Gene Expression Profiles Induced by a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα) Pemafibrate
Abstract
1. Introduction
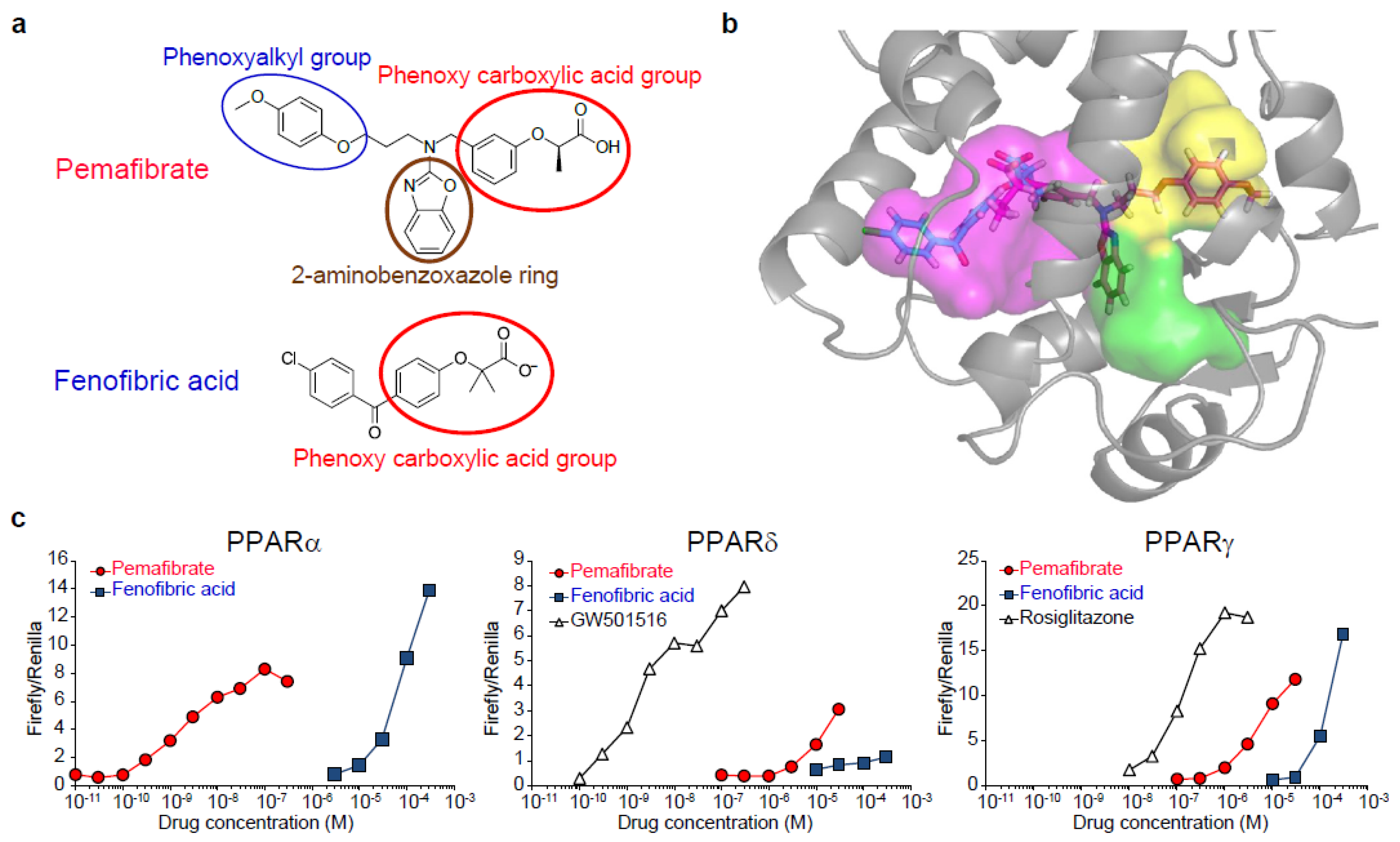
2. Pemafibrate as a Novel SPPARMα
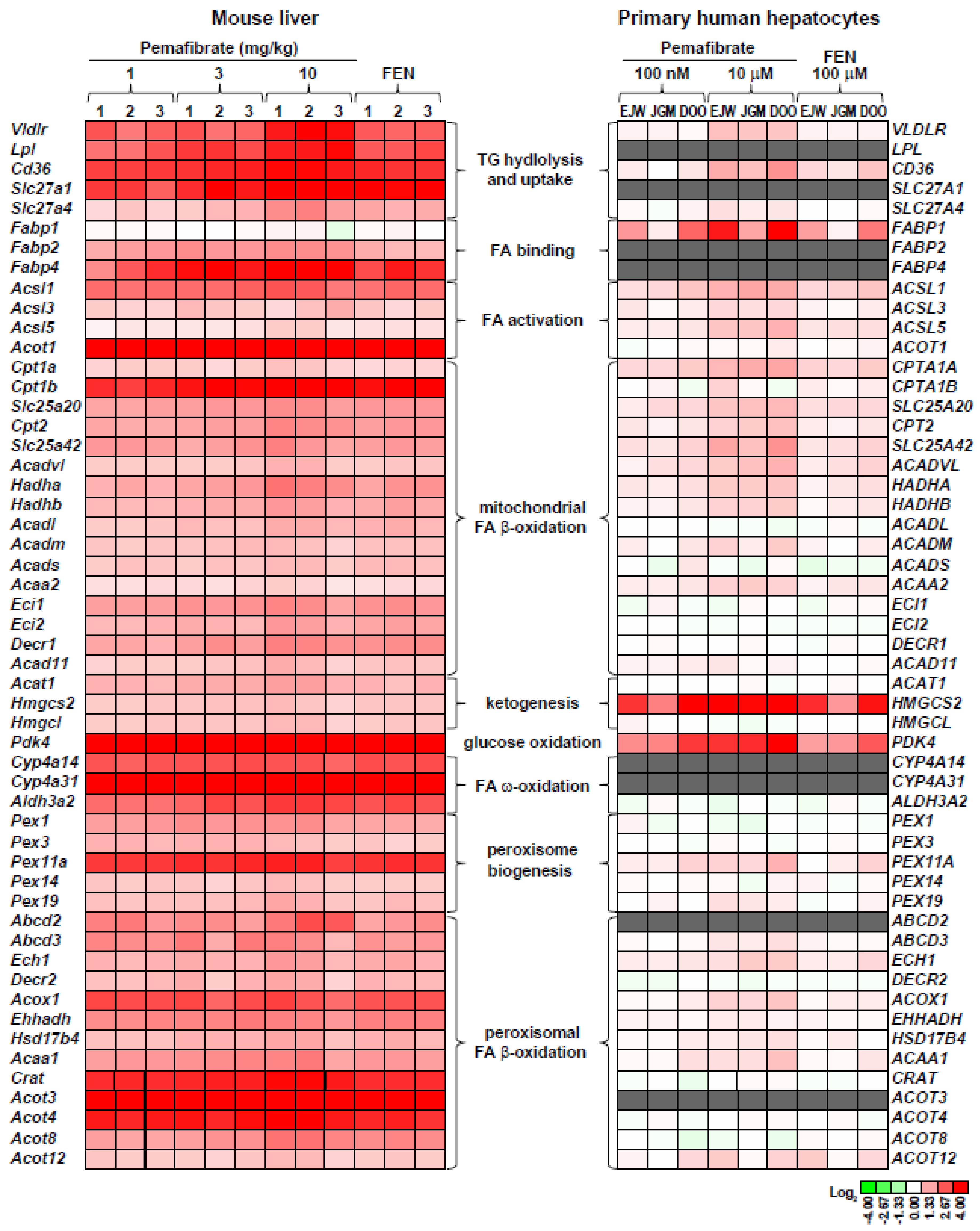
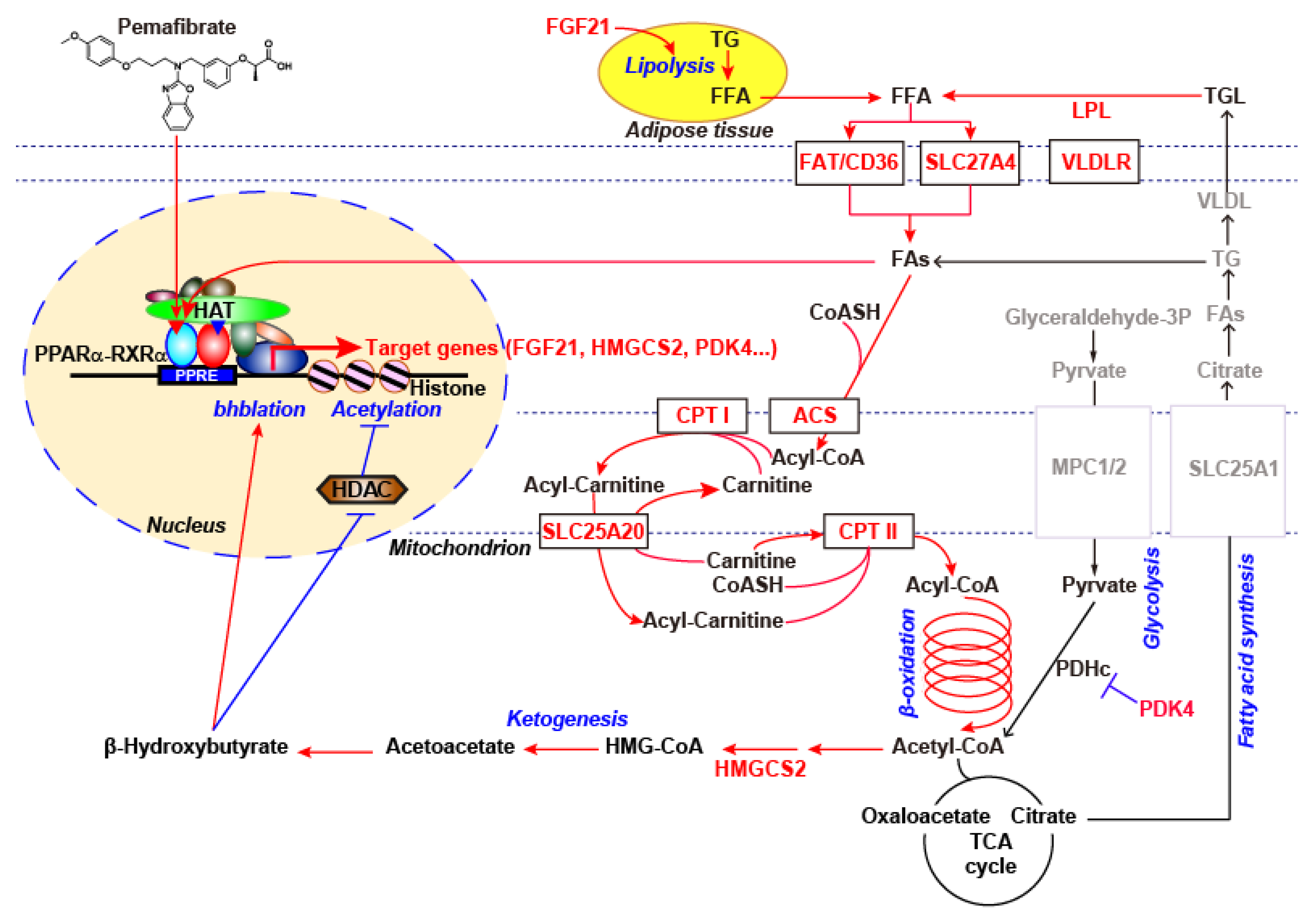
3. Pemafibrate Regulates the Availability of FA and Glucose Oxidation
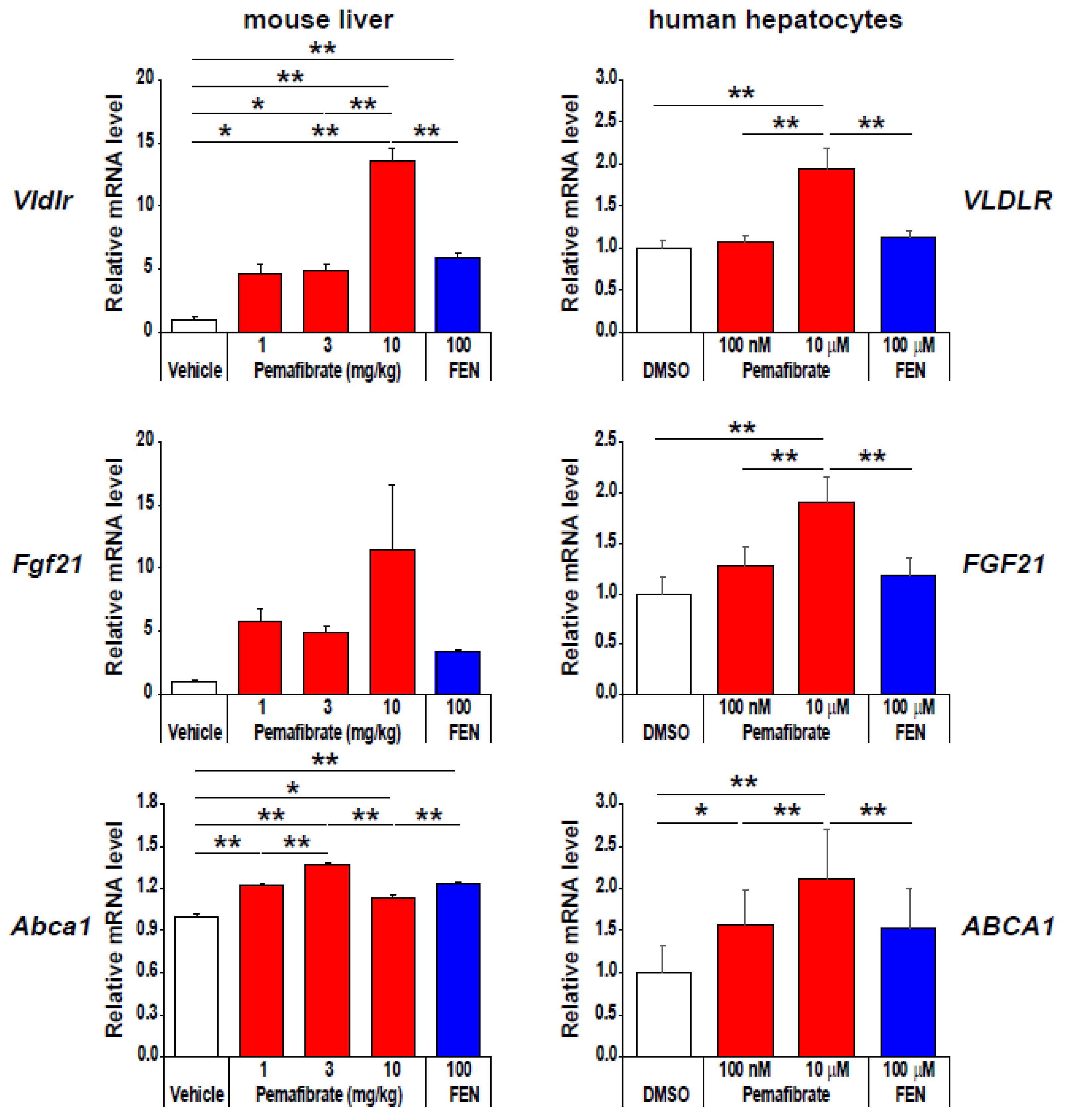
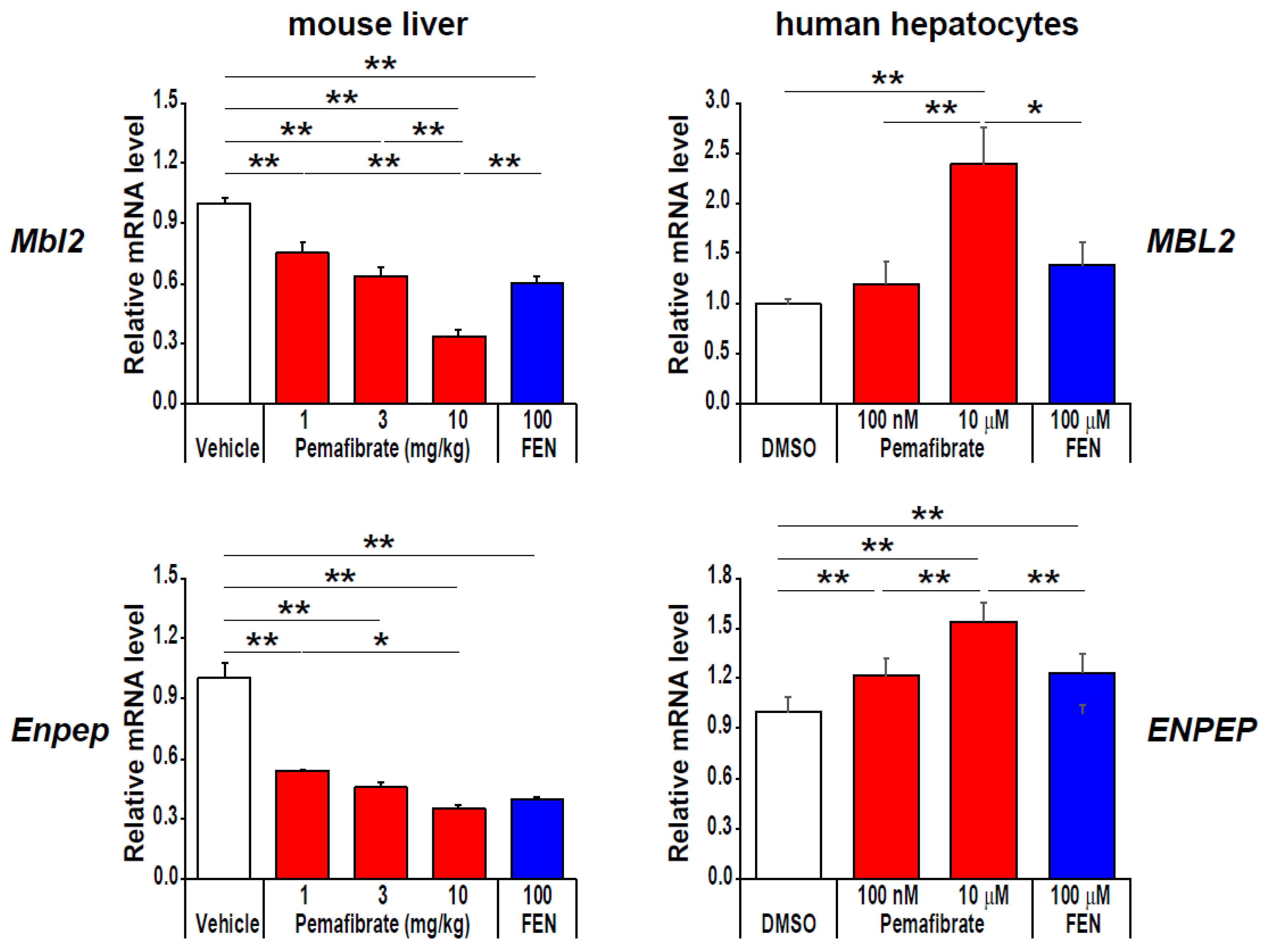
4. Pharmacologically Favorable Target Genes of Pemafibrate as a SPPARMα
5. Possible Mechanism for the Gene Expression Regulation Induced by Pemafibrate?
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCA1 | ATP binding cassette subfamily A member 1 |
| Abcd2 | ATP binding cassette subfamily D member 2 |
| ABCG1 | ATP binding cassette subfamily G member 1 |
| Acaa1 | acetyl-CoA acyltransferase 1 |
| Acadl | acyl-Coenzyme A dehydrogenase long-chain |
| Acadm | acyl-Coenzyme A dehydrogenase medium-chain |
| Acads | acyl-Coenzyme A dehydrogenase short chain |
| Acadvl | acyl-Coenzyme A dehydrogenase very long-chain |
| Acad11 | acyl-Coenzyme A dehydrogenase family member 11 |
| Acat1 | acetyl-CoA acetyltransferase 1 |
| Acot3 | acyl-CoA thioesterase 3 |
| Acox1 | acyl-Coenzyme A oxidase 1 |
| Acsl1 | acyl-CoA synthetase long-chain family member 1 |
| Acsf3 | acyl-CoA synthetase family member 3 |
| ADR | adverse drug reaction |
| Aldh3a2 | aldehyde dehydrogenase 3 family member A2 |
| APA | aminopeptidase A |
| ASCVD | atherosclerotic cardiovascular disease |
| CBP/p300 | cAMP-response element binding protein (CREB) binding protein |
| C/EBPβ | CCAAT enhancer binding protein β |
| CKD | chronic kidney disease |
| COL1A2 | collagen type I alpha 2 chain |
| COUP-TFs | chicken ovalbumin upstream promotor-transcription factors |
| Cpt1 | carnitine palmitoyltransferase I |
| Crat | carnitine acetyltransferase |
| CREBH | cAMP-responsive element-binding protein 3 like 3 |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| Cyp4a10 | cytochrome P450, family 4, subfamily a, polypeptide 10 |
| DAG | diacylglycerol |
| DBD | DNA binding domain |
| Decr1 | 2,4-dienoyl-CoA reductase 1 |
| DKK1 | dickkopf WNT signaling pathway inhibitor 1 |
| DR1 | direct repeat 1 |
| Ech1 | enoyl-CoA hydratase 1 |
| eGFR | estimated glomerular filtration rate |
| Ehhadh | enoyl-Coenzyme A, hydratase/3-hydroxyacyl Coenzyme A dehydrogenase |
| EndMT | endothelial-mesenchymal transition |
| ENPEP | glutamyl aminopeptidase |
| ET-1 | endothelin-1 |
| FA | fatty acid |
| FAT | fatty acid translocase |
| Fabp2 | fatty acid-binding protein 2 |
| FGF21 | fibroblast growth factor 21 |
| Fn1 | fibronectin 1 |
| GRIP1/TIF2 | glucocorticoid receptor interacting protein1/transcriptional intermediary factor 2 |
| Hadha | hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit alpha |
| Hadhb | hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit beta |
| HAT | histone acetyl transferase |
| HCECs | human coronary endothelial cells |
| HDAC | histone deacetylases |
| HDL-C | high-density lipoprotein cholesterol |
| Hmgcl | 3-hydroxy-3-methylglutaryl-CoA lyase |
| HMGCS2 | 3-hydroxy-3-methylglutaryl-CoA synthase 2 |
| HNF4s | hepatocyte nuclear factor 4s |
| Hsd17b4 | hydroxysteroid 17-beta dehydrogenase 4 |
| HUVECs | human umbilical vein endothelial cells |
| H3K9bhb | β-hydroxybutyrated histone H3 lysine 9 |
| IFNγ | interferon gamma |
| IL6 | interleukin 6 |
| LDL | low-density lipoprotein |
| LDLR | low-density lipoprotein receptor |
| Lpl | lipoprotein lipase |
| MBL2 | mannose-binding lectin 2 |
| MCP-1 | monocyte chemoattractant protein-1 |
| NAD(P)H | nicotinamide adenine dinucleotide phosphate |
| Ncf1 | neutrophil cytosolic factor 1 |
| Nox4 | NADPH oxidase 4 |
| 8-OHdG | 8-hydroxy-2’- deoxyguanosine |
| PDK4 | pyruvate dehydrogenase kinase 4 |
| Pex1 | peroxisome biogenesis factor 1 |
| PDH | pyruvate dehydrogenase |
| PGC1α | peroxisome proliferative activated receptor gamma coactivator 1α |
| PIC | preinitiation complex |
| PKC | protein kinase C |
| PPARα | peroxisome proliferator-activated receptor α |
| PPREs | PPAR response elements |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| ROS | reactive oxygen species |
| RXR | retinoid X receptor |
| SBP | systolic blood pressure |
| Slc27a1 | solute carrier family 27 member 1 |
| Slc25a20 | solute carrier family 25 member 20 |
| SPPARMα | selective peroxisome proliferator-activated receptor α modulator |
| SRC/p160 | steroid receptor coactivator |
| TG | triglyceride |
| Tgfb1 | transforming growth factor beta 1 |
| TNFα | tumor necrosis factor α |
| VCAM1 | vascular cell adhesion molecule 1 |
| VLDLR | very-low-density lipoprotein receptor |
References
- Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: A meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019, 393, 407–415. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [PubMed]
- Fruchart, J.C.; Sacks, F.; Hermans, M.P.; Assmann, G.; Brown, W.V.; Ceska, R.; Chapman, M.J.; Dodson, P.M.; Fioretto, P.; Ginsberg, H.N.; et al. The Residual Risk Reduction Initiative: A call to action to reduce residual vascular risk in patients with dyslipidemia. Am. J. Cardiol. 2008, 102, 1K–34K. [Google Scholar] [CrossRef] [PubMed]
- Alagona, P., Jr. Beyond LDL cholesterol: The role of elevated triglycerides and low HDL cholesterol in residual CVD risk remaining after statin therapy. Am. J. Manag. Care 2009, 15, S65–S73. [Google Scholar]
- Reiner, Z. Managing the residual cardiovascular disease risk associated with HDL-cholesterol and triglycerides in statin-treated patients: A clinical update. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 799–807. [Google Scholar] [CrossRef]
- Hermans, M.P.; Valensi, P. Elevated triglycerides and low high-density lipoprotein cholesterol level as marker of very high risk in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 118–129. [Google Scholar] [CrossRef]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Borén, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A.; et al. European Atherosclerosis Society Consensus Panel. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef]
- Vrablík, M.; Češka, R. Treatment of hypertriglyceridemia: A review of current options. Physiol. Res. 2015, 64, S331–S340. [Google Scholar]
- Katsiki, N.; Nikolic, D.; Montalto, G.; Banach, M.; Mikhailidis, D.P.; Rizzo, M. The role of fibrate treatment in dyslipidemia: An overview. Curr. Pharm. Des. 2013, 19, 3124–3131. [Google Scholar] [CrossRef]
- McCullough, P.A.; Ahmed, A.B.; Zughaib, M.T.; Glanz, E.D.; Di Loreto, M.J. Treatment of hypertriglyceridemia with fibric acid derivatives: Impact on lipid subfractions and translation into a reduction in cardiovascular events. Rev. Cardiovasc. Med. 2011, 12, 173–185. [Google Scholar]
- Nakaya, N.; Goto, Y. A retrospective meta-analysis of the efficacy and tolerability of fenofibrate 300 mg/d on high-density lipoprotein cholesterol levels in randomized, double-blind, comparative studies conducted in Japan. Curr. Res. Clin. Exp. 2003, 64, 634–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davidson, M.H.; Armani, A.; McKenney, J.M.; Jacobson, T.A. Safety considerations with fibrate therapy. Am. J. Cardiol. 2007, 99, 3C–18C. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, J.; Odin, J.A.; Hayashi, P.H.; Chalasani, N.; Fontana, R.J.; Barnhart, H.; Cirulli, E.T.; Kleiner, D.E.; Hoofnagle, J.H. Identification and Characterization of Fenofibrate-Induced Liver Injury. Dig. Dis. Sci. 2017, 62, 3596–3604. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.; Saraf, S.; Ramachandran, S.; Raju, J.; Ramachandran, S. Fibrates and estimated glomerular filtration rate: Observations from an outpatient clinic setting and clinical implications. Postgrad. Med. J. 2012, 88, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Abe, K.; Toma, T.; Nishikawa, M.; Ozawa, H.; Okuda, A.; Araki, T.; Oda, S.; Inoue, K.; Shibuya, K.; et al. Design and synthesis of highly potent and selective human peroxisome proliferator-activated receptor α agonists. Bioorg. Med. Chem. Lett. 2007, 17, 4689–4693. [Google Scholar] [CrossRef]
- Fruchart, J.C. Peroxisome proliferator-activated receptor-α (PPARα): At the crossroads of obesity, diabetes and cardiovascular disease. Atherosclerosis 2009, 205, 1–8. [Google Scholar] [CrossRef]
- Fruchart, J.C. Selective peroxisome proliferator-activated receptor α modulators (SPPARMα): The next generation of peroxisome proliferator-activated receptor α-agonists. Cardiovasc. Diabetol. 2013, 12, 82. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Takei, K.; Arulmozhiraja, S.; Sladek, V.; Matsuo, N.; Han, S.I.; Matsuzaka, T.; Sekiya, M.; Tokiwa, T.; Shoji, M.; et al. Molecular association model of PPARα and its new specific and efficient ligand, pemafibrate: Structural basis for SPPARMα. Biochem. Biophys. Res. Commun. 2018, 499, 239–245. [Google Scholar] [CrossRef]
- Raza-Iqbal, S.; Tanaka, T.; Anai, M.; Inagaki, T.; Matsumura, Y.; Ikeda, K.; Taguchi, A.; Gonzalez, F.J.; Sakai, J.; Kodama, T. Transcriptome Analysis of K-877 (a Novel Selective PPARα Modulator (SPPARMα))-Regulated Genes in Primary Human Hepatocytes and the Mouse Liver. J. Atheroscler. Thromb. 2015, 22, 754–772. [Google Scholar] [CrossRef]
- Fruchart, J.C. Pemafibrate (K-877), a novel selective peroxisome proliferator-activated receptor α modulator for management of atherogenic dyslipidaemia. Cardiovasc. Diabetol. 2017, 16, 124. [Google Scholar] [CrossRef]
- Takei, K.; Han, S.I.; Murayama, Y.; Satoh, A.; Oikawa, F.; Ohno, H.; Osaki, Y.; Matsuzaka, T.; Sekiya, M.; Iwasaki, H.; et al. Selective peroxisome proliferator-activated receptor-α modulator K-877 efficiently activates the peroxisome proliferator-activated receptor-α pathway and improves lipid metabolism in mice. J. Diabetes Investig. 2017, 8, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Hennuyer, N.; Duplan, I.; Paquet, C.; Vanhoutte, J.; Woitrain, E.; Touche, V.; Colin, S.; Vallez, E.; Lestavel, S.; Lefebvre, P.; et al. The novel selective PPARα modulator (SPPARMα) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis 2016, 249, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.G. Peroxisome proliferators: Paradigms and prospects. Toxicol. Lett. 1993, 68, 193–201. [Google Scholar] [CrossRef]
- Misra, P.; Viswakarma, N.; Reddy, J.K. Peroxisome proliferator-activated receptor-α signaling in hepatocarcinogenesis. Subcell. Biochem. 2013, 69, 77–99. [Google Scholar] [PubMed]
- Peters, J.M.; Shah, Y.M.; Gonzalez, F.J. The role of peroxisome proliferator-activated receptors in carcinogenesis and chemoprevention. Nat. Rev. Cancer 2012, 12, 181–195. [Google Scholar] [CrossRef]
- Takei, K.; Nakagawa, Y.; Wang, Y.; Han, S.I.; Satoh, A.; Sekiya, M.; Matsuzaka, T.; Shimano, H. Effects of K-877, a novel selective PPARα modulator, on small intestine contribute to the amelioration of hyperlipidemia in low-density lipoprotein receptor knockout mice. J. Pharm. Sci. 2017, 133, 214–222. [Google Scholar] [CrossRef]
- Sairyo, M.; Kobayashi, T.; Masuda, D.; Kanno, K.; Zhu, Y.; Okada, T.; Koseki, M.; Ohama, T.; Nishida, M.; Sakata, Y.; et al. A Novel Selective PPARα Modulator (SPPARMα), K-877 (Pemafibrate), Attenuates Postprandial Hypertriglyceridemia in Mice. J. Atheroscler. Thromb. 2018, 25, 142–152. [Google Scholar] [CrossRef]
- Fruchart, J.C.; Santos, R.D.; Aguilar-Salinas, C.; Aikawa, M.; Al Rasadi, K.; Amarenco, P.; Barter, P.J.; Ceska, R.; Corsini, A.; Després, J.P.; et al. The selective peroxisome proliferator-activated receptor α modulator (SPPARMα) paradigm: Conceptual framework and therapeutic potential: A consensus statement from the International Atherosclerosis Society (IAS) and the Residual Risk Reduction Initiative (R3i) Foundation. Cardiovasc. Diabetol. 2019, 18, 71. [Google Scholar]
- Ishibashi, S.; Yamashita, S.; Arai, H.; Araki, E.; Yokote, K.; Suganami, H.; Fruchart, J.C.; Kodama, T.; K-877-04 Study Group. Effects of K-877, a novel selective PPARα modulator (SPPARMα), in dyslipidaemic patients: A randomized, double blind, active- and placebo-controlled, phase 2 trial. Atherosclerosis 2016, 249, 36–43. [Google Scholar] [CrossRef]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S.; K-877 Study Group. Efficacy and safety of K-877, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), in combination with statin treatment: Two randomised, double-blind, placebo-controlled clinical trials in patients with dyslipidaemia. Atherosclerosis 2017, 261, 144–152. [Google Scholar]
- Ishibashi, S.; Arai, H.; Yokote, K.; Araki, E.; Suganami, H.; Yamashita, S.; K-877 Study Group. Efficacy and safety of pemafibrate (K-877), a selective peroxisome proliferator-activated receptor α modulator, in patients with dyslipidemia: Results from a 24-week, randomized, double blind, active-controlled, phase 3 trial. J. Clin. Lipidol. 2018, 12, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Araki, E.; Yamashita, S.; Arai, H.; Yokote, K.; Satoh, J.; Inoguchi, T.; Nakamura, J.; Maegawa, H.; Yoshioka, N.; Tanizawa, Y.; et al. Effects of Pemafibrate, a Novel Selective PPARα Modulator, on Lipid and Glucose Metabolism in Patients with Type 2 Diabetes and Hypertriglyceridemia: A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes Care 2018, 41, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S.; K-877 Study Group. Efficacy and Safety of Pemafibrate Versus Fenofibrate in Patients with High Triglyceride and Low HDL Cholesterol Levels: A Multicenter, Placebo-Controlled, Double-Blind, Randomized Trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Matsuba, I.; Matsuba, R.; Ishibashi, S.; Yamashita, S.; Arai, H.; Yokote, K.; Suganami, H.; Araki, E. Effects of a novel selective peroxisome proliferator-activated receptor-α modulator, pemafibrate, on hepatic and peripheral glucose uptake in patients with hypertriglyceridemia and insulin resistance. J. Diabetes Investig. 2018, 9, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, S.; Masuda, D.; Matsuzawa, Y. Clinical Applications of a Novel Selective PPARα Modulator, Pemafibrate, in Dyslipidemia and Metabolic Diseases. J. Atheroscler. Thromb. 2019, 26, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Ida, S.; Kaneko, R.; Murata, K. Efficacy and safety of pemafibrate administration in patients with dyslipidemia: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2019, 18, 38. [Google Scholar] [CrossRef]
- Araki, E.; Yamashita, S.; Arai, H.; Yokote, K.; Satoh, J.; Inoguchi, T.; Nakamura, J.; Maegawa, H.; Yoshioka, N.; Tanizawa, Y.; et al. Efficacy and safety of pemafibrate in people with type 2 diabetes and elevated triglyceride levels: 52-week data from the PROVIDE study. Diabetes Obes. Metab. 2019, 21, 1737–1744. [Google Scholar] [CrossRef]
- Yokote, K.; Yamashita, S.; Arai, H.; Araki, E.; Suganami, H.; Ishibashi, S.; K-Study Group. Long-Term Efficacy and Safety of Pemafibrate, a Novel Selective Peroxisome Proliferator-Activated Receptor-α Modulator (SPPARMα), in Dyslipidemic Patients with Renal Impairment. Int. J. Mol. Sci. 2019, 20, 706. [Google Scholar] [CrossRef]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef]
- Holden, P.R.; Tugwood, J.D. Peroxisome proliferator-activated receptor α: Role in rodent liver cancer and species differences. J. Mol. Endocrinol. 1999, 22, 1–8. [Google Scholar] [CrossRef]
- Lawrence, J.W.; Li, Y.; Chen, S.; DeLuca, J.G.; Berger, J.P.; Umbenhauer, D.R.; Moller, D.E.; Zhou, G. Differential gene regulation in human versus rodent hepatocytes by peroxisome proliferator-activated receptor (PPAR) α. PPARα fails to induce peroxisome proliferation-associated genes in human cells independently of the level of receptor expression. J. Biol. Chem. 2001, 276, 31521–31527. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.H.; Savas, U.; Griffin, K.J.; Johnson, E.F. Identification of peroxisome proliferator-responsive human genes by elevated expression of the peroxisome proliferator-activated receptor α in HepG2 cells. J. Biol. Chem. 2001, 276, 27950–27958. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernández-Fernández, C.; Mouriño-Bayolo, D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Ferdinandusse, S.; Denis, S.; Van Roermund, C.W.; Wanders, R.J.; Dacremont, G. Identification of the peroxisomal beta-oxidation enzymes involved in the degradation of long-chain dicarboxylic acids. J. Lipid Res. 2004, 45, 1104–1111. [Google Scholar] [CrossRef]
- Yeldandi, A.V.; Rao, M.S.; Reddy, J.K. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat. Res. 2000, 448, 159–177. [Google Scholar] [CrossRef]
- Raucy, J.L.; Lasker, J.; Ozaki, K.; Zoleta, V. Regulation of CYP2E1 by ethanol and palmitic acid and CYP4A11 by clofibrate in primary cultures of human hepatocytes. Toxicol. Sci. 2004, 79, 233–241. [Google Scholar] [CrossRef]
- Savas, U.; Hsu, M.H.; Johnson, E.F. Differential regulation of human CYP4A genes by peroxisome proliferators and dexamethasone. Arch. Biochem. Biophys. 2003, 409, 212–220. [Google Scholar] [CrossRef]
- Pettersen, I.K.N.; Tusubira, D.; Ashrafi, H.; Dyrstad, S.E.; Hansen, L.; Liu, X.Z.; Nilsson, L.I.H.; Løvsletten, N.G.; Berge, K.; Wergedahl, H.; et al. Upregulated PDK4 expression is a sensitive marker of increased fatty acid oxidation. Mitochondrion 2019, 49, 97–110. [Google Scholar] [CrossRef]
- Attia, R.R.; Sharma, P.; Janssen, R.C.; Friedman, J.E.; Deng, X.; Lee, J.S.; Elam, M.B.; Cook, G.A.; Park, E.A. Regulation of pyruvate dehydrogenase kinase 4 (PDK4) by CCAAT/enhancer-binding protein beta (C/EBPbeta). J. Biol. Chem. 2011, 286, 23799–23807. [Google Scholar] [CrossRef]
- Holness, M.J.; Bulmer, K.; Smith, N.D.; Sugden, M.C. Investigation of potential mechanisms regulating protein expression of hepatic pyruvate dehydrogenase kinase isoforms 2 and 4 by fatty acids and thyroid hormone. Biochem. J. 2003, 369, 687–695. [Google Scholar] [CrossRef]
- Vilà-Brau, A.; De Sousa-Coelho, A.L.; Mayordomo, C.; Haro, D.; Marrero, P.F. Human HMGCS2 regulates mitochondrial fatty acid oxidation and FGF21 expression in HepG2 cell line. J. Biol. Chem. 2011, 286, 20423–20430. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, D.; Chung, D.; Tang, Z.; Huang, H.; Dai, L.; Qi, S.; Li, J.; Colak, G.; Chen, Y.; et al. Metabolic Regulation of Gene Expression by Histone Lysine β-Hydroxybutyrylation. Mol. Cell 2016, 62, 194–206. [Google Scholar] [CrossRef]
- Webb, J.C.; Patel, D.D.; Jones, M.D.; Knight, B.L.; Soutar, A.K. Characterization and tissue-specific expression of the human ‘very low density lipoprotein (VLDL) receptor’ mRNA. Hum. Mol. Genet. 1994, 3, 531–537. [Google Scholar]
- Tiebel, O.; Oka, K.; Robinson, K.; Sullivan, M.; Martinez, J.; Nakamuta, M.; Ishimura-Oka, K.; Chan, L. Mouse very low-density lipoprotein receptor (VLDLR): Gene structure, tissue-specific expression and dietary and developmental regulation. Atherosclerosis 1999, 145, 239–251. [Google Scholar] [CrossRef]
- Gao, Y.; Shen, W.; Lu, B.; Zhang, Q.; Hu, Y.; Chen, Y. Upregulation of hepatic VLDLR via PPARα is required for the triglyceride-lowering effect of fenofibrate. J. Lipid Res. 2014, 55, 1622–1633. [Google Scholar] [CrossRef]
- Merkel, M.; Weinstock, P.H.; Chajek-Shaul, T.; Radner, H.; Yin, B.; Breslow, J.L.; Goldberg, I.J. Lipoprotein lipase expression exclusively in liver. A mouse model for metabolism in the neonatal period and during cachexia. J. Clin. Investig. 1998, 102, 893–901. [Google Scholar] [CrossRef]
- Wang, S.; Smith, J.D. ABCA1 and nascent HDL biogenesis. Biofactors 2014, 40, 547–554. [Google Scholar] [CrossRef]
- Babashamsi, M.M.; Koukhaloo, S.Z.; Halalkhor, S.; Salimi, A.; Babashamsi, M. ABCA1 and metabolic syndrome; a review of the ABCA1 role in HDL-VLDL production, insulin-glucose homeostasis, inflammation and obesity. Diabetes Metab. Syndr. 2019, 13, 1529–1534. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, C. Regulation of ABCA1 functions by signaling pathways. Biochim. Biophys. Acta 2012, 1821, 522–529. [Google Scholar] [CrossRef]
- Brunham, L.R.; Singaraja, R.R.; Duong, M.; Timmins, J.M.; Fievet, C.; Bissada, N.; Kang, M.H.; Samra, A.; Fruchart, J.C.; McManus, B.; et al. Tissue-specific roles of ABCA1 influence susceptibility to atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 548–554. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Véniant, M.M.; Komorowski, R.; Chen, P.; Stanislaus, S.; Winters, K.; Hager, T.; Zhou, L.; Wada, R.; Hecht, R.; Xu, J. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology 2012, 153, 4192–4203. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARα-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Lundåsen, T.; Hunt, M.C.; Nilsson, L.M.; Sanyal, S.; Angelin, B.; Alexson, S.E.; Rudling, M. PPARα is a key regulator of hepatic FGF21. Biochem. Biophys. Res. Commun. 2007, 360, 437–440. [Google Scholar]
- Yamashita, S.; Arai, H.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S.; K-877 Study Group. Effects of pemafibrate (K-877) on cholesterol efflux capacity and postprandial hyperlipidemia in patients with atherogenic dyslipidemia. J. Clin. Lipidol. 2018, 12, 1267–1279. [Google Scholar] [CrossRef]
- Kim, H.; Mendez, R.; Zheng, Z.; Chang, L.; Cai, J.; Zhang, R.; Zhang, K. Liver-enriched transcription factor CREBH interacts with peroxisome proliferator-activated receptor α to regulate metabolic hormone FGF21. Endocrinology 2014, 155, 769–782. [Google Scholar] [CrossRef]
- Ip, W.K.; Takahashi, K.; Ezekowitz, R.A.; Stuart, L.M. Mannose-binding lectin and innate immunity. Immunol. Rev. 2009, 230, 9–21. [Google Scholar]
- Hansen, T.K. Growth hormone and mannan-binding lectin: Emerging evidence for hormonal regulation of humoral innate immunity. Minerva. Endocrinologica 2003, 28, 75–84. [Google Scholar]
- Fernández-Real, J.M.; Straczkowski, M.; Vendrell, J.; Soriguer, F.; Pérez Del Pulgar, S.; Gallart, L.; López-Bermejo, A.; Kowalska, I.; Manco, M.; Cardona, F.; et al. Protection from inflammatory disease in insulin resistance: The role of mannan-binding lectin. Diabetologia 2006, 49, 2402–2411. [Google Scholar] [CrossRef][Green Version]
- Holmes, R.S.; Spradling-Reeves, K.D.; Cox, L.A. Mammalian Glutamyl Aminopeptidase Genes (ENPEP) and Proteins: Comparative Studies of a Major Contributor to Arterial Hypertension. J. Data Min. Genomics Proteom. 2017, 8, 2. [Google Scholar] [CrossRef]
- Mizutani, S.; Ishii, M.; Hattori, A.; Nomura, S.; Numaguchi, Y.; Tsujimoto, M.; Kobayshi, H.; Murohara, T.; Wright, J.W. New insights into the importance of aminopeptidase A in hypertension. Heart Fail. Rev. 2008, 13, 273–284. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Goto, Y.; Maruyama, M.; Hattori, A. Biochemical and enzymatic properties of the M1 family of aminopeptidases involved in the regulation of blood pressure. Heart. Fail. Rev. 2008, 13, 285–291. [Google Scholar] [CrossRef]
- Mitsui, T.; Nomura, S.; Okada, M.; Ohno, Y.; Kobayashi, H.; Nakashima, Y.; Murata, Y.; Takeuchi, M.; Kuno, N.; Nagasaka, T.; et al. Hypertension and angiotensin II hypersensitivity in aminopeptidase A-deficient mice. Mol. Med. 2003, 9, 57–62. [Google Scholar] [CrossRef]
- Surendran, P.; Drenos, F.; Young, R.; Warren, H.; Cook, J.P.; Manning, A.K.; Grarup, N.; Sim, X.; Barnes, D.R.; Witkowska, K.; et al. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat. Genet. 2016, 48, 1151–1161. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Cheng, H.; Harris, R.C. Renal Endothelial Dysfunction in Diabetic Nephropathy. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 22–33. [Google Scholar] [CrossRef]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef]
- Maki, T.; Maeda, Y.; Sonoda, N.; Makimura, H.; Kimura, S.; Maeno, S.; Takayanagi, R.; Inoguchi, T. Renoprotective effect of a novel selective PPARα modulator K-877 in db/db mice: A role of diacylglycerol-protein kinase C-NAD(P)H oxidase pathway. Metabolism 2017, 71, 33–45. [Google Scholar] [CrossRef]
- Kitajima, K.; Miura, S.; Mastuo, Y.; Uehara, Y.; Saku, K. Newly developed PPAR-agonist (R)-K-13675 inhibits the secretion of inflammatory markers without affecting cell proliferation or tube formation. Atherosclerosis 2009, 203, 75–81. [Google Scholar] [CrossRef]
- Kovacic, J.C.; Dimmeler, S.; Harvey, R.P.; Finkel, T.; Aikawa, E.; Krenning, G.; Baker, A.H. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 190–209. [Google Scholar] [CrossRef]
- Cho, J.G.; Lee, A.; Chang, W.; Lee, M.S.; Kim, J. Endothelial to Mesenchymal Transition Represents a Key Link in the Interaction between Inflammation and Endothelial Dysfunction. Front. Immunol. 2018, 9, 294. [Google Scholar] [CrossRef]
- Thomas, A.A.; Biswas, S.; Feng, B.; Chen, S.; Gonder, J.; Chakrabarti, S. lncRNA H19 prevents endothelial-mesenchymal transition in diabetic retinopathy. Diabetologia 2019, 62, 517–530. [Google Scholar] [CrossRef]
- Gong, H.; Lyu, X.; Wang, Q.; Hu, M.; Zhang, X. Endothelial to mesenchymal transition in the cardiovascular system. Life Sci. 2017, 184, 95–102. [Google Scholar] [CrossRef]
- Li, Y.; Lui, K.O.; Zhou, B. Reassessing endothelial-to-mesenchymal transition in cardiovascular diseases. Nat. Rev. Cardiol. 2018, 15, 445–456. [Google Scholar] [CrossRef]
- Cheng, S.L.; Shao, J.S.; Behrmann, A.; Krchma, K.; Towler, D.A. Dkk1 and MSX2-Wnt7b signaling reciprocally regulate the endothelial-mesenchymal transition in aortic endothelial cells. Arter. Thromb. Vasc. Biol. 2013, 33, 1679–1689. [Google Scholar] [CrossRef]
- Glineur, C.; Gross, B.; Neve, B.; Rommens, C.; Chew, G.T.; Martin-Nizard, F.; Rodríguez-Pascual, F.; Lamas, S.; Watts, G.F.; Staels, B. Fenofibrate inhibits endothelin-1 expression by peroxisome proliferator-activated receptor α-dependent and independent mechanisms in human endothelial cells. Arter. Thromb. Vasc. Biol. 2013, 33, 621–628. [Google Scholar] [CrossRef]
- Widyantoro, B.; Emoto, N.; Nakayama, K.; Anggrahini, D.W.; Adiarto, S.; Iwasa, N.; Yagi, K.; Miyagawa, K.; Rikitake, Y.; Suzuki, T.; et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation 2010, 121, 2407–2418. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Capece, D.; Zazzeroni, F.; Liakouli, V.; Pantano, I.; Berardicurti, O.; Carubbi, F.; Pecetti, G.; et al. The Endothelial-mesenchymal Transition in Systemic Sclerosis Is Induced by Endothelin-1 and Transforming Growth Factor-β and May Be Blocked by Macitentan, a Dual Endothelin-1 Receptor Antagonist. J. Rheumatol. 2015, 42, 1808–1816. [Google Scholar] [CrossRef]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. FIELD study investigators. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Chew, E.Y.; Davis, M.D.; Danis, R.P.; Lovato, J.F.; Perdue, L.H.; Greven, C.; Genuth, S.; Goff, D.C.; Leiter, L.A.; Ismail-Beigi, F.; et al. Action to Control Cardiovascular Risk in Diabetes Eye Study Research Group. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014, 121, 2443–2451. [Google Scholar]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.G. Peroxisome proliferator activated receptor-α (PPARα) and PPAR gamma coactivator-1α (PGC-1α) regulation of cardiac metabolism in diabetes. Pediatr. Cardiol. 2011, 32, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Viswakarma, N.; Jia, Y.; Bai, L.; Gao, Q.; Lin, B.; Zhang, X.; Misra, P.; Rana, A.; Jain, S.; Gonzalez, F.J.; et al. The Med1 subunit of the mediator complex induces liver cell proliferation and is phosphorylated by AMP kinase. J. Biol. Chem. 2013, 288, 27898–27911. [Google Scholar] [CrossRef]
- Mukherjee, R.; Sun, S.; Santomenna, L.; Miao, B.; Walton, H.; Liao, B.; Locke, K.; Zhang, J.H.; Nguyen, S.H.; Zhang, L.T.; et al. Ligand and coactivator recruitment preferences of peroxisome proliferator activated receptor α. J. Steroid Biochem. Mol. Biol. 2002, 81, 217–225. [Google Scholar] [CrossRef]
- Surapureddi, S.; Yu, S.; Bu, H.; Hashimoto, T.; Yeldandi, A.V.; Kashireddy, P.; Cherkaoui-Malki, M.; Qi, C.; Zhu, Y.J.; Rao, M.S.; et al. Identification of a transcriptionally active peroxisome proliferator-activated receptor α-interacting cofactor complex in rat liver and characterization of PRIC285 as a coactivator. Proc. Natl. Acad. Sci. USA 2002, 99, 11836–11841. [Google Scholar] [CrossRef]
- Jia, Y.; Qi, C.; Kashireddi, P.; Surapureddi, S.; Zhu, Y.J.; Rao, M.S.; Le Roith, D.; Chambon, P.; Gonzalez, F.J.; Reddy, J.K. Transcription coactivator PBP, the peroxisome proliferator-activated receptor (PPAR)-binding protein, is required for PPARα-regulated gene expression in liver. J. Biol. Chem. 2004, 279, 24427–244234. [Google Scholar] [CrossRef]
- Pawlak, M.; Baugé, E.; Bourguet, W.; De Bosscher, K.; Lalloyer, F.; Tailleux, A.; Lebherz, C.; Lefebvre, P.; Staels, B. The transrepressive activity of peroxisome proliferator-activated receptor α is necessary and sufficient to prevent liver fibrosis in mice. Hepatology 2014, 60, 1593–1606. [Google Scholar] [CrossRef]
- Delerive, P.; De Bosscher, K.; Besnard, S.; Vanden Berghe, W.; Peters, J.M.; Gonzalez, F.J.; Fruchart, J.C.; Tedgui, A.; Haegeman, G.; Staels, B. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J. Biol. Chem. 1999, 274, 32048–32054. [Google Scholar] [CrossRef]
- Planavila, A.; Iglesias, R.; Giralt, M.; Villarroya, F. Sirt1 acts in association with PPARα to protect the heart from hypertrophy, metabolic dysregulation, and inflammation. Cardiovasc. Res. 2011, 90, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Paumelle, R.; Caron, S.; Hennuyer, N.; Mansouri, R.; Gervois, P.; Staels, B.; Haegeman, G.; De Bosscher, K. PPARα blocks glucocorticoid receptor α-mediated transactivation but cooperates with the activated glucocorticoid receptor α for transrepression on NF-κB. Proc. Natl. Acad. Sci. USA 2009, 106, 7397–7402. [Google Scholar] [CrossRef] [PubMed]
- Gervois, P.; Vu-Dac, N.; Kleemann, R.; Kockx, M.; Dubois, G.; Laine, B.; Kosykh, V.; Fruchart, J.C.; Kooistra, T.; Staels, B. Negative regulation of human fibrinogen gene expression by peroxisome proliferator-activated receptor α agonists via inhibition of CCAAT box/enhancer-binding protein β. J. Biol. Chem. 2001, 276, 33471–33477. [Google Scholar] [CrossRef] [PubMed]
- Dongol, B.; Shah, Y.; Kim, I.; Gonzalez, F.J.; Hunt, M.C. The acyl-CoA thioesterase I is regulated by PPARαand HNF4αvia a distal response element in the promoter. J. Lipid Res. 2007, 48, 1781–1791. [Google Scholar] [CrossRef]
- Marrapodi, M.; Chiang, J.Y. Peroxisome proliferator-activated receptor α (PPARα) and agonist inhibit cholesterol 7α-hydroxylase gene (CYP7A1) transcription. J. Lipid Res. 2000, 41, 514–520. [Google Scholar]
- Spann, N.J.; Kang, S.; Li, A.C.; Chen, A.Z.; Newberry, E.P.; Davidson, N.O.; Hui, S.T.; Davis, R.A. Coordinate transcriptional repression of liver fatty acid-binding protein and microsomal triglyceride transfer protein blocks hepatic very low density lipoprotein secretion without hepatosteatosis. J. Biol. Chem. 2006, 281, 33066–33077. [Google Scholar] [CrossRef]
- IJpenberg, A.; Tan, N.S.; Gelman, L.; Kersten, S.; Seydoux, J.; Xu, J.; Metzger, D.; Canaple, L.; Chambon, P.; Wahli, W.; et al. In vivo activation of PPAR target genes by RXR homodimers. EMBO J. 2004, 23, 2083–2091. [Google Scholar] [CrossRef]
- Pradhan, A.D.; Paynter, N.P.; Everett, B.M.; Glynn, R.J.; Amarenco, P.; Elam, M.; Ginsberg, H.; Hiatt, W.R.; Ishibashi, S.; Koenig, W.; et al. Rationale and design of the Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients with Diabetes (PROMINENT) study. Am. Heart J. 2018, 206, 80–93. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, Y.; Raza-Iqbal, S.; Tanaka, T.; Murakami, K.; Anai, M.; Osawa, T.; Matsumura, Y.; Sakai, J.; Kodama, T. Gene Expression Profiles Induced by a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα) Pemafibrate. Int. J. Mol. Sci. 2019, 20, 5682. https://doi.org/10.3390/ijms20225682
Sasaki Y, Raza-Iqbal S, Tanaka T, Murakami K, Anai M, Osawa T, Matsumura Y, Sakai J, Kodama T. Gene Expression Profiles Induced by a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα) Pemafibrate. International Journal of Molecular Sciences. 2019; 20(22):5682. https://doi.org/10.3390/ijms20225682
Chicago/Turabian StyleSasaki, Yusuke, Sana Raza-Iqbal, Toshiya Tanaka, Kentaro Murakami, Motonobu Anai, Tsuyoshi Osawa, Yoshihiro Matsumura, Juro Sakai, and Tatsuhiko Kodama. 2019. "Gene Expression Profiles Induced by a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα) Pemafibrate" International Journal of Molecular Sciences 20, no. 22: 5682. https://doi.org/10.3390/ijms20225682
APA StyleSasaki, Y., Raza-Iqbal, S., Tanaka, T., Murakami, K., Anai, M., Osawa, T., Matsumura, Y., Sakai, J., & Kodama, T. (2019). Gene Expression Profiles Induced by a Novel Selective Peroxisome Proliferator-Activated Receptor α Modulator (SPPARMα) Pemafibrate. International Journal of Molecular Sciences, 20(22), 5682. https://doi.org/10.3390/ijms20225682




