Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury
Abstract
:1. Introduction
2. Results
2.1. Effects of MSC-CM on the Histopathology of Endotoxin-Induced ALI
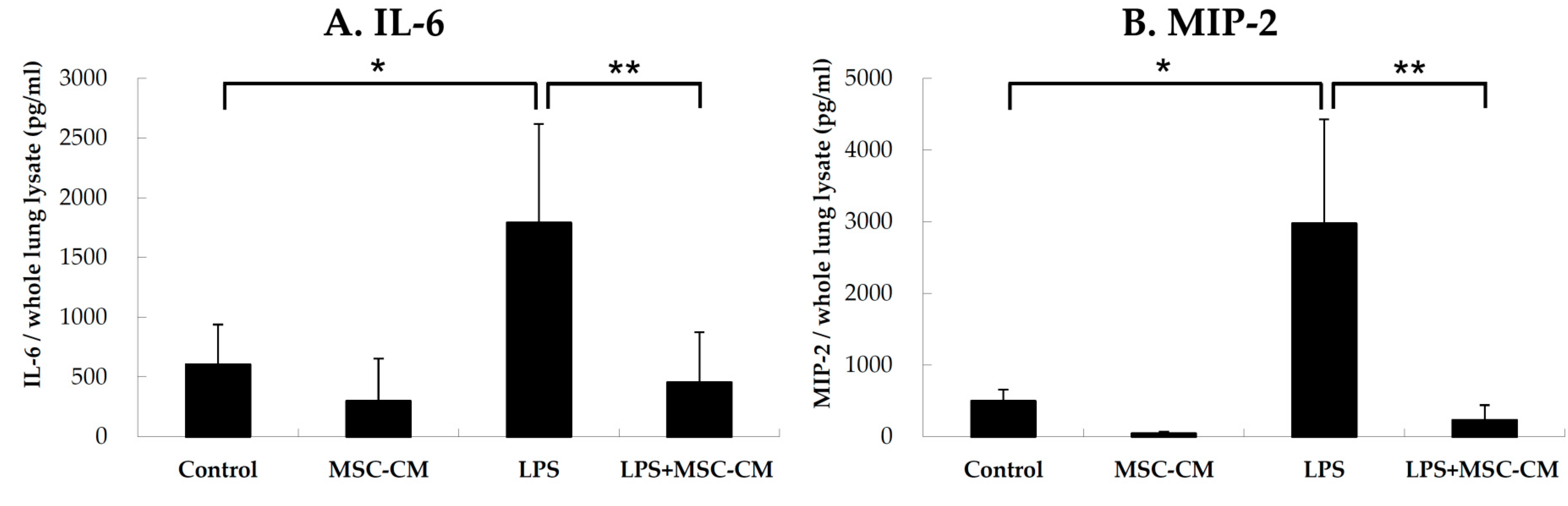
2.2. Effects of MSC-CM on Proinflammatory Cytokines in Lung Tissues
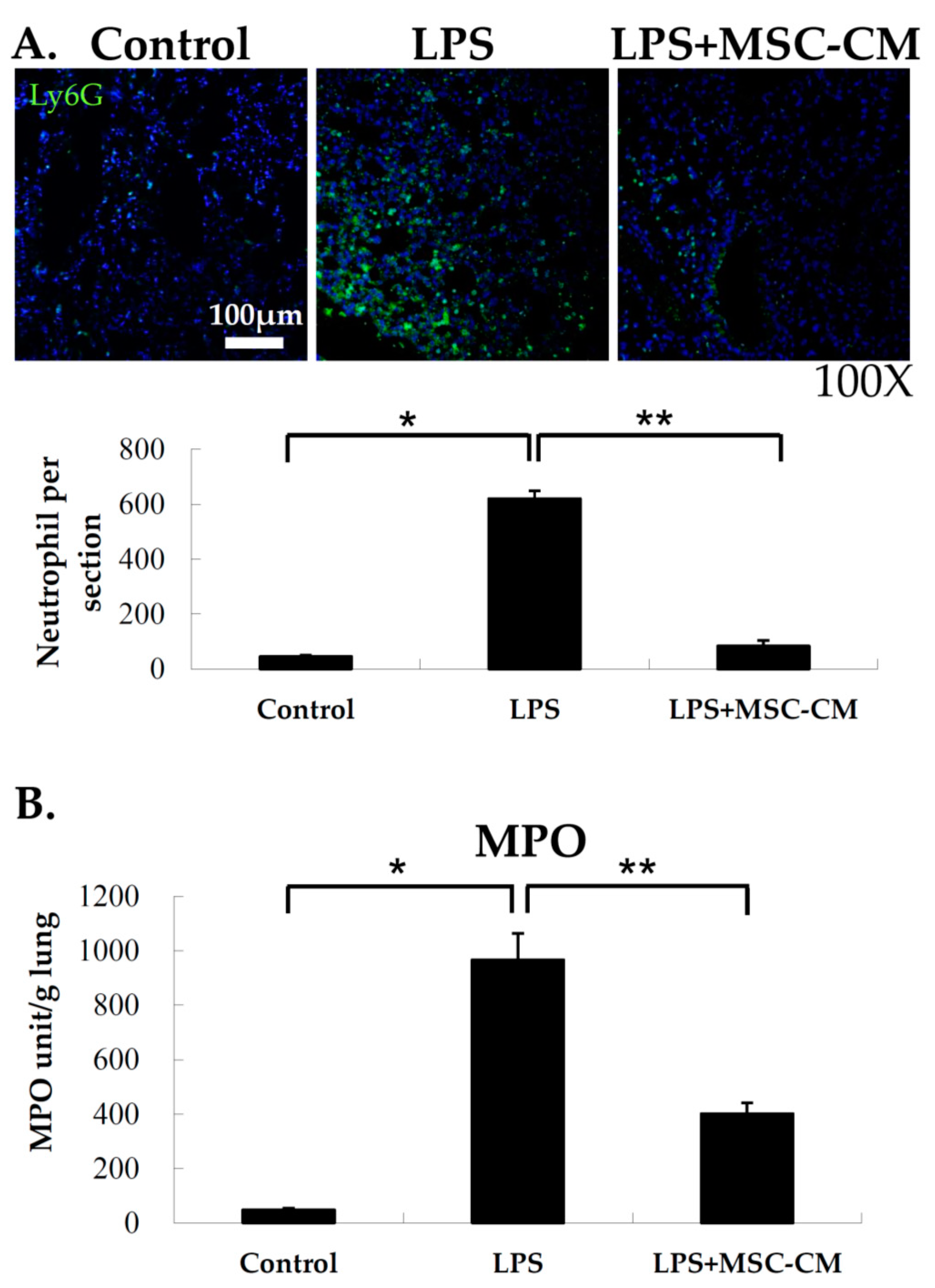
2.3. Effects of MSC-CM on Neutrophil Accumulation in Lung Tissues
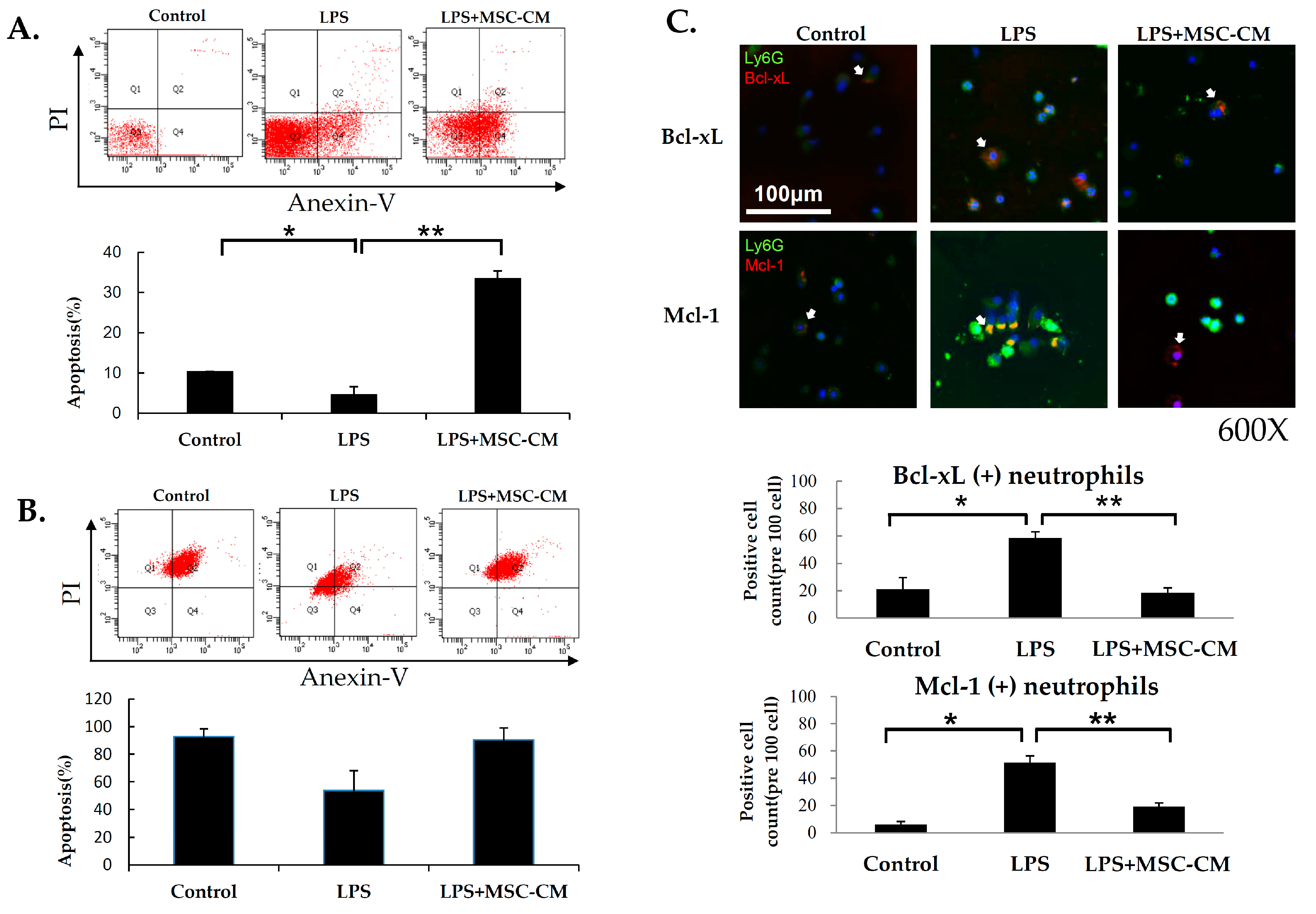
2.4. Effects of MSC-CM on Neutrophil Apoptosis in ALI
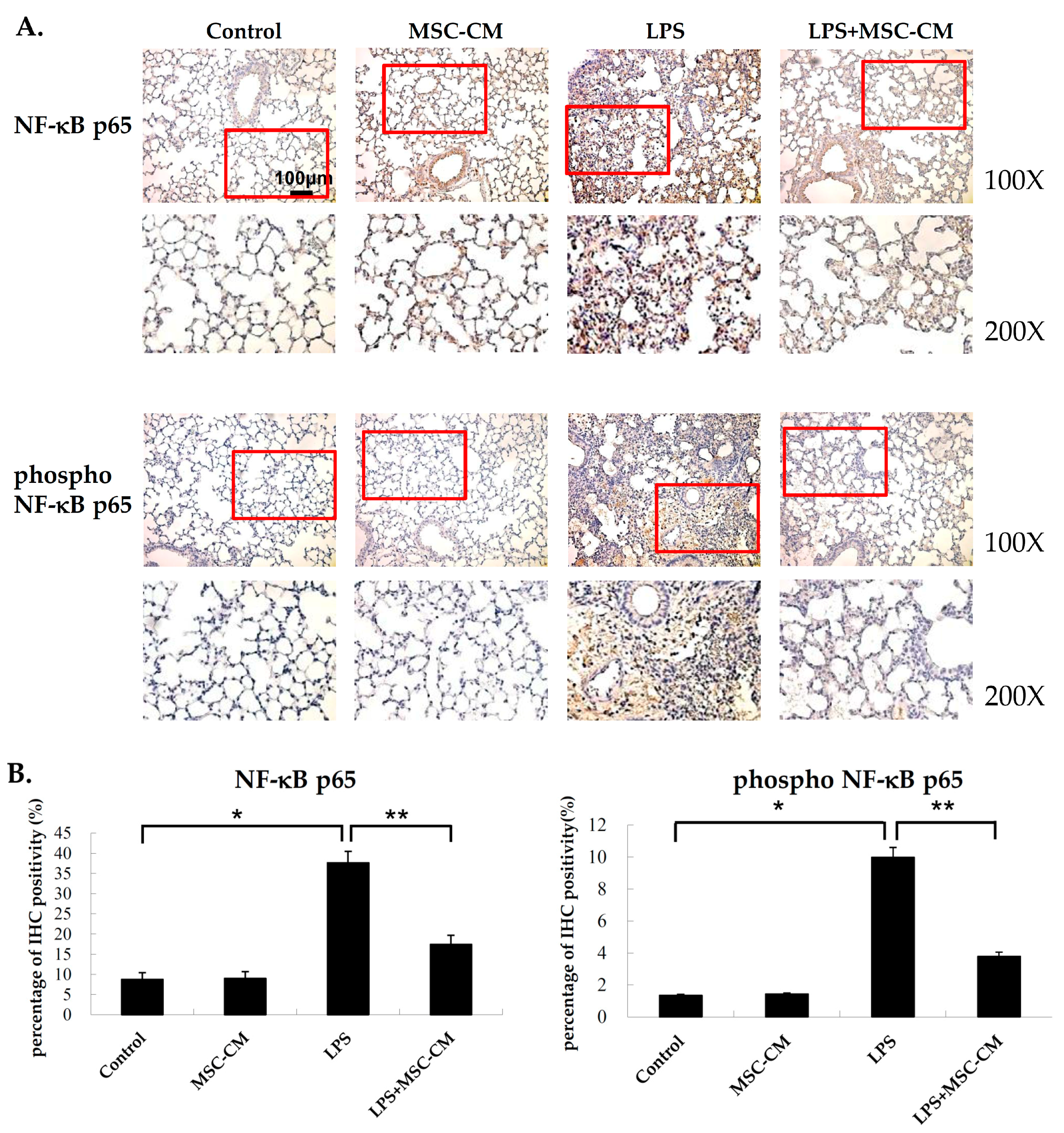
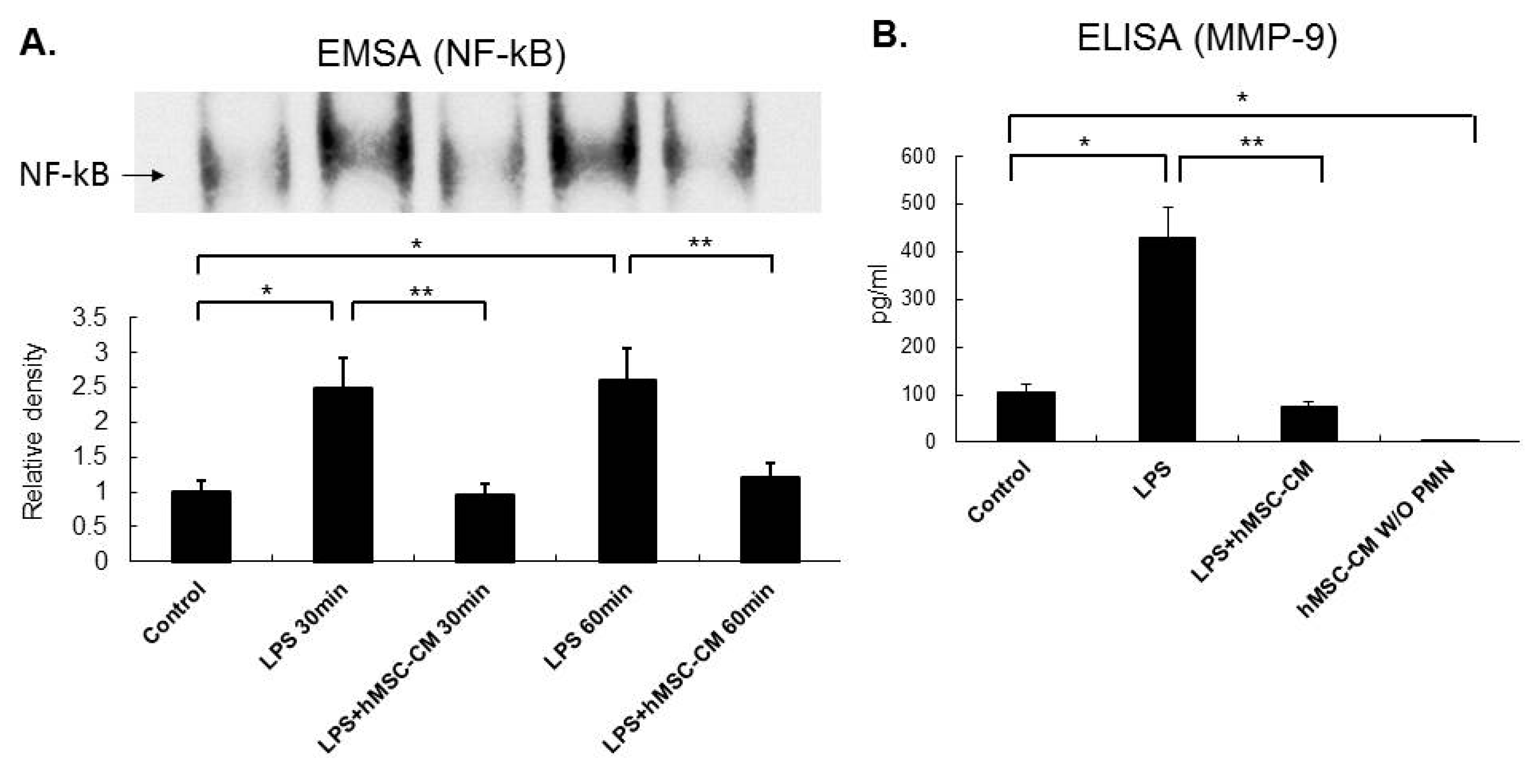
2.5. Effects of MSC-CM on the Expression of NF-kB and MMP-9 in Mouse and Human Neutrophils
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Experimental Design
4.3. Generation of Murine and Human MSC-CM
4.4. Isolation of Neutrophils from BALF
4.5. Histology and IHC of Lung Tissue Sections
4.6. Lung Injury Scoring of Lung Tissue Sections
4.7. Enzyme-Linked Immunosorbent Assay (ELISA) Assay
4.8. Confocal Microscopy
4.9. Myeloperoxidase (MPO) Activity Assay
4.10. Flow Cytometry
4.11. Patients with ARDS and Isolation of Human Neutrophils
4.12. Electrophoretic Mobility Shift Assay (EMSA)
4.13. Apoptosis Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Availability of Data and Materials
Ethics Approval and Consent to Participate
Conflicts of Interest
References
- Sweeney, R.M.; McAuley, D.F. Acute respiratory distress syndrome. Lancet 2016, 388, 2416–2430. [Google Scholar] [CrossRef]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef]
- Williams, A.E.; Chambers, R.C. The mercurial nature of neutrophils: Still an enigma in ards? Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 306, L217–L230. [Google Scholar] [CrossRef]
- Williams, A.E.; Jose, R.J.; Mercer, P.F.; Brealey, D.; Parekh, D.; Thickett, D.R.; O’Kane, C.; McAuley, D.F.; Chambers, R.C. Evidence for chemokine synergy during neutrophil migration in ards. Thorax 2017, 72, 66–73. [Google Scholar] [CrossRef]
- Kangelaris, K.N.; Prakash, A.; Liu, K.D.; Aouizerat, B.; Woodruff, P.G.; Erle, D.J.; Rogers, A.; Seeley, E.J.; Chu, J.; Liu, T.; et al. Increased expression of neutrophil-related genes in patients with early sepsis-induced ards. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L1102–L1113. [Google Scholar] [CrossRef]
- Chopra, M.; Reuben, J.S.; Sharma, A.C. Acute lung injury:Apoptosis and signaling mechanisms. Exp. Biol. Med. 2009, 234, 361–371. [Google Scholar] [CrossRef]
- Rojas, M.; Xu, J.; Woods, C.R.; Mora, A.L.; Spears, W.; Roman, J.; Brigham, K.L. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am. J. Respir Cell Mol. Biol. 2005, 33, 145–152. [Google Scholar] [CrossRef]
- Gupta, N.; Su, X.; Popov, B.; Lee, J.W.; Serikov, V.; Matthay, M.A. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J. Immunol. 2007, 179, 1855–1863. [Google Scholar] [CrossRef]
- Xu, J.; Woods, C.R.; Mora, A.L.; Joodi, R.; Brigham, K.L.; Iyer, S.; Rojas, M. Prevention of endotoxin-induced systemic response by bone marrow-derived mesenchymal stem cells in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L131–L141. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Abratte, C.M.; Schimenti, J.C.; Felippe, M.J.; Cassano, J.M.; Southard, T.L.; Cross, J.A.; Fortier, L.A. Induced pluripotent stem cells have similar immunogenic and more potent immunomodulatory properties compared with bone marrow-derived stromal cells in vitro. Regen. Med. 2014, 9, 621–635. [Google Scholar] [CrossRef]
- Su, V.Y.; Chiou, S.H.; Lin, C.S.; Chen, W.C.; Yu, W.K.; Chen, Y.W.; Chen, C.Y.; Yang, K.Y. Induced pluripotent stem cells reduce neutrophil chemotaxis via activating grk2 in endotoxin-induced acute lung injury. Respirology 2017, 22, 1156–1164. [Google Scholar] [CrossRef]
- Yang, K.Y.; Shih, H.C.; How, C.K.; Chen, C.Y.; Hsu, H.S.; Yang, C.W.; Lee, Y.C.; Perng, R.P.; Peng, C.H.; Li, H.Y.; et al. Iv delivery of induced pluripotent stem cells attenuates endotoxin-induced acute lung injury in mice. Chest 2011, 140, 1243–1253. [Google Scholar] [CrossRef]
- Su, V.Y.; Yang, K.Y.; Chiou, S.H.; Chen, N.J.; Mo, M.H.; Lin, C.S.; Wang, C.T. Induced pluripotent stem cells regulate triggering receptor expressed on myeloid cell-1 expression and the p38 mitogen-activated protein kinase pathway in endotoxin-induced acute lung injury. Stem Cells 2019. [Google Scholar] [CrossRef]
- Haslett, C. Granulocyte apoptosis and inflammatory disease. Br. Med Bull. 1997, 53, 669–683. [Google Scholar] [CrossRef]
- Fialkow, L.; Fochesatto Filho, L.; Bozzetti, M.C.; Milani, A.R.; Rodrigues Filho, E.M.; Ladniuk, R.M.; Pierozan, P.; de Moura, R.M.; Prolla, J.C.; Vachon, E.; et al. Neutrophil apoptosis: A marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit. Care 2006, 10, R155. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Liles, W.C.; Radella, F., 2nd; Steinberg, K.P.; Ruzinski, J.T.; Jonas, M.; Chi, E.Y.; Hudson, L.D.; Martin, T.R. Neutrophil apoptosis in the acute respiratory distress syndrome. Am. J. Respir Crit Care Med. 1997, 156, 1969–1977. [Google Scholar] [CrossRef]
- Esmann, L.; Idel, C.; Sarkar, A.; Hellberg, L.; Behnen, M.; Moller, S.; van Zandbergen, G.; Klinger, M.; Kohl, J.; Bussmeyer, U.; et al. Phagocytosis of apoptotic cells by neutrophil granulocytes: Diminished proinflammatory neutrophil functions in the presence of apoptotic cells. J. Immunol. 2010, 184, 391–400. [Google Scholar] [CrossRef]
- Teixeira, C.F.; Landucci, E.C.; Antunes, E.; Chacur, M.; Cury, Y. Inflammatory effects of snake venom myotoxic phospholipases a2. Toxicon: Off. J. Int. Soc. Toxinol. 2003, 42, 947–962. [Google Scholar] [CrossRef]
- Lin, W.C.; Lin, C.F.; Chen, C.L.; Chen, C.W.; Lin, Y.S. Inhibition of neutrophil apoptosis via sphingolipid signaling in acute lung injury. J. Pharmacol. Exp. Ther. 2011, 339, 45–53. [Google Scholar] [CrossRef]
- Perl, M.; Hohmann, C.; Denk, S.; Kellermann, P.; Lu, D.; Braumuller, S.; Bachem, M.G.; Thomas, J.; Knoferl, M.W.; Ayala, A.; et al. Role of activated neutrophils in chest trauma-induced septic acute lung injury. Shock 2012, 38, 98–106. [Google Scholar] [CrossRef]
- Tak, P.P.; Firestein, G.S. Nf-kappab: A key role in inflammatory diseases. J. Clin. Invest. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kupfner, J.G.; Arcaroli, J.J.; Yum, H.K.; Nadler, S.G.; Yang, K.Y.; Abraham, E. Role of nf-kappab in endotoxemia-induced alterations of lung neutrophil apoptosis. J. Immunol. 2001, 167, 7044–7051. [Google Scholar] [CrossRef]
- Ward, C.; Chilvers, E.R.; Lawson, M.F.; Pryde, J.G.; Fujihara, S.; Farrow, S.N.; Haslett, C.; Rossi, A.G. Nf-kappab activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 1999, 274, 4309–4318. [Google Scholar] [CrossRef]
- Castro-Alcaraz, S.; Miskolci, V.; Kalasapudi, B.; Davidson, D.; Vancurova, I. Nf-kappa b regulation in human neutrophils by nuclear i kappa b alpha: Correlation to apoptosis. J. Immunol. 2002, 169, 3947–3953. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Blackwell, T.R.; Christman, J.W. Impaired activation of nuclear factor-kappab in endotoxin-tolerant rats is associated with down-regulation of chemokine gene expression and inhibition of neutrophilic lung inflammation. J. Immunol. 1997, 158, 5934–5940. [Google Scholar] [PubMed]
- Blackwell, T.S.; Blackwell, T.R.; Holden, E.P.; Christman, B.W.; Christman, J.W. In vivo antioxidant treatment suppresses nuclear factor-kappa b activation and neutrophilic lung inflammation. J Immunol. 1996, 157, 1630–1637. [Google Scholar] [PubMed]
- Lee, J.W.; Fang, X.; Krasnodembskaya, A.; Howard, J.P.; Matthay, M.A. Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells 2011, 29, 913–919. [Google Scholar] [CrossRef] [PubMed]
- Wolpe, S.D.; Sherry, B.; Juers, D.; Davatelis, G.; Yurt, R.W.; Cerami, A. Identification and characterization of macrophage inflammatory protein 2. Proc. Natl Acad Sci USA 1989, 86, 612–616. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Ono, S.; Mochizuki, H.; Aosasa, S.; Majima, T.; Ueno, C.; Matsumoto, A. Role of macrophage inflammatory protein 2 in acute lung injury in murine peritonitis. J. Surg Res. 2002, 103, 61–67. [Google Scholar] [CrossRef]
- Raffaghello, L.; Bianchi, G.; Bertolotto, M.; Montecucco, F.; Busca, A.; Dallegri, F.; Ottonello, L.; Pistoia, V. Human mesenchymal stem cells inhibit neutrophil apoptosis: A model for neutrophil preservation in the bone marrow niche. Stem Cells 2008, 26, 151–162. [Google Scholar] [CrossRef]
- Liu, S.; Su, X.; Pan, P.; Zhang, L.; Hu, Y.; Tan, H.; Wu, D.; Liu, B.; Li, H.; Li, H.; et al. Neutrophil extracellular traps are indirectly triggered by lipopolysaccharide and contribute to acute lung injury. Sci. Rep. 2016, 6, 37252. [Google Scholar] [CrossRef]
- Hung, S.C.; Pochampally, R.R.; Chen, S.C.; Hsu, S.C.; Prockop, D.J. Angiogenic effects of human multipotent stromal cell conditioned medium activate the pi3k-akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells 2007, 25, 2363–2370. [Google Scholar] [CrossRef]
- Yang, K.Y.; Arcaroli, J.J.; Abraham, E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am. J. Respir Crit Care Med. 2003, 167, 1567–1574. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, V.Y.-F.; Lin, C.-S.; Hung, S.-C.; Yang, K.-Y. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. Int. J. Mol. Sci. 2019, 20, 2208. https://doi.org/10.3390/ijms20092208
Su VY-F, Lin C-S, Hung S-C, Yang K-Y. Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. International Journal of Molecular Sciences. 2019; 20(9):2208. https://doi.org/10.3390/ijms20092208
Chicago/Turabian StyleSu, Vincent Yi-Fong, Chi-Shiuan Lin, Shih-Chieh Hung, and Kuang-Yao Yang. 2019. "Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury" International Journal of Molecular Sciences 20, no. 9: 2208. https://doi.org/10.3390/ijms20092208
APA StyleSu, V. Y.-F., Lin, C.-S., Hung, S.-C., & Yang, K.-Y. (2019). Mesenchymal Stem Cell-Conditioned Medium Induces Neutrophil Apoptosis Associated with Inhibition of the NF-κB Pathway in Endotoxin-Induced Acute Lung Injury. International Journal of Molecular Sciences, 20(9), 2208. https://doi.org/10.3390/ijms20092208





