The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies
Abstract
1. Introduction
2. Therapeutic Properties of MSCs
2.1. Immunomodulation—A Key Process in Tissue Regeneration
2.2. Secretory Activity of MSCs—Growth Factors and Extracellular Vesicles (EVs)
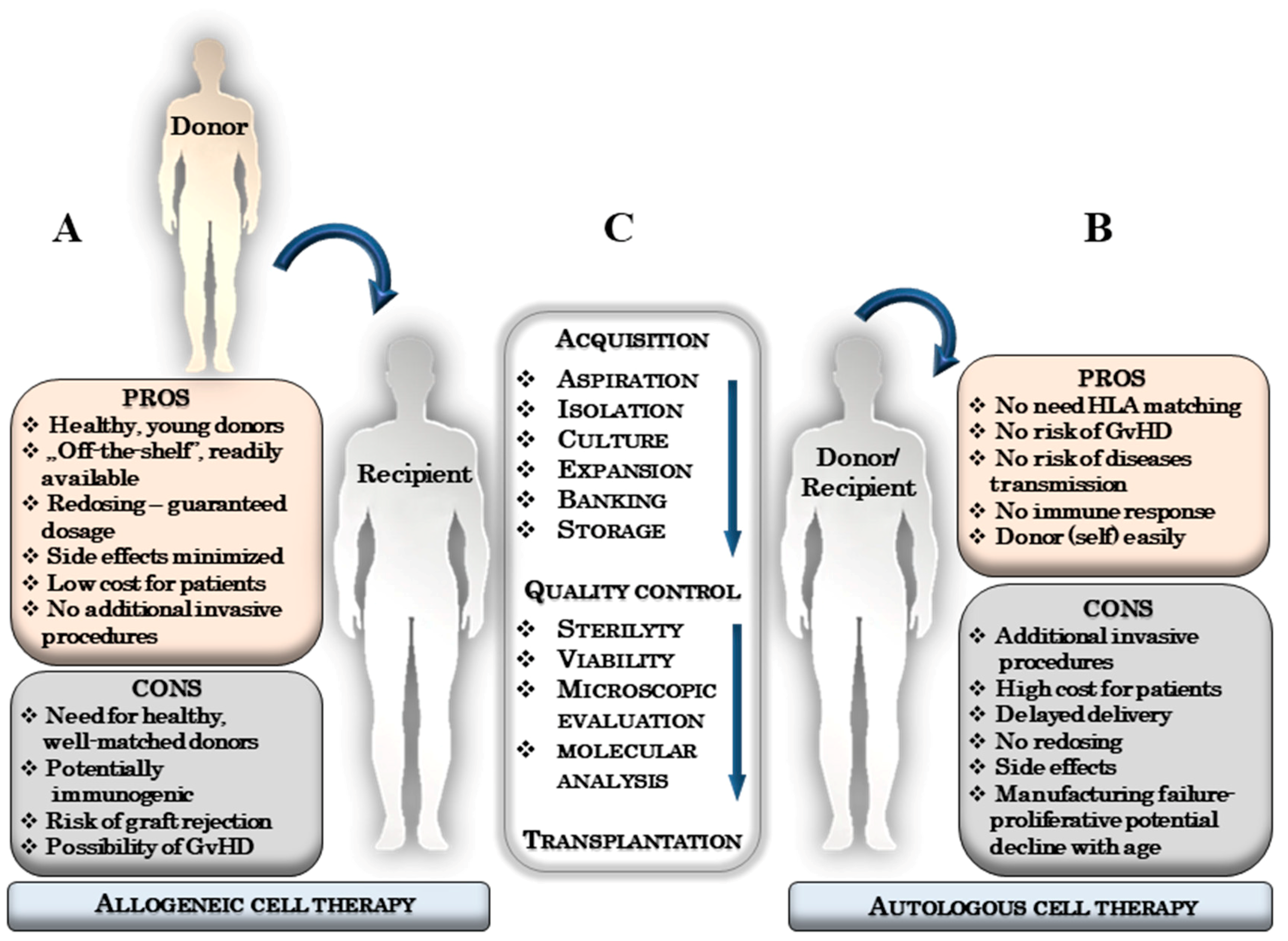
3. The Essence of an Autologous and Allogeneic Stem Cells Therapies
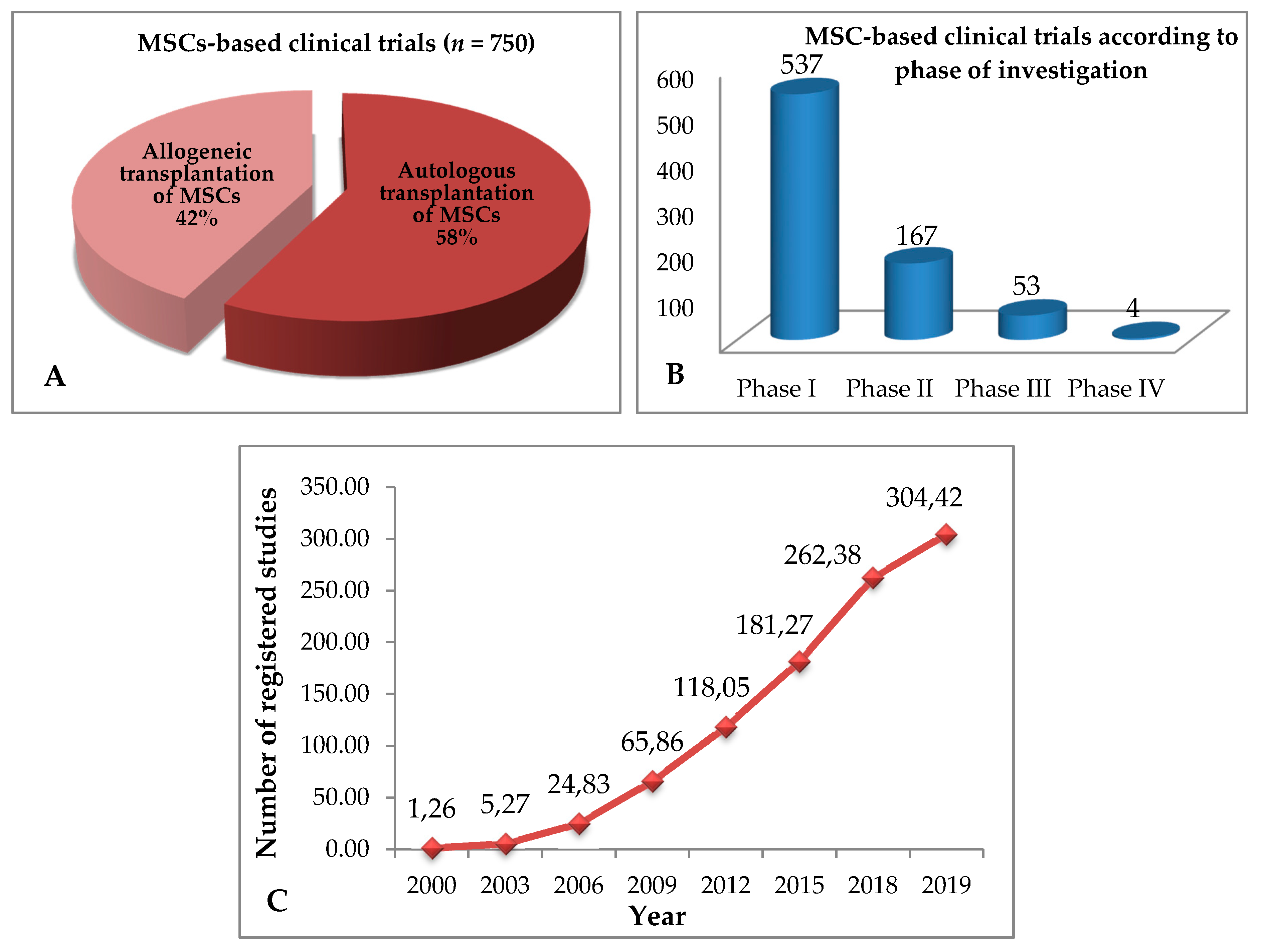
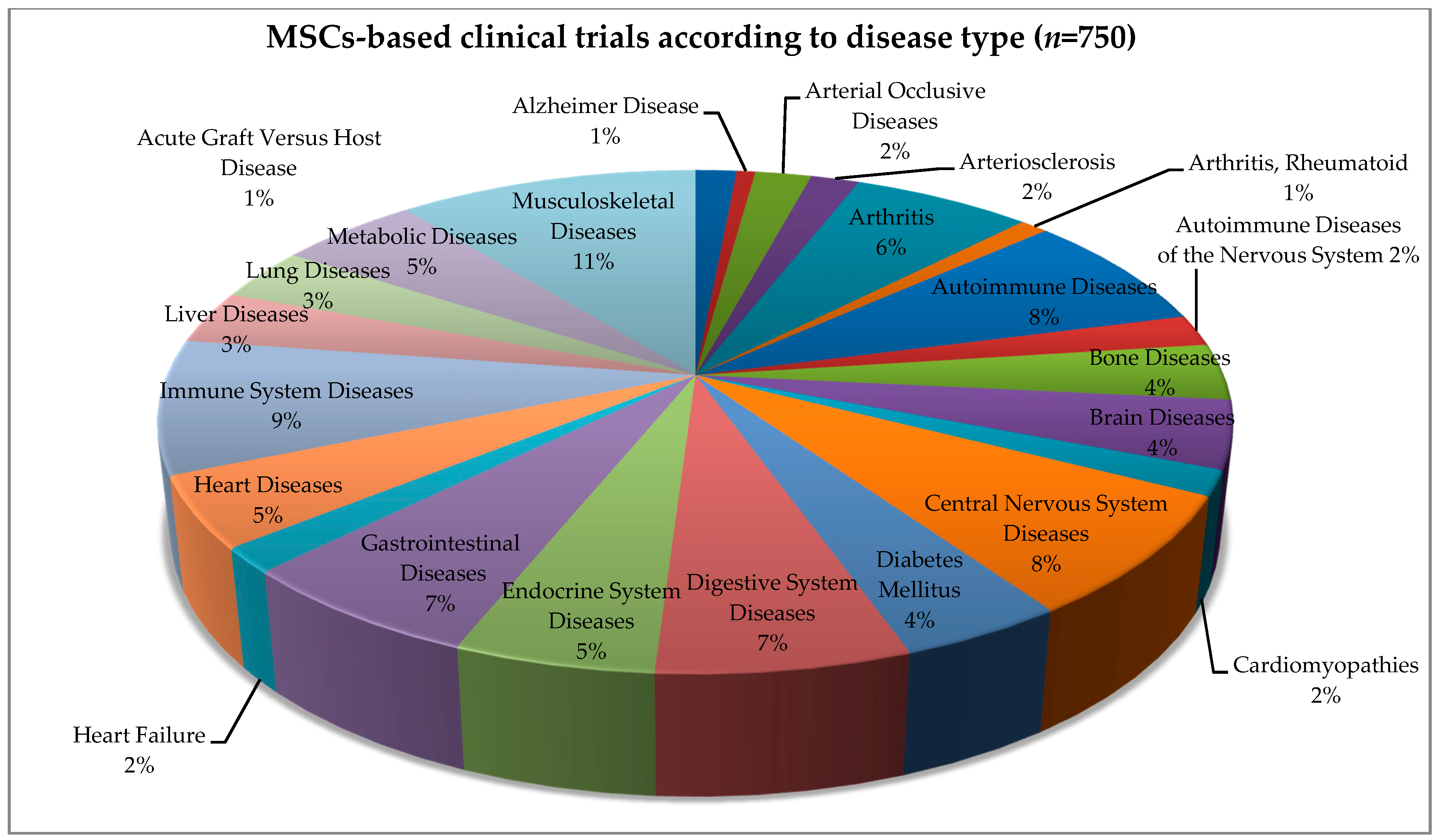
4. Allogeneic and Autologous Stem Cell Transplant—Clinical Trials
5. HLA Matching—Old and New Application Challenges
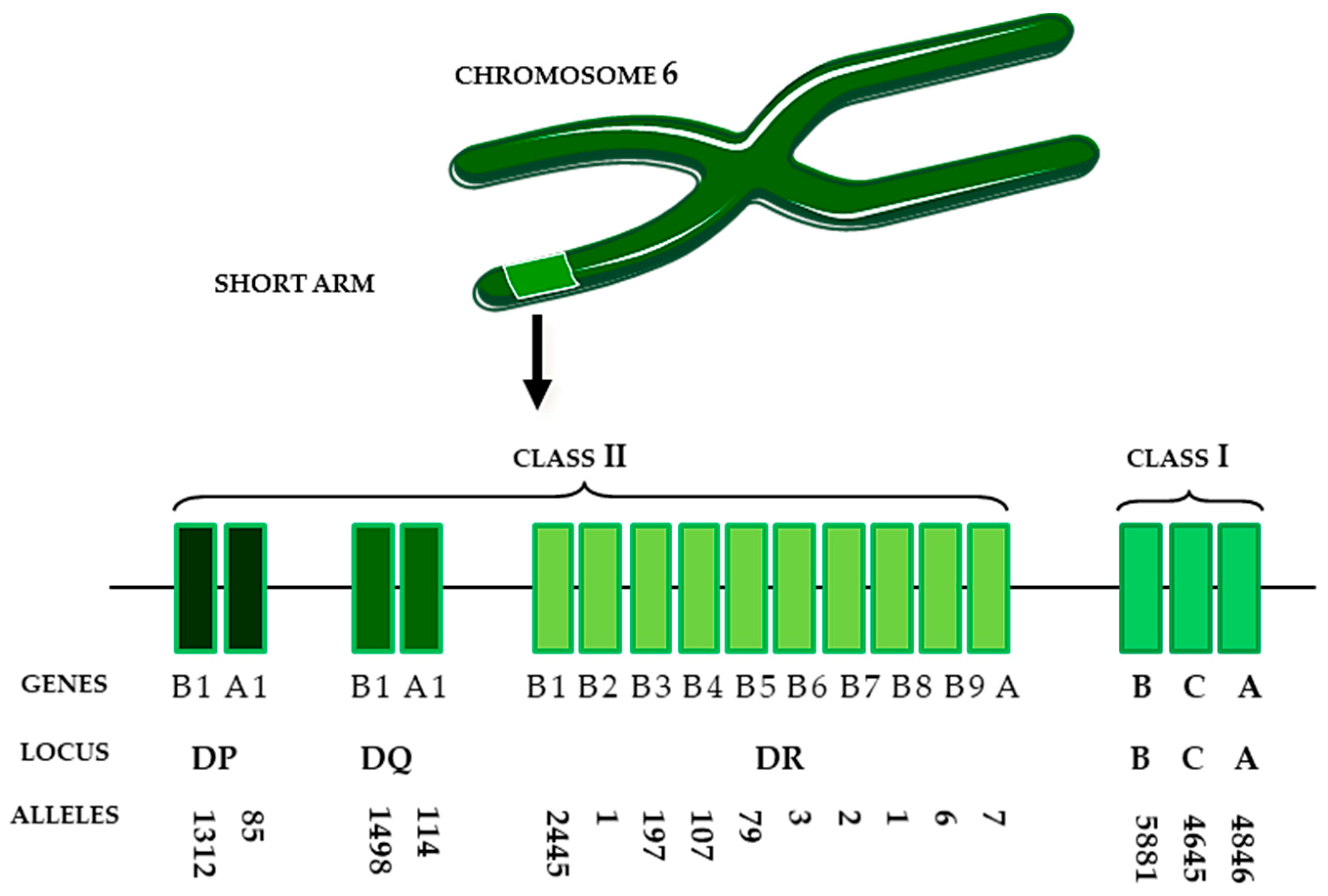
A Brief Story about Human Leukocyte Antigens (HLA)
6. Genes and Proteins—a Blessing or a Curse
7. Organ Transplantation—Focus on DSA
8. Hematopoietic Transplantation; from a Perfect Match to Haplocompatibility
9. Immuno-Privileged Status of MSCs
10. Safety of MSCs-Based Therapy
11. Regulatory Issues for Clinical Trials in Humans
12. Conclusions—Future Issues for Consideration
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal Stem Cells |
| HLA | Human Leukocyte Antigens |
| GvHD | Graft versus Host Disease |
| DSA | Donor Specific Antibodies |
| EVs | Extracellular Vesicles |
| ATIMP | Advanced Therapy Investigational Product |
| GMP | Good Manufacturing Practice |
References
- Gremmels, H.; Fledderus, J.O.; Teraa, M.; Verhaar, M.C. Mesenchymal stromal cells for the treatment of critical limb ischemia: Context and perspective. Stem Cell Res. Ther. 2013, 4, 140. [Google Scholar] [CrossRef][Green Version]
- Musialek, P.; Mazurek, A.; Jarocha, D.; Tekieli, L.; Szot, W.; Kostkiewicz, M.; Banys, R.P.; Urbanczyk, M.; Kadzielski, A.; Trystula, M.; et al. Myocardial regeneration strategy using Wharton’s jelly mesenchymal stem cells as an off-the-shelf ‘unlimited’ therapeutic agent: Results from the Acute Myocardial Infarction First-in-Man Study. Pos. Kardiol. Interw. 2015, 11, 100–107. [Google Scholar] [CrossRef]
- Shroff, G.; Gupta, R. Human embryonic stem cells in the treatment of patients with spinal cord injury. Ann. Neurosci. 2015, 22, 208–216. [Google Scholar] [CrossRef]
- Mervin, C.Y. Human endothelial progenitor cells. Cold Spring Harb. Perspect. Med. 2012, 2, 17–32. [Google Scholar]
- Fisher, S.A.; Doree, C.; Mathur, A.; Martin-Rendon, E. Meta-analysis of cell therapy trials for patients with heart failure. Circ. Res. 2015, 116, 1361–1377. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.Y.; Hoe Ng, W.; Ellison-Hughes, G.M.; Tan, J.J. Cardiac stem cells for myocardial regeneration: They are not alone. Front. Cardiovasc. Med. 2017, 4, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.A.; Rubio, F.J. Cell therapy using induced pluripotent stem cells or somatic stem cells: This is the question. Curr. Stem Cell Res. Ther. 2012, 7, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Z.; Chi, Y.; Zhang, Q.; Xu, F.; Yang, Z.; Meng, L.; Yang, S.; Yan, S.; Mao, A.; et al. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013, 4, e950. [Google Scholar] [CrossRef]
- Atala, A.; Cetrulo, K.; Taghizadeh, R.; Cetrulo, C.; Murphy, S. Perinatal Stem Cells; Elsevier Books: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Guillot, P.V.; De Bari, C.; Dell’Accio, F.; Kurata, H.; Polak, J.; Fisk, N.M. Comparative osteogenic transcription profiling of various fetal and adult mesenchymal stem cell sources. Differentiation 2008, 76, 946–957. [Google Scholar] [CrossRef]
- Yen, B.L.; Huang, H.I.; Chien, C.C.; Jui, H.Y.; Ko, B.S.; Yao, M.; Shun, C.T.; Yen, M.L.; Lee, M.C.; Chen, Y.C. Isolation of multipotent cells from human term placenta. Stem Cells 2005, 23, 3–9. [Google Scholar] [CrossRef]
- Sessarego, N.; Parodi, A.; Podestà, M.; Benvenuto, F.; Mogni, M.; Raviolo, V.; Lituania, M.; Kunkl, A.; Ferlazzo, G.; Bricarelli, F.D.; et al. Multipotent mesenchymal stromal cells from amniotic fluid: Solid perspectives for clinical application. Haematologica 2008, 93, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Troyer, D.L.; Weiss, M.L. Wharton’s jelly-derived cells are a primitive stromal cell population. Stem Cells 2008, 26, 591–599. [Google Scholar] [CrossRef] [PubMed]
- De Coppi, P.; Bartsch, G., Jr.; Siddiqui, M.M.; Xu, T.; Santos, C.C.; Perin, L.; Mostoslavsky, G.; Serre, A.C.; Snyder, E.Y.; Yoo, J.J.; et al. Isolation of amniotic stem cell lines with potential for therapy. Nat. Biotechnol. 2007, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Soler, R.; Morera, C.; Alberca, M.; Sanchez, A.; Garcia-Sancho, J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: A pilot study. Transplantation 2011, 92, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; Velazquez, D.L.D.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The Poseidon randomized trial. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martin-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: A randomized controlled trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Noriega, D.C.; Ardura, F.; Hernández-Ramajo, R.; Martín-Ferrero, M.Á.; Sánchez-Lite, I.; Toribio, B.; Alberca, M.; García, V.; Moraleda, J.M.; Sánchez, A.; et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: A randomized controlled trial. Transplantation 2017, 101, 1945–1951. [Google Scholar] [CrossRef]
- García-Sancho, J.; Sánchez, A.; Vega, A.; Noriega, C.D.; Nocito, M. Influence of HLA matching on the efficacy of allogeneic mesenchymal stromal cell therapies for osteoarthritis and degenerative disc disease. Transplant. Direct 2017, 3, 205–215. [Google Scholar] [CrossRef]
- Florea, V.; Rieger, A.C.; DiFede, D.L.; El-Khorazaty, J.; Natsumeda, M.; Banerjee, M.N.; Tompkins, B.A.; Khan, A.; Schulman, I.H. Dose Comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT study). Circ. Res. 2017, 10, 1279–1290. [Google Scholar] [CrossRef]
- Bolli, R.; Chugh, A.R.; D’Amario, D.; Loughran, J.H.; Stoddard, M.F.; Ikram, S.; Beache, G.M.; Wagner, S.G.; Leri, A.; Hosoda, T.; et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): Initial results of a randomised phase 1 trial. Lancet 2011, 378, 1847–1857. [Google Scholar] [CrossRef]
- Makkar, R.R.; Smith, R.R.; Cheng, K.; Malliaras, K.; Thomson, L.E.J.; Berman, D.; Czer, L.S.C.; Marbán, L.; Mendizabal, A.; Johnston, P.V.; et al. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): A prospective, randomised phase 1 trial. Lancet 2012, 379, 895–904. [Google Scholar] [CrossRef]
- Da Silva, M.L.; Fontes, A.M.; Covas, D.T.; Caplan, A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tredget, E.E.; Wu, P.Y.G.; Wu, Y.; Wu, Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS ONE 2008, 3, e1886. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, S.; Enhe, J.; Ma, K.; Yang, S.; Sun, T.; Fu, X. Bone marrow-derived mesenchymal stem cell attenuates skin fibrosis development in mice. Int. Wound J. 2014, 11, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Sun, C.K.; Lin, Y.C.; Chang, L.T.; Chen, Y.L.; Tsai, T.H.; Chung, S.Y.; Chua, S.; Kao, Y.H.; Yen, C.H.; et al. Adiposederived mesenchymal stem cell protects kidneys against ischemiareperfusion injury through suppressing oxidative stress and inflammatory reaction. J. Transl. Med. 2011, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Linero, I.; Chaparro, O. Paracrine effect of mesenchymal stem cells derived from human adipose tissue in bone regeneration. PLoS ONE 2014, 9, e107001. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, J.; Xu, X.; Wang, S.; Xu, R.; Zhao, M.; Nie, W.; Wang, X.; Zhang, J.; Li, T.; et al. Safety and immunological responses to human mesenchymal stem cell therapy in difficult-to-treat HIV-1-infected patients. AIDS 2013, 27, 1283–1293. [Google Scholar] [CrossRef]
- De Windt, T.S.; Saris, D.B.F.; Slaper-Cortenbach, I.C.M.; van Rijen, M.H.P.; Gawlitta, D.; Creemers, L.B.; De Weger, R.A.; Dhert, W.J.; Vonk, L.A. Direct cell–cell contact with chondrocytes is a key mechanism in multipotent mesenchymal stromal cell-mediated chondrogenesis. Tissue Eng. Part A 2015, 21, 2536–2547. [Google Scholar] [CrossRef]
- Tse, W.; Pendleton, J.; Beyer, W.; Egalka, M.; Guinan, E. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation 2003, 75, 389–397. [Google Scholar] [CrossRef]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisant, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef]
- Zhao, Q.; Ren, H.; Han, Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J. Cell. Immunother. 2015, 2, 3–20. [Google Scholar] [CrossRef]
- Le Blanc, K.; Tammik, L.; Sundberg, B.; Haynesworth, S.E.; Ringden, O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand. J. Immunol. 2003, 57, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Klyushnenkova, E.; Mosca, J.D.; Zernetkina, V.; Majumdar, M.K.; Beggs, K.J.; Simonetti, D.W.; Deans, R.J.; McIntosh, K.R. T cell responses to allogeneic human mesenchymal stem cells: Immunogenicity, tolerance, and suppression. J. Biomed. Sci. 2005, 12, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Fu, Q.L.; Chow, Y.Y.; Sun, S.J.; Zeng, Q.X.; Li, H.B.; Shi, J.B.; Sun, Y.Q.; Wen, W.; Tse, H.F.; Lian, Q.; et al. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy 2012, 67, 1215–1222. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Soto-Gutierrez, A.; Parekkadan, B.; Kitagawa, Y.; Tompkins, R.G.; Kobayashi, N.; Yarmush, M.L. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010, 19, 667–679. [Google Scholar] [CrossRef]
- Chabannes, D.; Hill, M.; Merieau, E.; Rossignol, J.; Brion, R.; Soulillou, J.P.; Anegon, I.; Cuturi, M.C. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood 2007, 110, 3691–3694. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Deng, M.X.; He, J.; Zeng, Q.X.; Wen, W.; Wong, D.S.; Tse, H.-F.; Xu, G.; Lian, Q.; Shi, J.; et al. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cells 2012, 30, 2692–2699. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, 20–36. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 2006, 24, 386–398. [Google Scholar] [CrossRef]
- Ryan, J.M.; Barry, F.; Murphy, J.M.; Mahon, B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin. Exp. Immunol. 2007, 149, 353–363. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, O.; Lombardo, E.; Beraza, A.; Mancheno-Corvo, P.; Ramirez, C.; Menta, R.; Rico, L.; Camarillo, E.; García, L.; Abad, J.L.; et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng. Part A 2009, 15, 2795–2806. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.I.; Zhao, R.C.; Shi, Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Crop, M.J.; Korevaar, S.S.; de Kuiper, R.; Ijzermans, J.N.; Van Besouw, N.M.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Human mesenchymal stem cells are susceptible to lysis by CD8(+) T cells and NK cells. Cell Transpl. 2011, 20, 1547–1559. [Google Scholar] [CrossRef]
- Gotherstrom, C.; Lundqvist, A.; Duprez, I.R.; Childs, R.; Berg, L.; Le Blanc, K. Fetal and adult multipotent mesenchymal stromal cells are killed by different pathways. Cytotherapy 2011, 13, 269–278. [Google Scholar] [CrossRef]
- Kaundal, U.; Bagai, U.; Rakha, A. Immunomodulatory plasticity of mesenchymal stem cells: A potential key to successful solid organ transplantation. J. Transl. Med. 2018, 16, 31. [Google Scholar] [CrossRef]
- Pittenger, M.F.; MacKay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Zhao, K.; Lou, R.; Huang, F.; Peng, Y.; Jiang, Z.; Huang, K.; Wu, X.; Zhang, Y.; Fan, Z.; Zhou, H.; et al. Immunomodulation effects of mesenchymal stromal cells on acute graft-versus- host disease after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2015, 21, 97–104. [Google Scholar] [CrossRef]
- Atoui, R.; Chiu, R.C.J. Concise review: Immunomodulatory properties of mesenchymal stem cells in cellular transplantation: Update, controversies, and unknowns. Stem Cells Transl. Med. 2012, 1, 200–205. [Google Scholar] [CrossRef]
- Kim, H.; Lee, Y.T.; Hong, J.M.; Hwang, Y.I. Suppression of in vitro murine T cell proliferation by human adipose tissue-derived mesenchymal stem cells is dependent mainly on cyclooxygenase-2 expression. Anat. Cell Biol. 2013, 46, 262–271. [Google Scholar] [CrossRef]
- Benvenuto, F.; Ferrari, S.; Gerdoni, E.; Gualandi, F.; Frassoni, F.; Pistoia, V.; Mancardi, G.; Uccelli, A.; Mancardi, G.L. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells 2007, 25, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Marti, L.C.; Ribeiro, A.A.; Hamerschlak, N. Immunomodulatory effect of mesenchymal stem cells. Stem Cells 2011, 9, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Basciano, L.; Nemos, C.; Foliguet, B.; De Isla, N.; De Carvalho, M.; Tran, N.; Dalloul, A. Long term culture of mesenchymal stem cells in hypoxia promotes a genetic program maintaining their undifferentiated and multipotent status. BMC Cell Biol. 2011, 12, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Hennink, S.; Molendijk, I.; van Zuylen, V.L.; Bosse, T.; Vos, A.C.W.; De Jonge-Muller, E.S.M.; Roelofs, H.; et al. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells 2011, 29, 1549–1558. [Google Scholar] [CrossRef]
- Rutz, S.; Janke, M.; Kassner, N.; Hohnstein, T.; Krueger, M.; Scheffold, A. Notch regulates IL-10 production by T helper 1 cells. Proc. Natl. Acad. Sci. USA 2008, 105, 3497–3502. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Su, W.R.; Shi, S.H.; Wilder-Smith, P.; Xiang, A.P.; Wong, A.; Nguyen, A.L.; Kwon, C.W.; Le, A.D. Human gingiva-derived mesenchymal stem cells elicit polarization of m2 macrophages and enhance cutaneous wound healing. Stem Cells 2010, 28, 1856–1868. [Google Scholar] [CrossRef]
- Selleri, S.; Bifsha, P.; Civini, S.; Pacelli, C.; Dieng, M.M.; Lemieux, W.; Jin, P.; Bazin, R.; Patey, N.; Marincola, F.M.; et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget 2016, 7, 30193–30210. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells Int. 2018, 18, 305–324. [Google Scholar] [CrossRef]
- Nasef, A.; Mathieu, N.; Chapel, A.; Frick, J.; Franc¸ois, S.; Mazurier, C.; Boutarfa, A.; Bouchet, S.; Gorin, N.C.; Thierry, D.; et al. Immunosuppressive Effects of Mesenchymal Stem Cells: Involvement of HLA-G. Transplantation 2007, 84, 231–237. [Google Scholar] [CrossRef]
- Hunt, J.S.; Petroff, M.G.; Morales, P.; Sedlmayr, P.; Geraghty, D.E.; Ober, C. HLA-G in reproduction: Studies on the maternal-fetal interface. Hum. Immunol. 2000, 6, 1113–1117. [Google Scholar] [CrossRef]
- Machado, C.; Telles, P.; Nascimento, I. Immunological characteristics of mesenchymal stem cells. Rev. Bras. Hematol. Hemoter. 2013, 35, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Selmani, Z.; Naji, A.; Gaiffe, E.; Obert, L.; Tiberghien, P.; Rouas-Freiss, N.; Carosella, E.D.; Deschaseaux, F. HLA-G is a crucial immunosuppressive molecule secreted by adult human mesenchymal stem cells. Transplantation 2009, 87, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, Q.; Wang, Z.; Tong, H.; Ma, L.; Zhang, Y.; Shan, F.; Meng, Y.; Yuan, Z. Comparative analysis of human mesenchymal stem cells from fetal-bone marrow, adipose tissue, and Wharton’s jelly as sources of cell immunomodulatory therapy. Hum. Vaccines Immunother. 2016, 12, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.C.; Chou, H.L.; Chang, Y.H.; Hung, W.T.; Liu, H.W.; Chu, T.Y. Characterization of HLA-G and related immunosuppressive effects in human umbilical cord stroma-derived stem cells. Cell Transplant. 2016, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Fanchin, R.; Gallot, V.; Rouas-Freiss, N.; Frydman, R.; Carosella, E.D. Implication of HLA-G in human embryo implantation. Hum. Immunol. 2007, 68, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Steinborn, A.; Varkonyi, T.; Scharf, A.; Bahlmann, F.; Klee, A.; Sohn, C. Early detection of decreased soluble HLA-G levels in the maternal circulation predicts the occurrence of preeclampsia and intrauterine growth retardation during further course of pregnancy. Am. J. Reprod. Immunol. 2007, 57, 277–286. [Google Scholar] [CrossRef]
- Mallis, P.; Boulari, D.; Michalopoulos, E.; Dinou, A.; Spyropoulou-Vlachou, M.; Stavropoulos-Giokas, C. Evaluation of HLA-G Expression in multipotent mesenchymal stromal cells derived from vitrified Wharton’s jelly tissue. Bioengineering 2018, 5, 95. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, G.; Meng, F.; Wang, W.; Hao, P.; Xiang, Y.; Wang, Y.; Han, R.; Li, F.; Wang, L.; et al. Paracrine effects of mesenchymal stem cells on the activation of keratocytes. Br. J. Ophthalmol. 2017, 101, 1583–1590. [Google Scholar] [CrossRef]
- Wen, J.D.; Ying, C.; Zhou, X.Y.; Zong, J.L.; Cui, J.J.; Song, B.Q.; Li, X.; Yang, S.G.; Han, Z.B.; Han, Z.C. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 2016, 7, 163–178. [Google Scholar]
- Musiał-Wysocka, A.; Kot, M.; Sułkowski, M.; Majka, M. Regenerative potential of the product “Cardiocell” derived from the Wharton’s jelly mesenchymal stem cells for treating hindlimb ischemia. Int. J. Mol. Sci. 2019, 20, 4632. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, T.N.; Jay, S.M. Production of extracellular vesicles loaded with therapeutic cargo. Methods Mol. Biol. 2018, 1831, 37–47. [Google Scholar] [PubMed]
- Wendt, S.; Goetzenich, A.; Goettsch, C.; Stoppe, C.; Bleilevens, C.; Kraemer, S.; Benstoem, C. Evaluation of the cardioprotective potential of extracellular vesicles—A systematic review and meta-analysis. Sci. Rep. 2018, 8, 1570–1589. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Mavin, E.; Nicholson, L.; Green, K.; Dickinson, A.M.; Wang, X. Mesenchymal stromal cell-derived extracellular vesicles attenuate dendritic cell maturation and function. Front. Immunol. 2018, 9, 2538–2552. [Google Scholar] [CrossRef]
- Galieva, L.R.; James, V.; Mukhamedshina, Y.O.; Rizvanov, A.A. Therapeutic potential of extracellular vesicles for the treatment of nerve disorders. Front. Neurosci. 2019, 13, 163–178. [Google Scholar] [CrossRef]
- Chinen, J.; Buckley, R.H. Transplantation immunology: Solid organ and bone marrow. J. Allergy Clin. Immunol. 2010, 125, 324–335. [Google Scholar] [CrossRef]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef]
- Van den Akker, F.; de Jager, S.C.; Sluijter, J.P. Mesenchymal stem cell therapy for cardiac inflammation: Immunomodulatory properties and the influence of toll-like receptors. Mediat. Inflamm. 2013, 2013, 1810–1820. [Google Scholar] [CrossRef]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef]
- Hatzistergos, K.E.; Quevedo, H.; Oskouei, B.N.; Hu, Q.; Feigenbaum, G.S.; Margitich, I.S.; Mazhari, R.; Boyle, A.J.; Zambrano, J.P.; Rodriguez, J.E.; et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ. Res. 2010, 107, 913–922. [Google Scholar] [CrossRef]
- Poncelet, A.J.; Vercruysse., J.; Saliez., A.; Gianello., P. Although pig allogeneic mesenchymal stem cells are not immunogenic in vitro, intracardiac injection elicits an immune response in vivo. Transplantation 2007, 83, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.C.; Boyd, A.S.; Wood, K.J. Embryonic stem cells and their differentiated derivatives have a fragile immune privilege but still represent novel targets of immune attack. Stem Cells 2008, 26, 1939–1950. [Google Scholar] [CrossRef]
- Eliopoulos, N.; Stagg, J. Allogeneic marrow stromal cells are immune rejected by MHC class I and class II-mismatched recipient mice. Gene Ther. 2005, 106, 4057–4065. [Google Scholar] [CrossRef]
- Nauta, A.J.; Westerhuis, G.; Kruisselbrink, A.B.; Lurvink, E.G.; Willemze, R.; Fibbe, W.E. Donor derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood 2006, 108, 2114–2120. [Google Scholar] [CrossRef]
- Badillo, A.T.; Beggs, K.J.; Javazon, E.H.; Tebbets, J.C.; Flake, A.W. Murine bone marrow stromal progenitor cells elicit an in vivo cellular and humoral alloimmune response. Biol. Blood Marrow Transplant. 2007, 13, 412–422. [Google Scholar] [CrossRef]
- Zangi, L.; Margalit, R.; Beilhack, A.; Negrin, R.; Reisner, Y.; Reich-Zeliger, S.; Bachar-Lustig, E.; Reich-Zeliger, S.; Bachar-Lustig, E. Direct imaging of immune rejection and memory induction by allogeneic mesenchymal stromal cells. Stem Cells 2009, 27, 2865–2874. [Google Scholar] [CrossRef]
- Isakova, I.A.; Lanclos, C.; Bruhn, J.; Kuroda, M.J.; Baker, K.C.; Krishnappa, V.; Phinney, D.G. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS ONE 2014, 9, e87238. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Fortier, L.A.; Antczak, D.F.; Cassano, J.M.; Brosnahan, M.M.; Miller, D.; Schnabel, L.V. Equine allogeneic bone marrow-derived mesenchymal stromal cells elicit antibody responses in vivo. Stem Cell Res. Ther. 2015, 6, 1–11. [Google Scholar] [CrossRef]
- Lazarus, H.M. Bone marrow transplantation in low grade non-Hodgkin’s lymphoma. Leuk. Lymphoma 1995, 17, 199–210. [Google Scholar] [CrossRef]
- Ascheim, D.D.; Gelijns, A.C.; Goldstein, D.; Moye, L.A.; Smedira, N.; Lee, S.; Klodell, C.T.; Szady, A.; Parides, M.K.; O Jeffries, N.; et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation 2014, 129, 2287–2296. [Google Scholar] [CrossRef]
- Panés, J.; García-Olmo, D.; Van Assche, G.; Colombel, J.F.; Reinisch, W.; Baumgart, D.C.; Dignass, A.; Nachury, M.; Ferrante, M.; Kazemi-Shirazi, L.; et al. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fi stulas in Crohn’s disease: A phase 3 randomised, double-blind controlled trial. Lancet 2016, 388, 1281–1290. [Google Scholar] [CrossRef]
- Le Blanc, K.; Ringden, O. Immunomodulation by mesenchymal stem cells and clinical experience. J. Intern. Med. 2007, 262, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stemcells: A pilot study. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; DiFede, D.L.; Rieger, A.C.; Florea, V.; Landin, A.M.; El-Khorazaty, J.; Khan, A.; Mushtaq, M.; Lowery, M.H.; Byrnes, J.J.; et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J. Am. Coll. Cardiol. 2017, 69, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Peeters, C.M.; Leijs, M.J.; Reijman, M.; Van Osch, G.J.; Bos, P.K. Safety of intraarticular cell-therapy with culture-expanded stem cells in humans: A systematic literature review. Osteoarth. Cartil. 2013, 21, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Liang, J.; Li, X.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Lu, L.; Gilkeson, G.S.; et al. Allogeneic mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus: 4 years of experience. Cell Transplant. 2013, 22, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, Y.; Huang, L.; Yang, J.; Yang, R.; Deng, W.; Liang, B.; Dai, L.; Meng, Q.; Gao, L.; et al. Effects and safety of allogenic mesenchymal stem cells intravenous infusion in active ankylosing spondylitis patients who failed NSAIDs: A 20 week clinical trial. Cell Transplant. 2013, 23, 1293–1303. [Google Scholar] [CrossRef]
- Reinders, M.E.J.; Fibbe, W.E.; Rabelink, T.J. Multipotent mesenchymal stromal cell therapy in renal disease and kidney transplantation. Nephrol. Dial. Transplant. 2010, 25, 17–24. [Google Scholar] [CrossRef]
- Kebriaei, P.; Saliba, R.; Rondon, G.; Chiattone, A.; Luthra, R.; Anderlini, P.; Andersson, B.; Shpall, E.; Popat, U.; Jones, R.; et al. Long-term follow-up of allogeneic hematopoietic stem cell transplantation for patients with Philadelphia chromosome-positive acute lymphoblasticleukemia: Impactof tyrosine kinase inhibitors on treatment outcomes. Biol. Blood Marrow Transplant. 2012, 18, 584–592. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Muller, I.; Kordowich, S.; Holzwarth, C.; Isensee, G.; Lang, P.; Neunhoeffer, F.; Dominici, M.; Greil, J.; Handgretinger, R. Application of multipotent mesenchymal stromal cells in pediatric patients following allogeneic stem cell transplantation. Blood Cells Mol. Dis. 2008, 40, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lauden, L.; Boukouaci, W.; Borlado, L.R.; Lopez, I.P.; Sepulveda, P.; Tamouza, R.; Charron, D.; Al-Daccak, R. Allogenicity of human cardiac stem/progenitor cells orchestrated by programmed death ligand 1. Circ. Res. 2013, 112, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.R.; Barile, L.; Cho, H.C.; Leppo, M.K.; Hare, J.M.; Messina, E.; Giacomello, A.; Abraham, M.R.; Marban, E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 2007, 115, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Vijayan, K.; Colletti, E.J.; Harrington, D.A.; Matthiesen, T.S.; Simpson, D.; Goh, S.K.; Walker, B.L.; Almeida-Porada, G.; Wang, D.; et al. Characterization and functionality of cardiac progenitor cells in congenital heart patients. Circulation 2011, 123, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Jansen of Lorkeers, S.J.; Eding, J.E.; Vesterinen, H.M.; van der Spoel, T.I.; Sena, E.S.; Duckers, H.J.; Doevendans, P.A.; Macleod, M.R.; Chamuleau, S.A. Similar effect of autologous and allogeneic cell therapy for ischemic heart disease: Systematic review and meta-analysis of large animal studies. Circ. Res. 2015, 116, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Shen, R.; Song, L.; Lu, M.; Wang, J.; Zhao, S.; Tang, Y.; Meng, X.; Li, Z.; He, Z.X. Bone marrow mesenchymal stem cells (BM-MSCs) improve heart function in swine myocardial infarction model through paracrine effects. Sci. Rep. 2016, 6, 8250–8271. [Google Scholar] [CrossRef]
- Karantalis, V.; Hare, J.M. Use of mesenchymal stem cells for therapy of cardiac disease. Circ. Res. 2016, 116, 1413–1430. [Google Scholar] [CrossRef]
- Scolding, N.J.; Pasquini, M.; Reingold, S.C.; Cohen, J.A. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017, 140, 2776–2796. [Google Scholar] [CrossRef]
- Song, C.G.; Zhang, Y.Z.; Wu, H.N.; Cao, X.L.; Guo, C.J.; Li, Y.Q.; Zheng, M.H.; Han, H. Stem cells: A promising candidate to treat neurological disorders. Neural Regen. Res. 2018, 13, 1294–1304. [Google Scholar]
- Jarocha, D.; Milczarek, O.; Wedrychowicz, A.; Kwiatkowski, S.; Majka, M. Continuous improvement after multiple mesenchymal stem cell transplantations in a patient with complete spinal cord injury. Cell Transplant. 2015, 24, 661–672. [Google Scholar] [CrossRef]
- Milczarek, O.; Jarocha, D.; Starowicz-Filip, A.; Kwiatkowski, S.; Badyra, B.; Majka, M. Multiple autologous bone marrow-derived CD271+ mesenchymal stem cell transplantation overcomes drug-resistant epilepsy in children. Stem Cells Transl. Med. 2018, 7, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Wysocka, A.; Kot, M.; Sułkowski, M.; Badyra, B.; Majka, M. Molecular and Functional Verification of Wharton’s Jelly Mesenchymal Stem Cells (WJ-MSCs) Pluripotency. Int. J. Mol. Sci. 2019, 20, 1807. [Google Scholar] [CrossRef] [PubMed]
- Gorer, P.A. The detection of a hereditary antigenic difference in the blood of mice by means of human group a serum. J. Genet. 1936, 32, 17–31. [Google Scholar] [CrossRef]
- Terasaki, P.I. The history of HLA and transplantation. Hirosaki Med. J. 2013, 64, S45–S52. [Google Scholar]
- Choo, S.Y. The HLA system: Genetics, immunology, clinical testing, and clinical implications. Yonsei Med. J. 2007, 48, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Janeway, J.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology: The Immune System in Health and Disease, 5th ed.; Current Biology: London, UK, 2001; Chapter 9; Available online: http://www.ncbi.nlm.nih.gov/books/NBK27162/ (accessed on 14 May 2019).
- HLA. Available online: http://hla.alleles.org/nomenclature/index.html (accessed on 14 May 2019).
- Le Bouteiller, P.; Lenfant, F. Antigen-presenting function(s) of the non-classical HLA-E, -F and -G class I molecules: The beginning of a story. Res. Immunol. 1996, 147, 301–313. [Google Scholar] [CrossRef]
- Ohashi, K.; Saji, F.; Kato, M.; Wakimoto, A.; Tanizawa, O. HLA expression on human ejaculated sperm. Am. J. Reprod. Immunol. 1990, 23, 29–32. [Google Scholar] [CrossRef]
- Fiszer, D.; Kurpisz, M. Major histocompatibility complex expression on human, male germ cells: A review. Am. J. Reprod. Immunol. 1998, 40, 172–176. [Google Scholar] [CrossRef]
- Apps, R.; Murphy, S.P.; Fernando, R.; Gardner, L.; Ahad, T.; Moffett, A. Human leucocyte antigen (HLA) expression of primary trophoblast cells and placental cell lines, determined using single antigen beads to characterize allotype specificities of anti-HLA antibodies. Immunology 2009, 127, 26–39. [Google Scholar] [CrossRef]
- De Villartay, J.P.; Rouger, P.; Muller, J.Y.; Salmon, C. HLA antigens on peripheral red blood cells: Analysis by flow cytofluorometry using monoclonal antibodies. Tissue Antigens 1985, 26, 12–19. [Google Scholar] [CrossRef]
- Effros, R.B.; Dillard, L.; Zeller, E.; Naeim, F.; Walford, R.L. Strong HLA-DR expression in T cell cultures after activation is necessary for IL-2-dependent proliferation. Hum. Immunol. 1983, 8, 249–254. [Google Scholar] [CrossRef]
- Muczynski, K.A.; Ekle, D.M.; Coder, D.M.; Anderson, S.K. Normal human kidney HLA-DR-expressing renal microvascular endothelial cells: Characterization, isolation, and regulation of MHC class II expression. J. Am. Soc. Nephrol. 2003, 14, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Afzali, B.; Lombardi, G.; Lechler, R.I. Pathways of major histocompatibility complex allorecognition. Curr. Opin. Organ Transplant. 2008, 13, 438–444. [Google Scholar] [CrossRef]
- Opelz, G. Correlation of HLA matching with kidney graft survival in patients with or without cyclosporine treatment. Transplantation 1985, 40, 240–243. [Google Scholar] [CrossRef]
- Ansari, D.; Bućin, D.; Nilsson, J. Human leukocyte antigen matching in heart transplantation: Systematic review and meta-analysis. Transpl. Int. 2014, 27, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Squifflet, J.P.; Moudry, K.; Sutherland, D.E.R. Is HLA matching relevant in pancreas transplantation?—A registry analysis. Transpl. Int. 1988, 1, 26–29. [Google Scholar] [CrossRef]
- Jonker, M.; Hoogeboom, J.; Van Leeuwen, A.; Koch, C.T.; van Oud Alblas, D.B.; van Rood, J.J. Influence of matching for HLA-DR antigens on skin graft survival. Transplantation 1979, 27, 91–94. [Google Scholar] [CrossRef]
- Leffell, M.S.; Steinberg, A.G.; Bias, W.B.; Machan, C.H.; Zachary, A.A. The distribution of HLA antigens and phenotypes among donors and patients in the UNOS registry. Transplantation 1994, 58, 1119–1130. [Google Scholar] [CrossRef]
- Starzl, T.E.; Eliasziw, M.; Gjertson, D.; Terasaki, P.I.; Fung, J.J.; Trucco, M.; Martell, J.; McMichael, J.; Scantlebury, V.; Shapiro, R.; et al. HLA and cross-reactive antigen group matching for cadaver kidney allocation. Transplantation 1997, 64, 983–991. [Google Scholar] [CrossRef]
- Zachary, A.A.; Leffell, M.S. HLA mismatching strategies for solid organ transplantation—A balancing act. Front. Immunol. 2016, 7, 575–591. [Google Scholar] [CrossRef]
- Duquesnoy, R.J.; Kamoun, M.; Baxter-Lowe, L.A.; Woodle, E.S.; Bray, R.A.; Claas, F.H.J.; Eckels, D.D.; Friedewald, J.J.; Fuggle, S.V.; Gebel, H.M.; et al. Should HLA mismatch acceptability for sensitized transplant candidates be determined at the high-resolution rather than the antigen level? Am. J. Transplant. 2015, 15, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Claas, F.H.; Smeenk, R.J.; Schmidt, R.; van Steenbrugge, G.J.; Eernisse, J.G. Alloimmunization against the MHC antigens after platelet transfusions is due to contaminating leukocytes in the platelet suspension. Exp. Hematol. 1981, 9, 84–89. [Google Scholar] [PubMed]
- Little, A.M.; Green, A.; Harvey, J.; Hemmatpour, S.; Latham, K.; Marsh, S.G.E.; Poulton, K.; Sage, D. BSHI Guideline: HLA matching and donor selection for haematopoietic progenitor cell transplantation. Int. J. Immunogenet. 2016, 43, 263–286. [Google Scholar] [CrossRef] [PubMed]
- Claas, F.H. Clinical relevance of circulating donor-specific HLA antibodies. Curr. Opin. Organ Transplant. 2010, 15, 462–466. [Google Scholar] [CrossRef]
- Spellman, S.; Bray, R.; Rosen-Bronson, S.; Haagenson, M.; Klein, J.; Flesch, S.; Vierra-Green, C.; Anasetti, C. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood 2010, 115, 2704–2708. [Google Scholar] [CrossRef]
- Ciurea, S.O.; De Lima, M.; Cano, P.; Korbling, M.; Giralt, S.; Shpall, E.J.; Wang, X.; Thall, P.F.; Champlin, R.E. High risk of graft failure in patients with anti-hla antibodies undergoing haploidentical stem-cell transplantation. Transplantation 2009, 88, 1019–1024. [Google Scholar] [CrossRef]
- Leffell, M.S.; Cao, K.; Coppage, M.; Hansen, J.A.; Hart, J.M.; Pereira, N.; Pereira, S.; Reinsmoen, N.L.; Senitzer, D.; Smith, A.; et al. Incidence of humoral sensitization in HLA partially mismatched hematopoietic stem cell transplantation. Tissue Antigens 2009, 74, 494–498. [Google Scholar] [CrossRef]
- Horan, J.; Wang, T.; Haagenson, M.; Spellman, S.R.; Dehn, J.; Eapen, M.; Frangoul, H.; Gupta, V.; Hale, G.A.; Hurley, C.K.; et al. Evaluation of HLA matching in unrelated hematopoietic stem cell transplantation for nonmalignant disorders. Blood 2012, 120, 2918–2924. [Google Scholar] [CrossRef]
- Moreau, A.; Varey, E.; Anegon, I.; Cuturi, M.C. Effector mechanisms of rejection. Cold Spring Harb. Perspect. Med. 2013, 2013, 015461. [Google Scholar] [CrossRef]
- Ochando, J.C.; Krieger, N.R.; Bromberg, J.S. Direct versus indirect allorecognition: Visualization of dendritic cell distribution and interactions during rejection and tolerization. Am. J. Transplant. 2006, 6, 2488–2496. [Google Scholar] [CrossRef]
- Herrera, O.B.; Golshayan, D.; Tibbott, R.; Salcido Ochoa, F.; James, M.J.; Marelli-Berg, F.M.; Lechler, R.I. A novel pathway of alloantigen presentation by dendritic cells. J. Immunol. 2004, 173, 4828–4837. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.A.; Herrera, O.B.; Golshayan, D.; Lombardi, G.; Lechler, R.I. A novel pathway of antigen presentation by dendritic and endothelial cells: Implications for allorecognition and infectious diseases. Transplantation 2006, 82, S15–S18. [Google Scholar] [CrossRef] [PubMed]
- Swijnenburg, R.J.; Tanaka, M.; Vogel, H.; Baker, J.; Kofidis, T.; Gunawan, F.; Lebl, D.R.; Caffarelli, A.D.; De Bruin, J.L.; Fedoseyeva, E.V.; et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 2005, 112, 166–182. [Google Scholar]
- Kofidis, T.; DeBruin, J.L.; Tanaka, M.; Zwierzchoniewska, M.; Weissman, I.; Fedoseyeva, E.; Haverich, A.; Robbins, R.C. They are not stealthy in the heart: Embryonic stem cells trigger cell infiltration, humoral and T-lymphocyte-based host immune response. Eur. J. Cardiothorac. Surg. 2005, 28, 461–479. [Google Scholar] [CrossRef]
- Drukker, M.; Katz, G.; Urbach, A.; Schuldiner, M.; Markel, G.; Itskovitz-Eldor, J.; Reubinoff, B.; Mandelboim, O.; Benvenisty, N. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9864–9884. [Google Scholar] [CrossRef]
- Bocelli-Tyndall, C.; Zajac, P.; Di Maggio, N.; Trella, E.; Benvenuto, F.; Iezzi, G.; Scherberich, A.; Barbero, A.; Schaeren, S.; Pistoia, V.; et al. Fibroblast growth factor 2 and platelet-derived growth factor, but not platelet lysate, induce proliferation-dependent, functional class II major histocompatibility complex antigen in human mesenchymal stem cells. Arthritis Rheum. 2010, 62, 3815–3828. [Google Scholar] [CrossRef]
- Suarez-Alvarez, B.; Rodriguez, R.M.; Calvanese, V.; Blanco-Gelaz, M.A.; Suhr, S.T.; Ortega, F.; Otero, J.; Cibelli, J.B.; Moore, H.; Fraga, M.F.; et al. Epigenetic mechanisms regulate MHC and antigen processing molecules in human embryonic and induced pluripotent stem cells. PLoS ONE 2010, 5, e10192. [Google Scholar] [CrossRef]
- Golpanian, S.; Di Fede, D.L.; Khan, A.; Schulman, I.H.; Landin, A.M.; Tompkins, B.A.; Heldman, A.W.; Miki, R.; Goldstein, B.J.; Mushtaq, M.; et al. Allogeneic human mesenchymal stem cell infusions for aging frailty. J. Gerontol. Ser. A 2017, 72, 1505–1512. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M.; et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Campisi, J.; D’Adda di Fagagna, F. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Bertolo, A.; Baur, M.; Guerrero, J.; Pötzel, T.; Stoyanov, J. Autofluorescence is a Reliable in vitro Marker of Cellular Senescence in Human Mesenchymal Stromal Cells. Sci. Rep. 2019, 9, 2074. [Google Scholar] [CrossRef] [PubMed]
- Giai Via, A.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal Stem Cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar]
- Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the Approximation of the Laws, Regulations and Administrative Provisions of the Member States Relating to the Implementation of Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32001L0020 (accessed on 24 September 2019).
- Commission Directive 2003/94/EC of 8 October 2003 Laying Down the Principles and Guidelines of Good Manufacturing Practice in Respect of Medicinal Products for Human Use and Investigational Medicinal Products for HUMAN Use. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32003L0094 (accessed on 24 September 2019).
- Daley, G.Q.; Hyun, I.; Apperley, J.F.; Barker, R.A.; Benvenisty, N.; Bredenoord, A.L.; Breuer, C.K.; Caulfield, T.; Cedars, M.I.; Frey-Vasconcells, J.; et al. Setting global standards for stem cell research and clinical translation: The 2016 ISSCR guidelines. Stem Cell Rep. 2016, 6, 787–797. [Google Scholar] [CrossRef] [PubMed]
- EudraLex. The Rules Governing Medicinal Products in the European Union Good Manufacturing Practice Guidelines on Good Manufacturing Practice specific to Advanced Therapy Medicinal Products; European Commission: Brussels, Belgium, 2017; Volume 4, pp. 1–88. Available online: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/2017_11_22_guidelines_gmp_for_atmps (accessed on 24 September 2019).
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kot, M.; Baj-Krzyworzeka, M.; Szatanek, R.; Musiał-Wysocka, A.; Suda-Szczurek, M.; Majka, M. The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies. Int. J. Mol. Sci. 2019, 20, 5680. https://doi.org/10.3390/ijms20225680
Kot M, Baj-Krzyworzeka M, Szatanek R, Musiał-Wysocka A, Suda-Szczurek M, Majka M. The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies. International Journal of Molecular Sciences. 2019; 20(22):5680. https://doi.org/10.3390/ijms20225680
Chicago/Turabian StyleKot, Marta, Monika Baj-Krzyworzeka, Rafał Szatanek, Aleksandra Musiał-Wysocka, Magdalena Suda-Szczurek, and Marcin Majka. 2019. "The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies" International Journal of Molecular Sciences 20, no. 22: 5680. https://doi.org/10.3390/ijms20225680
APA StyleKot, M., Baj-Krzyworzeka, M., Szatanek, R., Musiał-Wysocka, A., Suda-Szczurek, M., & Majka, M. (2019). The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies. International Journal of Molecular Sciences, 20(22), 5680. https://doi.org/10.3390/ijms20225680





