Nucleoplasmic Reticulum Formation in Human Endometrial Cells is Steroid Hormone Responsive and Recruits Nascent Components
Abstract
1. Introduction
2. Results and Discussion
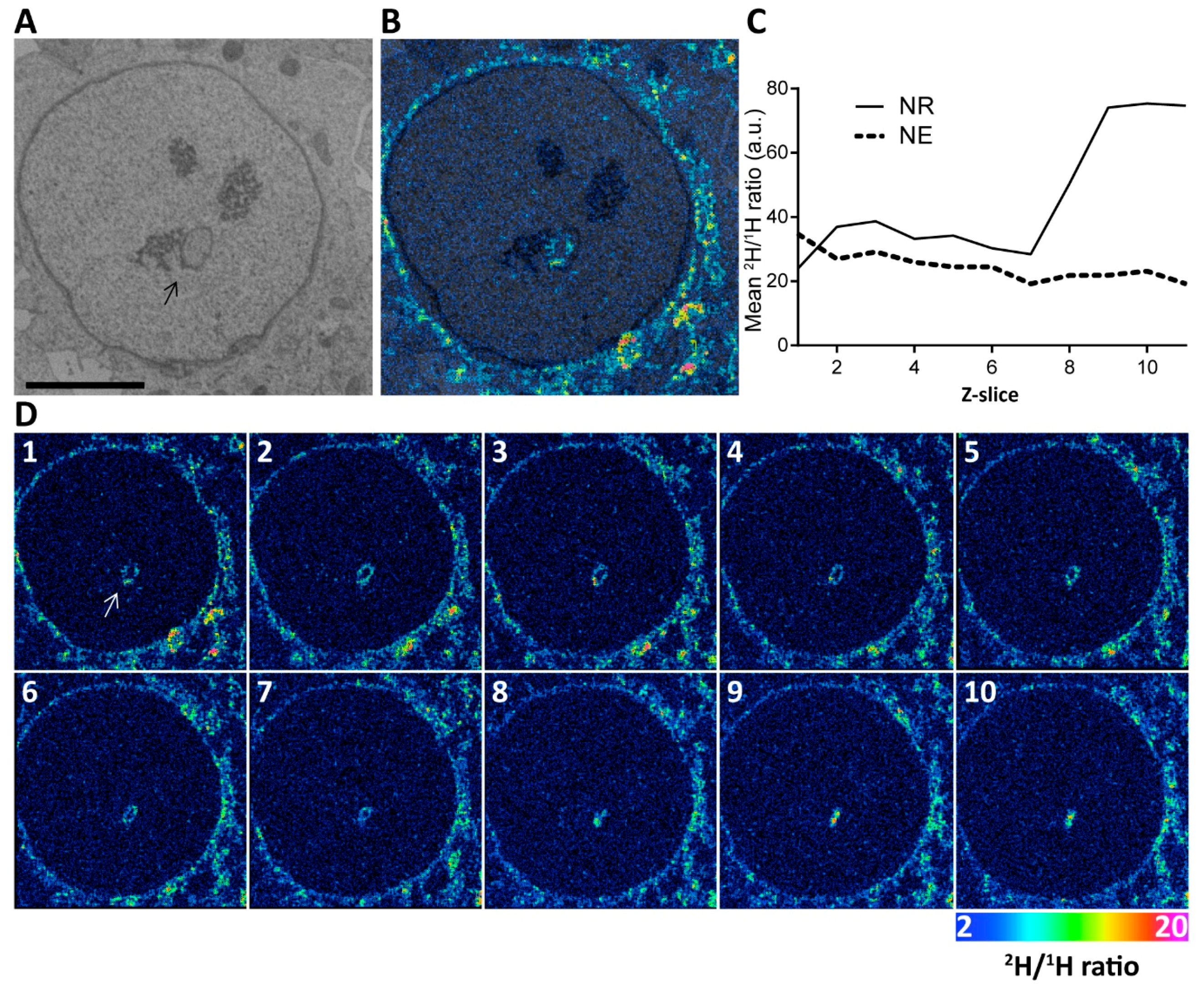
2.1. Nuclei of Ishikawa Cells Contain NR Structures
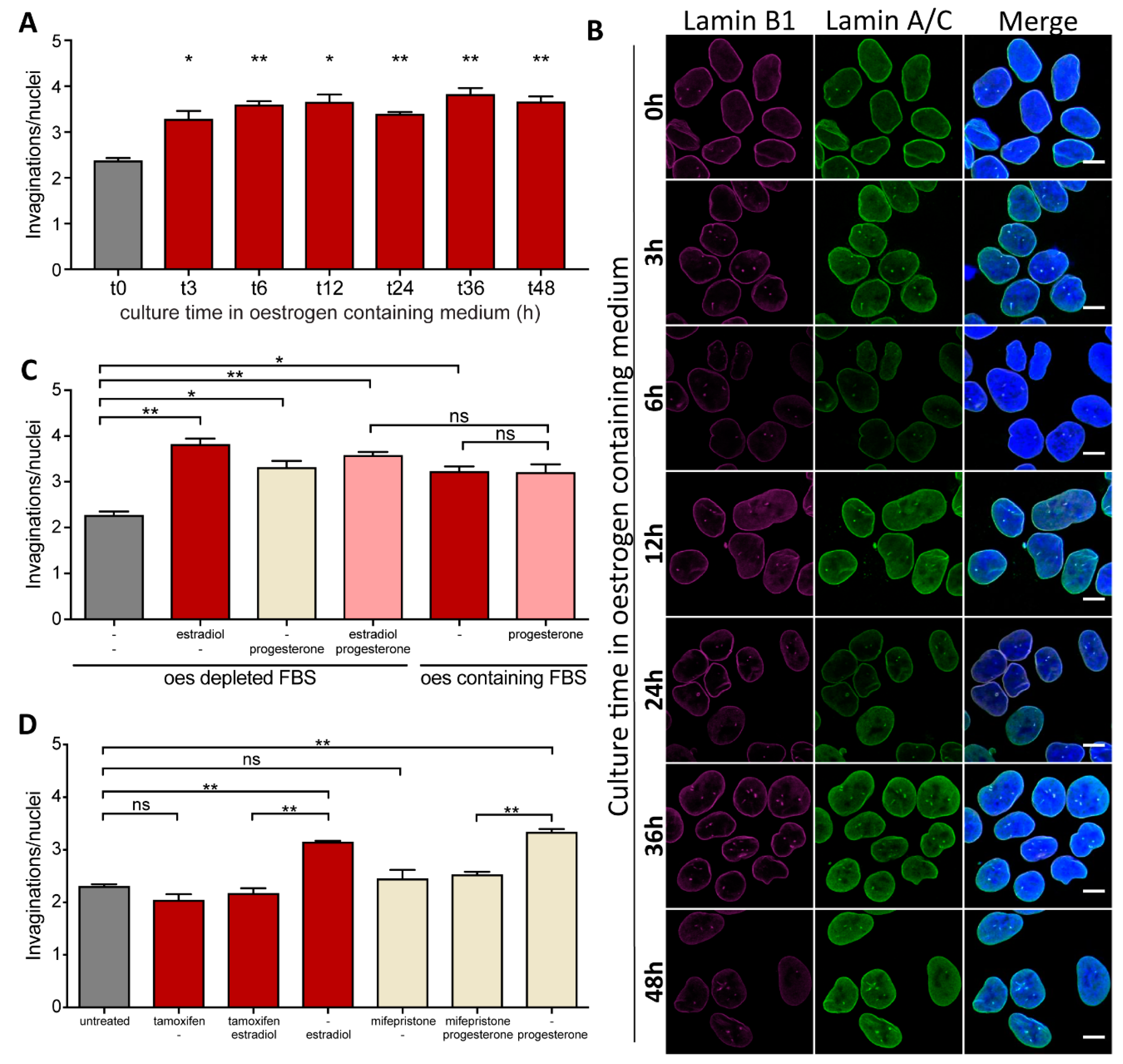
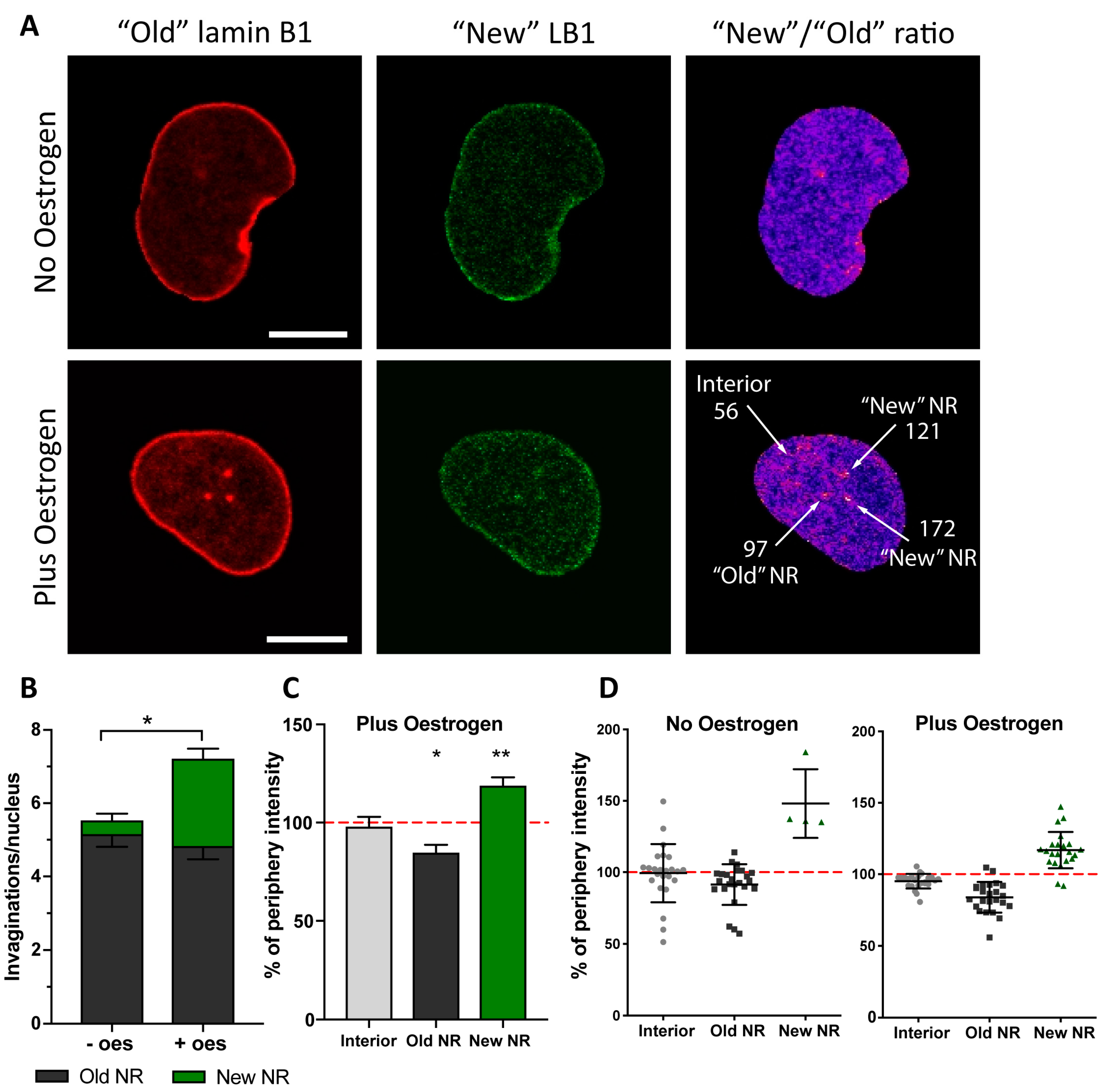
2.2. Formation of NR Tubules in Endometrial Cell Line Is Responsive to Hormones
3. Materials and Methods
3.1. Mammalian Cell Culture, Transfection and Treatments
3.2. Generation of mNeonGreen-LmnA Cell Line by CRISPR-Cas9
3.3. Transfection and Cell Sorting
3.4. Immunofluorescence and Confocal Microscopy
3.5. Live Cell Microscopy of Photoconvertible Maple3-Lamin B1
3.6. Flow Cytometry
3.7. Correlative Backscattered Electron Microscopy and NanoSIMS Imaging
3.8. Statistics
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Prunuske, A.J.; Ullman, K.S. The nuclear envelope: Form and reformation. Curr. Opin. Cell Biol. 2006, 18, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Roux, K.J.; Burke, B. Blurring the boundary: The nuclear envelope extends its reach. Science 2007, 318, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Grossman, E.; Medalia, O.; Zwerger, M. Functional architecture of the nuclear pore complex. Annu. Rev. Biophys. 2012, 41, 557–584. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, E.C.; Foisner, R. Proteins that associate with lamins: Many faces, many functions. Exp. Cell Res. 2007, 313, 2167–2179. [Google Scholar] [CrossRef]
- Fricker, M.; Hollinshead, M.; White, N.; Vaux, D. Interphase nuclei of many mammalian cell types contain deep, dynamic, tubular membrane-bound invaginations of the nuclear envelope. J. Cell Biol. 1997, 136, 531–544. [Google Scholar] [CrossRef]
- Fricker, M.; Hollinshead, M.; White, N.; Vaux, D. The convoluted nucleus. Trends Cell Biol. 1997, 7, 181. [Google Scholar] [CrossRef]
- Langevin, H.M.; Storch, K.N.; Snapp, R.R.; Bouffard, N.A.; Badger, G.J.; Howe, A.K.; Taatjes, D.J. Tissue stretch induces nuclear remodeling in connective tissue fibroblasts. Histochem. Cell Biol. 2010, 133, 405–415. [Google Scholar] [CrossRef]
- Storch, K.; Taatjes, D.; Bouffard, N.; Locknar, S.; Bishop, N.; Langevin, H. Alpha smooth muscle actin distribution in cytoplasm and nuclear invaginations of connective tissue fibroblasts. Histochem Cell Biol. 2007, 127, 523–530. [Google Scholar] [CrossRef]
- Bussolati, G.; Marchiò, C.; Gaetano, L.; Lupo, R.; Sapino, A. Pleomorphism of the nuclear envelope in breast cancer: A new approach to an old problem. J. Cell Mol. Med. 2008, 12, 209–218. [Google Scholar] [CrossRef]
- Malhas, A.N.; Vaux, D.J. Nuclear envelope invaginations and cancer. In Cancer Biology and the Nuclear Envelope; Schirmer, E., de las Heras, J., Eds.; Springer: New York, NY, USA, 2014; AEMB; Volume 773, pp. 523–535. [Google Scholar]
- Malhas, A.; Goulbourne, C.; Vaux, D.J. The nucleoplasmic reticulum: Form and function. Trends Cell Biol. 2011, 21, 362–373. [Google Scholar] [CrossRef]
- Kittur, N.; Zapantis, G.; Aubuchon, M.; Santoro, N.; Bazett-Jones, D.P.; Meier, U.T. The nucleolar channel system of human endometrium is related to endoplasmic reticulum and R-rings. Mol. Biol. Cell 2007, 18, 2296–2304. [Google Scholar] [CrossRef] [PubMed]
- Terzakis, J.A. The nucleolar channel system of the human endometrium. J. Cell Biol. 1965, 27, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Guffanti, E.; Kittur, N.; Brodt, Z.N.; Polotsky, A.J.; Kuokkanen, S.M.; Heller, D.S.; Young, S.L.; Santoro, N.; Meier, U.T. Nuclear pore complex proteins mark the implantation window in human endometrium. J. Cell Sci. 2008, 121, 2037–2045. [Google Scholar] [CrossRef] [PubMed]
- Isaac, C.; Pollard, J.W.; Meier, U.T. Intranuclear endoplasmic reticulum induced by Nopp140 mimics the nucleolar channel system of human endometrium. J. Cell Sci. 2001, 114, 4253–4264. [Google Scholar] [PubMed]
- Dockery, P.; Pritchard, K.; Warren, M.A.; Li, T.C.; Cooke, I.D. Uterus and endometrium: Changes in nuclear morphology in the human endometrial glandular epithelium in women with unexplained infertility. Hum. Rep. 1996, 11, 2251–2256. [Google Scholar] [CrossRef][Green Version]
- Kohorn, E.I.; Rice, S.I.; Hemperly, S.; Gordon, M. The relation of the structure of progestational steroids to nucleolar differentiation in human endometrium. J. Clin. Endocrinol. Metab. 1972, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Feria-Velasco, A.; Aznar-Ramos, R.; González-Angulo, A. Ultrastructural changes found in the endometrium of women using megestrol acetate for contraception. Contraception 1972, 5, 187–201. [Google Scholar] [CrossRef]
- Wynn, R.M. Intrauterine devices: Effects on ultrastructure of human endometrium. Science 1967, 156, 1508–1510. [Google Scholar] [CrossRef]
- Nejat, E.J.; Szmyga, M.J.; Zapantis, G.; Meier, U.T. Progesterone Threshold Determines Nucleolar Channel System Formation in Human Endometrium. Reprod. Sci. 2014, 21, 915–920. [Google Scholar] [CrossRef][Green Version]
- Pryse-Davies, J.; Ryder, T.A.; MacKenzi, M.L. In vivo production of the nucleolar channel system in post menopausal endometrium. Cell Tissue Res. 1979, 203, 493–498. [Google Scholar] [CrossRef]
- Nishida, M.; Kasahara, K.; Kaneko, M.; Iwasaki, H.; Hayashi, K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi. 1985, 37, 1103–1111. [Google Scholar] [PubMed]
- Drozdz, M.M.; Jiang, H.; Pytowski, L.; Grovenor, C.; Vaux, D.J. Formation of a nucleoplasmic reticulum requires de novo assembly of nascent phospholipids and shows preferential incorporation of nascent lamins. Sci. Rep. 2017, 7, 7454. [Google Scholar] [CrossRef] [PubMed]
- Chu, A.J.; Rooney, S.A. Estrogen stimulation of surfactant synthesis. Pediatr. Pulmonol. 1985, 1, S110–S114. [Google Scholar] [PubMed]
- Chu, A.J.; Rooney, S.A. Stimulation of cholinephosphate cytidylyltransferase activity by estrogen in fetal rabbit lung is mediated by phospholipids. Biochim. Biophys. Acta. 1985, 834, 346–356. [Google Scholar] [CrossRef]
- Goulbourne, C.N.; Malhas, A.N.; Vaux, D.J. The induction of a nucleoplasmic reticulum by prelamin A accumulation requires CTP:phosphocholine cytidylyltransferase-α. J. Cell. Sci. 2011, 124, 4253–4266. [Google Scholar] [CrossRef]
- Lagace, T.A.; Ridgway, N.D. The rate-limiting enzyme in phosphatidylcholine synthesis regulates proliferation of the nucleoplasmic reticulum. Mol. Biol. Cell 2005, 16, 1120–1130. [Google Scholar] [CrossRef]
- Haider, A.; Wei, Y.-C.; Lim, K.; Barbosa, A.D.; Liu, C.-H.; Weber, U.; Mlodzik, M.; Oras, K.; Collier, S.; Hussain, M.M.; et al. PCYT1A regulates phosphatidylcholine homeostasis from the inner nuclear membrane in response to membrane stored curvature elastic stress. Dev. Cell 2018, 45, 481–495.e8. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Lin, C.Y.; Gootenberg, J.S.; Konermann, S.; Trevino, A.E.; Scott, D.A.; Inoue, A.; Matoba, S.; Zhang, Y.; et al. Double nicking by RNA-guided CRISPR Cas9 for enhanced genome editing specificity. Cell 2013, 154, 1380–1389. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012, 9, 676. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytowski, L.; Drozdz, M.M.; Jiang, H.; Hernandez, Z.; Kumar, K.; Knott, E.; Vaux, D.J. Nucleoplasmic Reticulum Formation in Human Endometrial Cells is Steroid Hormone Responsive and Recruits Nascent Components. Int. J. Mol. Sci. 2019, 20, 5839. https://doi.org/10.3390/ijms20235839
Pytowski L, Drozdz MM, Jiang H, Hernandez Z, Kumar K, Knott E, Vaux DJ. Nucleoplasmic Reticulum Formation in Human Endometrial Cells is Steroid Hormone Responsive and Recruits Nascent Components. International Journal of Molecular Sciences. 2019; 20(23):5839. https://doi.org/10.3390/ijms20235839
Chicago/Turabian StylePytowski, Lior, Marek M. Drozdz, Haibo Jiang, Zayra Hernandez, Kurun Kumar, Emily Knott, and David J. Vaux. 2019. "Nucleoplasmic Reticulum Formation in Human Endometrial Cells is Steroid Hormone Responsive and Recruits Nascent Components" International Journal of Molecular Sciences 20, no. 23: 5839. https://doi.org/10.3390/ijms20235839
APA StylePytowski, L., Drozdz, M. M., Jiang, H., Hernandez, Z., Kumar, K., Knott, E., & Vaux, D. J. (2019). Nucleoplasmic Reticulum Formation in Human Endometrial Cells is Steroid Hormone Responsive and Recruits Nascent Components. International Journal of Molecular Sciences, 20(23), 5839. https://doi.org/10.3390/ijms20235839





