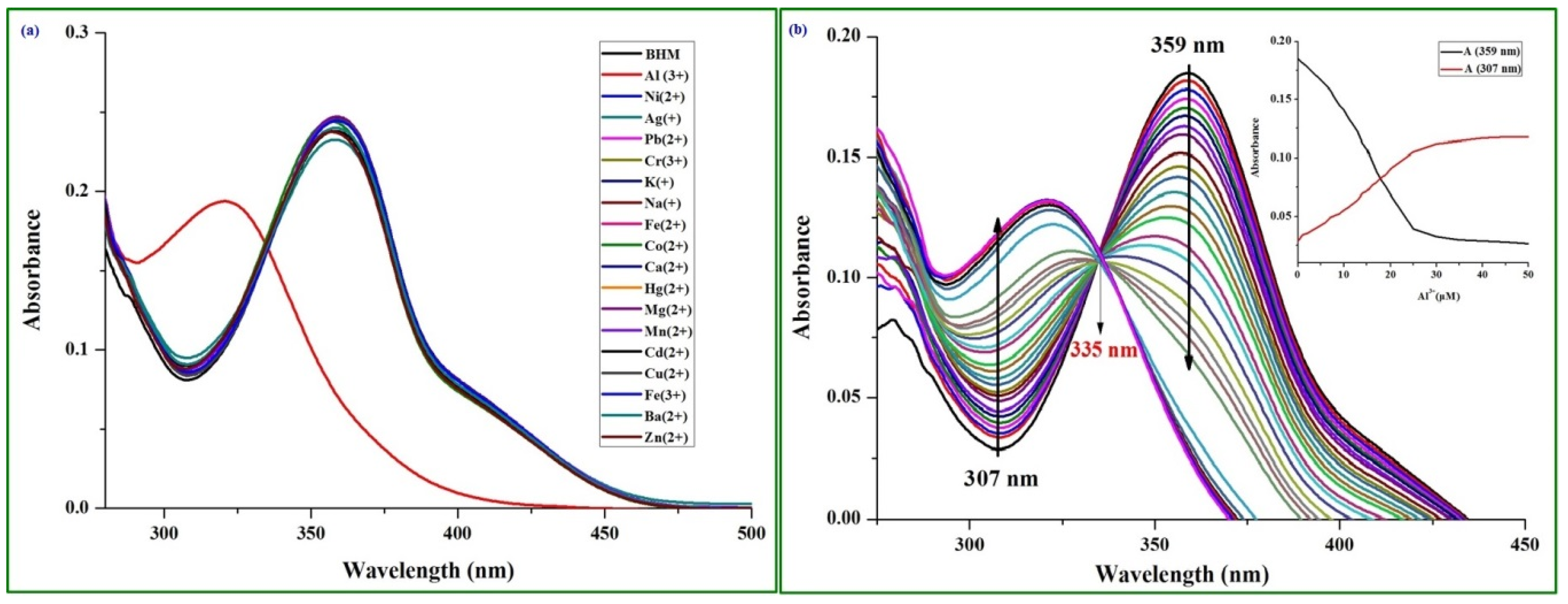
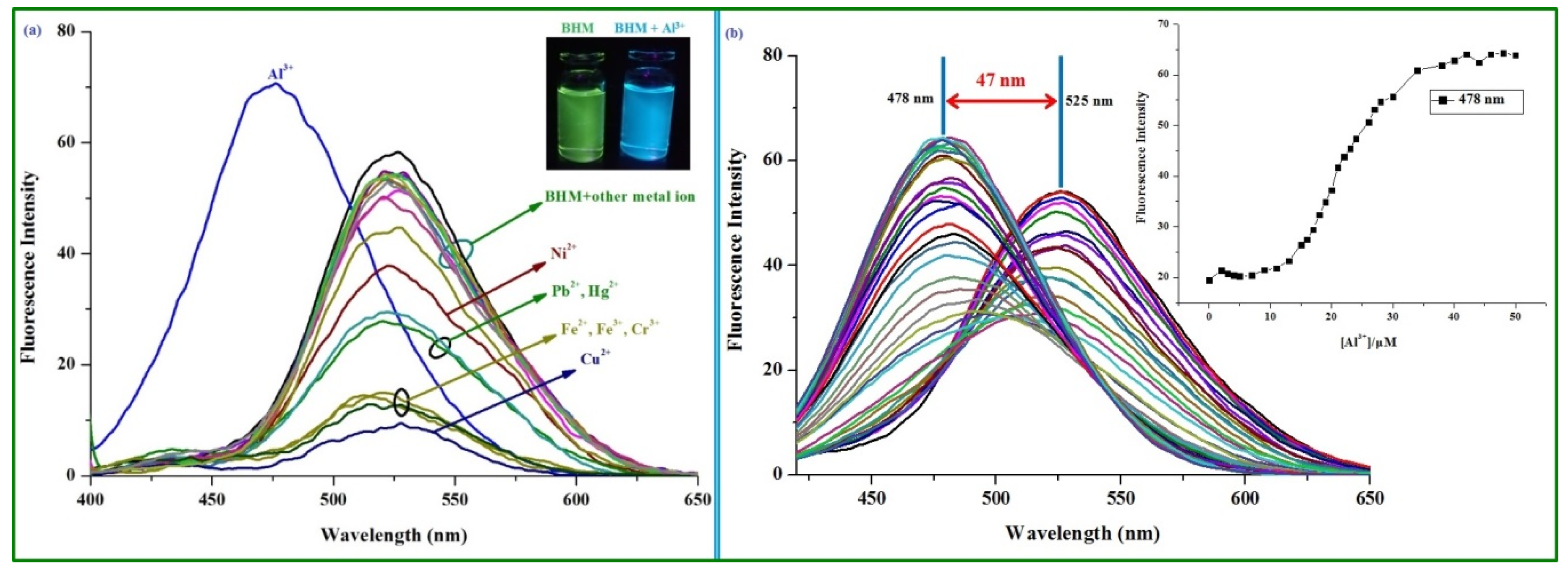
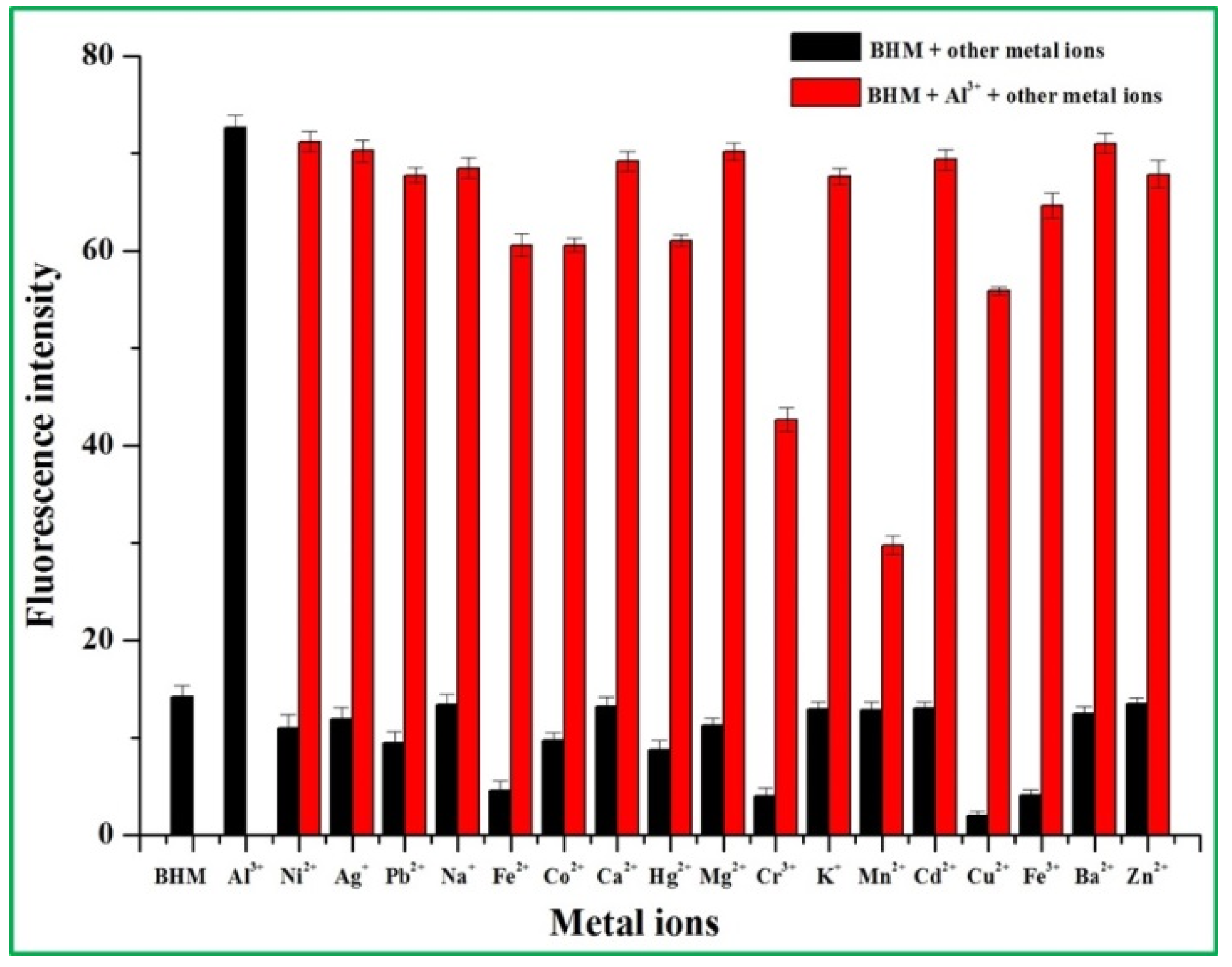
2.2. Spectra Performance Investigation of the BHM to Metal Ions
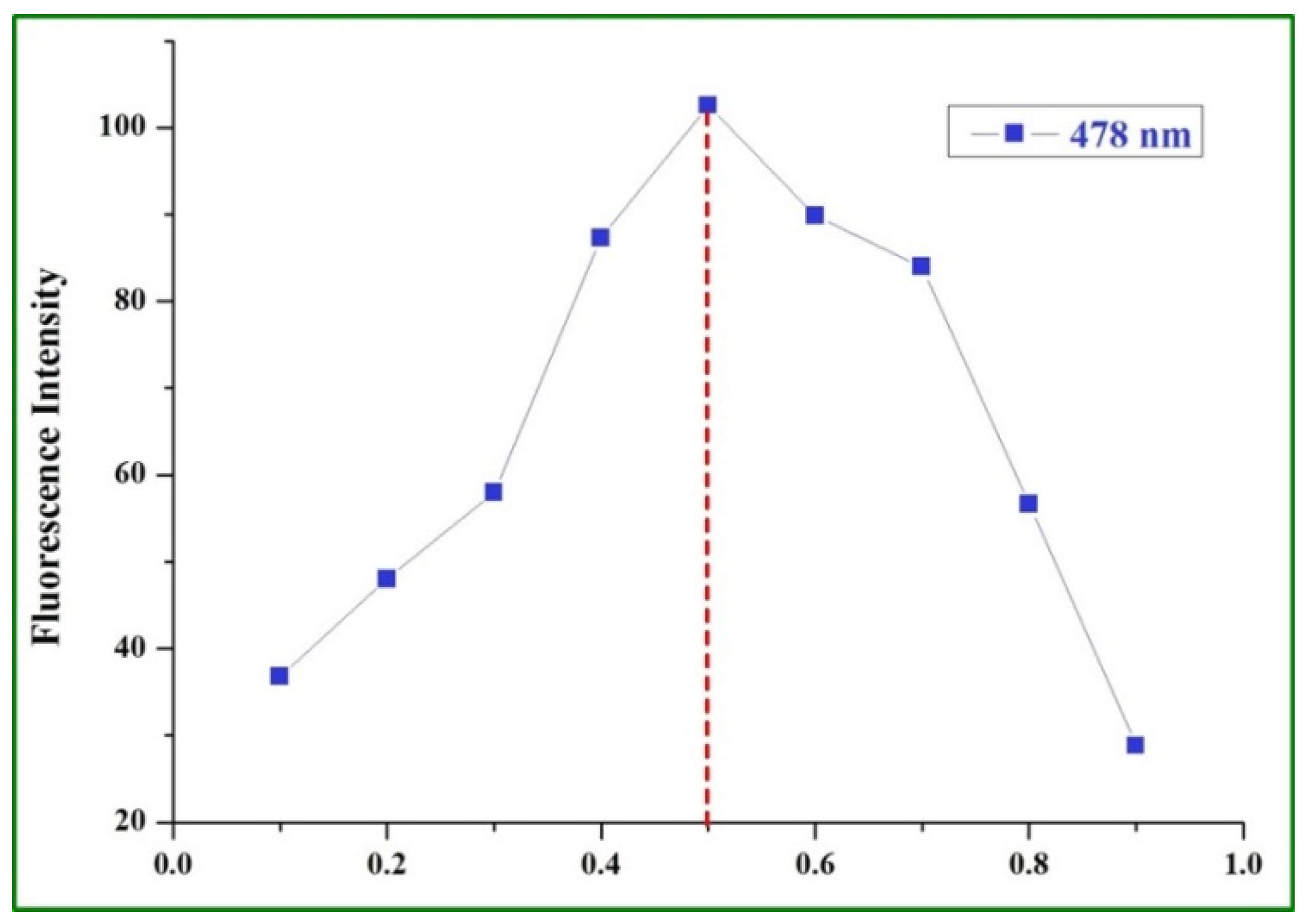
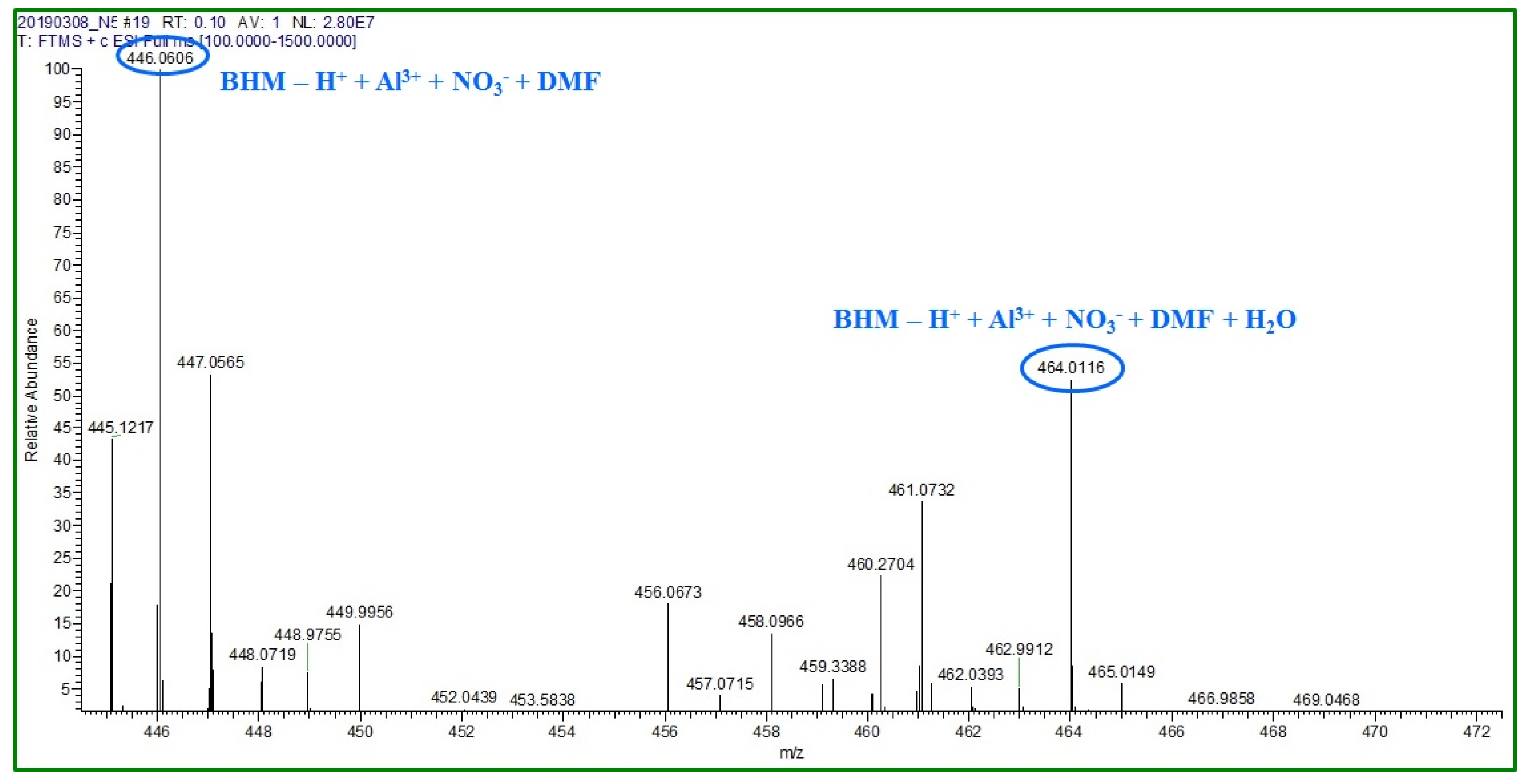
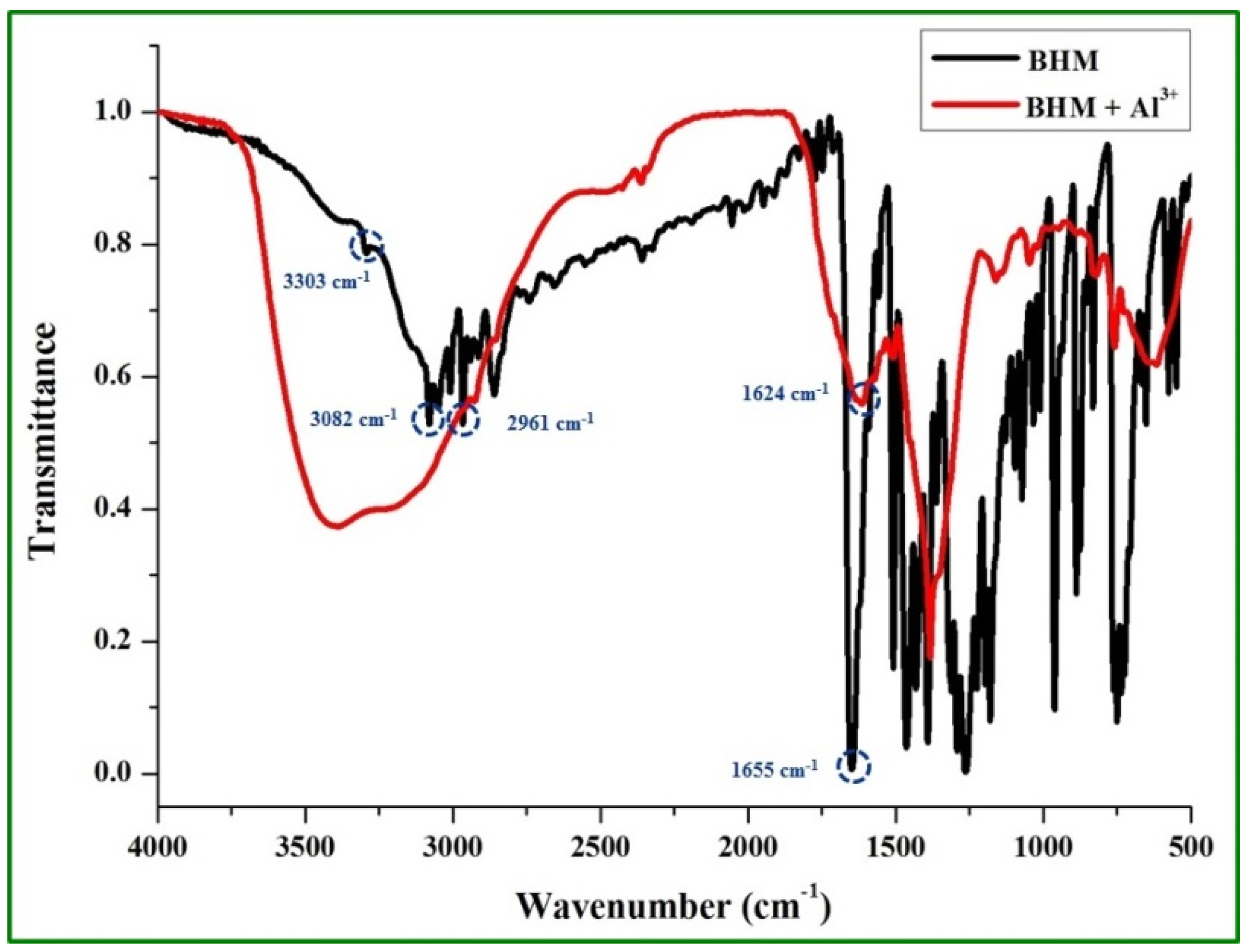
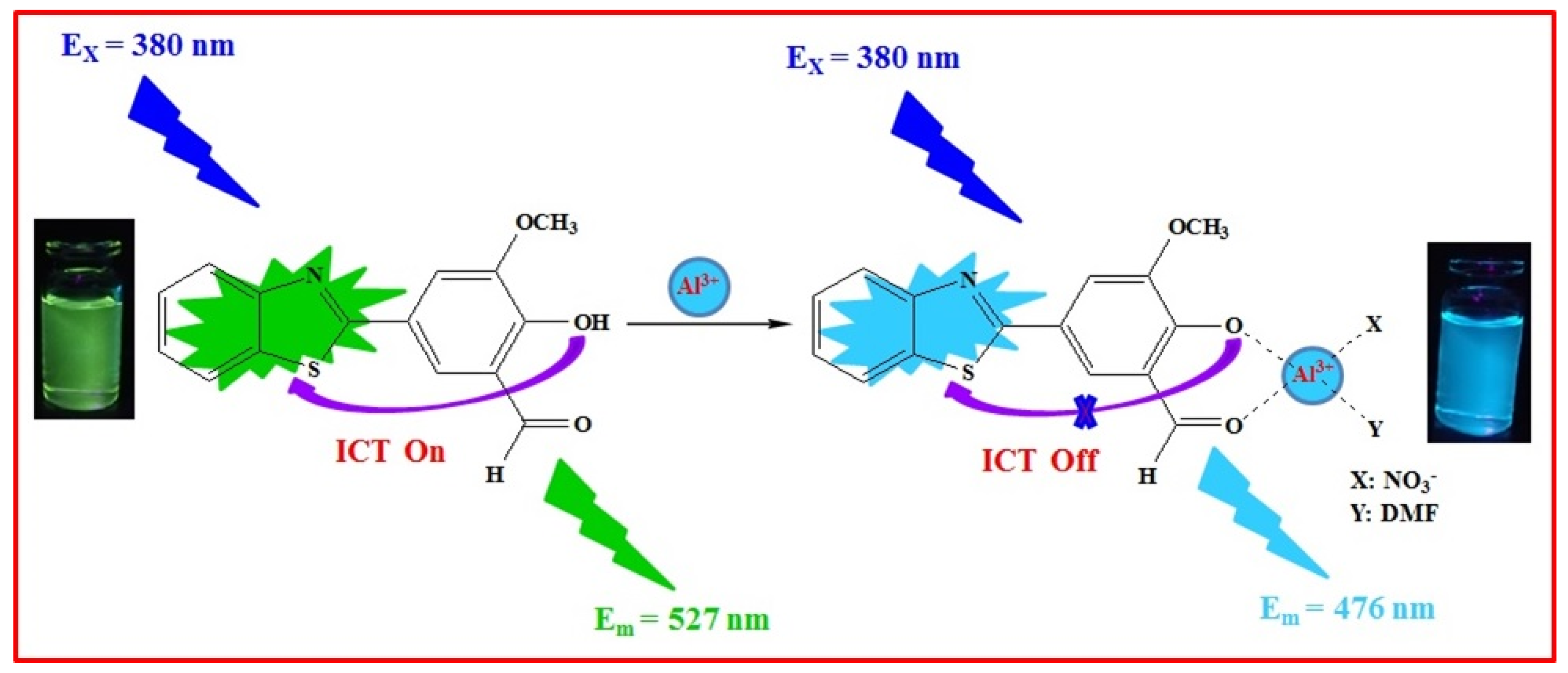
2.3. Sensing Mechanism of the BHM to Al3+
3. Materials and Methods
3.1. Materials and Apparatus
3.2. General Information
3.3. Synthesis
3.3.1. Synthesis of Probe BHM (5-(benzo[d]thiazol-2-yl)-2-hydroxy-3-methoxybenzaldehyde)
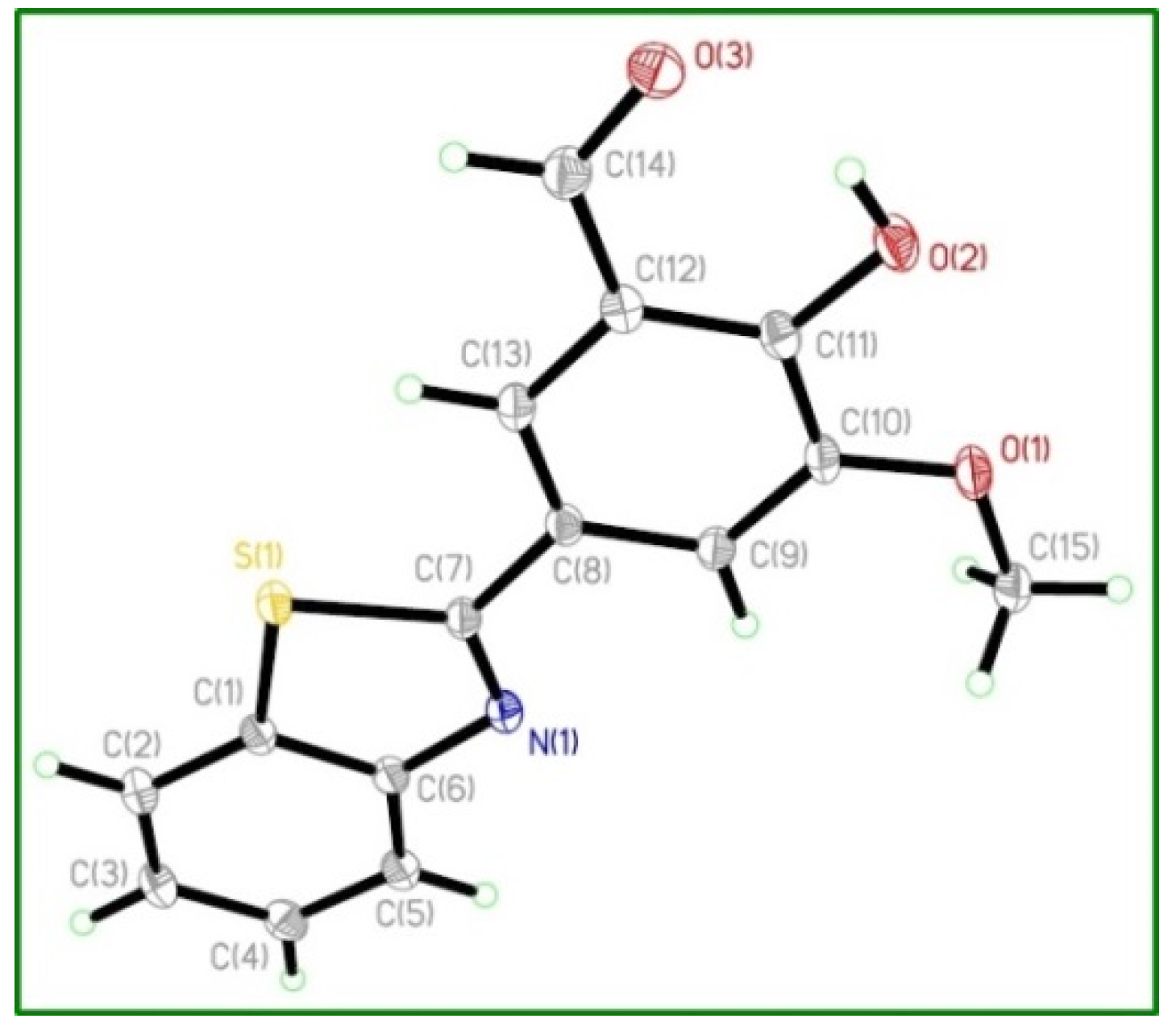
3.3.2. Preparation of the Crystal BHM
3.4. Cell Culture and Staining
3.5. X-Ray Diffraction Studies
4. Applications
4.1. Application in Water Samples
4.2. Application in Cell Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Azadbakht, R.; Talebi, M.; Karimi, J.; Golbedaghi, R. Synthesis and characterization of a new organic nanoparticle as fluorescent chemosensor for aluminum ions. Inorg. Chim. Acta 2016, 453, 728–734. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hicks, C.L.; Florence, R.L. Aluminum bioavailability from basic sodium aluminum phosphate, an approved food additive emulsifying agent, incorporated in cheese. Food Chem. Toxicol. 2008, 46, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Wu, B.; Zhou, W.; Wang, Q.; Yu, H.; Deng, K.; Li, J.M.; Zhuo, R.X.; Huang, S.W. Turn-on fluorescent probe-encapsulated micelle as colloidally stable nano-chemosensor for highly selective detection of Al3+ in aqueous solution and living cell imaging. Sens. Actuators B Chem. 2018, 271, 225–238. [Google Scholar] [CrossRef]
- Sont, M.G.; White, S.M.; Flamm, W.G.; Burdock, G.A. Safety evaluation of dietary aluminum. Regul. Toxicol. Pharmacol. 2001, 33, 66–79. [Google Scholar]
- Filippini, T.; Tancredi, S.; Malagoli, C.; Cilloni, S.; Malavolti, M.; Violi, F.; Vescovi, L.A.; Bargellini, M. Vinceti, Aluminum and tin: Food contamination and dietary intake in an Italian Population. J. Trace Elem. Med. Biol. 2019, 52, 293–301. [Google Scholar] [CrossRef]
- Levesque, L.; Mizzen, C.A.; McLachlan, D.R.; Fraser, P.E. Ligand specific effects on aluminum incorporation and toxicity in neurons and astrocytes. Brain Res. 2000, 877, 191–202. [Google Scholar] [CrossRef]
- Colomina, M.T.; Peris-Sampedro, F. Aluminum and Alzheimer’s disease. Adv. Neurobiol. 2017, 18, 183–197. [Google Scholar]
- Cavaleri, F. Review of Amyotrophic Lateral Sclerosis, Parkinson’s and Alzheimer’s diseases helps further define pathology of the novel paradigm for Alzheimer’s with heavy metals as primary disease cause. Med. Hypotheses 2015, 85, 779–790. [Google Scholar] [CrossRef]
- Mold, M.; Umar, D.; King, A.; Exley, C. Aluminium in brain tissue in autism. J. Trace Elem. Med. Biol. 2018, 46, 76–82. [Google Scholar] [CrossRef]
- Yang, M.H.; Chen, S.C.; Lin, Y.F.; Lee, Y.C.; Huang, M.Y.; Chen, K.C.; Wu, H.Y.; Lin, P.C.; Gozes, I.; Tyan, Y.C. Reduction of aluminum ion neurotoxicity through a small peptide application-NAP treatment of Alzheimer’s disease. J. Food Drug Anal. 2019, 27, 551–564. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, N. A review of mechanisms for fluorescent ‘‘turn-on’’ probes to detect Al3+ ions. RSC Adv. 2016, 6, 106413–106434. [Google Scholar] [CrossRef]
- Das, S.; Dutta, M.; Das, D. Fluorescent probes for selective determination of trace level Al3+: Recent developments and future prospects. Anal. Methods 2013, 5, 6262–6285. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, H.; Jin, L.; Wang, W.; Chen, Y. A novel turn on and reversible sensor for Al3+ and its applications in bioimaging. J. Lumin. 2018, 203, 113–120. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, H.; Liu, P.; Hu, Q.; Hu, J. A highly selective and sensitive turn-on probe for aluminum(III) based on quinoline Schiff’s base and its cell imaging Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 190, 104–110. [Google Scholar] [CrossRef]
- Shree, G.J.; Sivaraman, G.; Siva, A.; Chellappa, D. Anthracene- and pyrene-bearing imidazoles as turn-on fluorescent chemosensor for aluminum ion in living cells. Dyes Pigments 2019, 163, 204–212. [Google Scholar] [CrossRef]
- Liu, Y.; Bi, A.; Gao, T.; Cao, X.; Zeng, W. A novel self-assembled nanoprobe for the detection of aluminum ions in real water samples and living cells. Talanta 2019, 194, 38–45. [Google Scholar] [CrossRef]
- Qin, J.C.; Yan, J.; Wang, B.D.; Yang, Z.Y. Rhodamine–naphthalene conjugate as a novel ratiometric fluorescent probe for recognition of Al3+. Tetrahedron Lett. 2016, 57, 1935–1939. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, Y.; Wang, Y.Z.; Qiu, F.Z.; Tang, X.L.; Zhang, G.L.; Liu, W.S. A novel ratiometric fluorescent probe for selective detection of Hg2+, Cr3+ and Al3+ and its bioimaging application in living cells. Sens. Actuators B Chem. 2017, 253, 1055–1062. [Google Scholar] [CrossRef]
- Wang, D.; Fan, X.; Sun, S.; Du, S.; Li, H.; Zhu, J.; Tang, Y.; Chang, M.; Xu, Y. Substituent effect: A new strategy to construct a ratiometricfluorescent probe for detection of Al3+ and imaging in vivo. Sens. Actuators B Chem. 2018, 264, 304–311. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, L.; Mu, L.; Zeng, X.; Redshaw, C.; Wei, G. A ratiometric Al3+ ion probe based on the coumarin-quinoline FRET system. J. Photochem. Photobiol. A Chem. 2016, 328, 217–224. [Google Scholar] [CrossRef]
- Pang, B.J.; Li, C.R.; Yang, Z.Y. A novel chromone and rhodamine derivative as fluorescent probe for the detection of Zn(II) and Al(III) based on two different mechanisms. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 204, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Manna, A.; Sain, D.; Guchhait, N.; Goswami, S. FRET based selective and ratiometric detection of Al(III) with live-cell imaging. New J. Chem. 2017, 41, 14266–14271. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, R.; Kumar, A.; Pandey, D.S. Pyridylphenyl appended imidazoquinazoline based ratiometric fluorescence “turn on” chemosensor for Hg2+ and Al3+ in aqueous media. RSC Adv. 2014, 4, 55967–55970. [Google Scholar] [CrossRef]
- Das, S.; Goswami, S.; Aich, K.; Ghoshal, K.; Quah, C.K.; Bhattacharyya, M.; Fun, H.K. ESIPT and CHEF based highly sensitive and selective ratiometric sensor for Al3+ with imaging in human blood cells. New J. Chem. 2015, 39, 8582–8587. [Google Scholar] [CrossRef]
- Jeyanthi, D.; Iniya, M.; Krishnaveni, K.; Chellappa, D. A ratiometric fluorescent sensor for selective recognition of Al3+ ions based on a simple benzimidazole platform. RSC Adv. 2013, 3, 20984–20989. [Google Scholar] [CrossRef]
- Gupta, N.; Kaur, T.; Bhalla, V.; Parihar, R.D.; Ohri, P.; Kaur, G.; Kumar, M. A naphthalimide-based solid state luminescent probe for ratiometric detection of aluminum ions: In vitro and in vivo applications. Chem. Commun. 2017, 53, 12646–12649. [Google Scholar] [CrossRef]
- Maity, D.; Govindaraju, T. A differentially selective sensor with fluorescence turn-on response to Zn2+ and dual-mode ratiometric response to Al3+ in aqueous media. Chem. Commun. 2012, 48, 1039–1041. [Google Scholar] [CrossRef]
- Na, S.Y.; Park, S.; Kim, S.Y.; Kim, H.J. A benzothiazole-based water soluble and pH-independent probe for nitroreductase with a dramatic change of chromogenic and fluorogenic properties. Dyes Pigments 2019, 161, 247–251. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, H.; Zhang, W.; Wang, B.; Jiang, Y. A novel benzothiazole-based fluorescent probe for cysteine detection and its application on test paper and in living cells. Talanta 2018, 176, 151–155. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.; Zhang, D.; Liu, Y.; Tang, J.; Li, Y.; Zhao, Y.; Ye, Y. A fluorescein-based “turn-on” fluorescence probe for hypochlorous acid detection and its application in cell imaging. Sens. Actuators B Chem. 2018, 265, 84–90. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, X.; Zhang, Y.; Li, H.; Dai, L.; Peng, X.; Peng, Z.; Xie, Y. A fluorescent sensor for fast detection of peroxynitrite by removing of C=N in a benzothiazole derivative. Anal. Chim. Acta 2018, 1014, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, W.; Yan, M.; Tu, Q.; Chen, S.W.; Li, T.; Yuan, M.S.; Wang, J. 2-Hydroxy benzothiazole modified rhodol: Aggregation-induced emission and dual-channel fluorescence sensing of Hg2+ and Ag+ ions. Sens. Actuators B Chem. 2018, 255, 2086–2094. [Google Scholar] [CrossRef]
- Hong, K.I.; Choi, W.H.; Jang, W.D. Hydroxythiophene-bearing benzothiazole: Selective and sensitive detection of periodate and its application as security ink. Dyes Pigments 2019, 162, 984–989. [Google Scholar] [CrossRef]
- Zeng, S.; Li, S.J.; Sun, X.J.; Li, M.Q.; Xing, Z.Y.; Li, J.L. A benzothiazole-based chemosensor for significant fluorescent turn-on and ratiometric detection of Al3+ and its application in cell imaging. Inorg. Chim. Acta 2019, 486, 654–662. [Google Scholar] [CrossRef]
- Li, N.N.; Ma, Y.Q.; Sun, X.J.; Li, M.Q.; Zeng, S.; Xing, Z.Y.; Li, J.L. A dual-function probe based on naphthalene for fluorescent turn-on recognition of Cu2+ and colorimetric detection of Fe3+ in neat H2O. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Li, S.J.; Sun, X.J.; Liu, T.T.; Xing, Z.Y. A dual-functional chemosensor for fluorescent on-off and ratiometric detection of Cu2+ and Hg2+ and its application in cell imaging. Dyes Pigments 2019, 170, 107642. [Google Scholar] [CrossRef]
- Kang, L.; Xing, Z.Y.; Ma, X.Y.; Liu, Y.T.; Zhang, Y. A highly selective colorimetric and fluorescent turn-on chemosensor for Al3+ based on naphthalimide derivative. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 167, 59–65. [Google Scholar] [CrossRef]
- Zeng, S.; Li, S.J.; Sun, X.J.; Li, M.Q.; Ma, Y.Q.; Xing, Z.Y.; Li, J.L. A naphthalene-quinoline based chemosensor for fluorescent “turn-on” and absorbance-ratiometric detection of Al3+ and its application in cells imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 205, 276–286. [Google Scholar] [CrossRef]
- Ye, F.; Liang, X.M.; Xu, K.X.; Pang, X.X.; Chai, Q.; Fu, Y. A novel dithiourea-appended naphthalimide “on-off” fluorescent probe for detecting Hg2+ and Ag+ and its application in cell imaging. Talanta 2019, 200, 494–502. [Google Scholar] [CrossRef]
- Fu, Y.; Pang, X.X.; Wang, Z.Q.; Chai, Q.; Ye, F. A highly sensitive and selective fluorescent probe for determination of Cu (II) and application in live cell imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 208, 198–205. [Google Scholar] [CrossRef]


| Water Samples | Amount of Standard Al3+ Added (μmol/L) | Total Al3+ Found (n = 3) (μmol/L) | Recovery of Al3+ (n = 3) Added (%) | RSD (%) | Relative Error (%) |
|---|---|---|---|---|---|
| Ultrapure water | 15 | 15.08 | 100.54 | 1.54 | 0.69 |
| 16 | 16.18 | 101.12 | 2.33 | 1.41 | |
| 17 | 16.95 | 99.72 | 2.39 | −0.34 | |
| 18 | 17.66 | 98.10 | 1.20 | −2.31 | |
| 19 | 19.03 | 100.19 | 2.25 | 0.23 | |
| 20 | 20.16 | 100.81 | 1.67 | 0.97 | |
| Tap water (Department of Chemistry) | 15 | 15.30 | 102.00 | 2.33 | 2.55 |
| 16 | 15.63 | 97.67 | 0.20 | −2.91 | |
| 17 | 16.98 | 99.93 | 1.42 | −0.07 | |
| 18 | 17.90 | 99.47 | 1.90 | −0.64 | |
| 19 | 18.81 | 99.01 | 2.84 | −1.19 | |
| 20 | 19.83 | 99.18 | 2.83 | −0.96 | |
| Songhua River water | 15 | 14.82 | 98.82 | 0.22 | −1.15 |
| 16 | 15.94 | 99.60 | 2.67 | −0.39 | |
| 17 | 17.33 | 101.99 | 2.14 | 1.95 | |
| 18 | 17.91 | 99.47 | 2.84 | −0.51 | |
| 19 | 18.99 | 99.97 | 1.51 | −0.02 | |
| 20 | 19.86 | 99.32 | 1.36 | −0.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Z.-N.; Wu, D.-Q.; Sun, X.-J.; Liu, T.-T.; Xing, Z.-Y. A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+ and Its Application in Water Samples and Cell Imaging. Int. J. Mol. Sci. 2019, 20, 5993. https://doi.org/10.3390/ijms20235993
Tian Z-N, Wu D-Q, Sun X-J, Liu T-T, Xing Z-Y. A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+ and Its Application in Water Samples and Cell Imaging. International Journal of Molecular Sciences. 2019; 20(23):5993. https://doi.org/10.3390/ijms20235993
Chicago/Turabian StyleTian, Zhen-Nan, Ding-Qi Wu, Xue-Jiao Sun, Ting-Ting Liu, and Zhi-Yong Xing. 2019. "A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+ and Its Application in Water Samples and Cell Imaging" International Journal of Molecular Sciences 20, no. 23: 5993. https://doi.org/10.3390/ijms20235993
APA StyleTian, Z.-N., Wu, D.-Q., Sun, X.-J., Liu, T.-T., & Xing, Z.-Y. (2019). A Benzothiazole-Based Fluorescent Probe for Ratiometric Detection of Al3+ and Its Application in Water Samples and Cell Imaging. International Journal of Molecular Sciences, 20(23), 5993. https://doi.org/10.3390/ijms20235993